Synthesis of N-aryl-1-aminoindoles via intermolecular redox amination†
Abstract
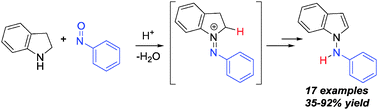
A redox amination strategy was developed for the synthesis of N-aryl-1-aminoindoles by N–N bond formation. Reaction of nitrosobenzenes with readily available indolines using Brønsted acid catalysis allows N–N bond formation under mild conditions. This method exploits the inherent reducing power of indoline to synthesize biologically relevant molecular architectures via redox amination. A one-pot synthesis of 1-aminoindoles starting from simple aniline and indolines is likewise described.

 Please wait while we load your content...
Please wait while we load your content...