Geometric and redox flexibility of pyridine as a redox-active ligand that can reversibly accept one or two electrons†
Abstract
A low-coordinate iron(I) species can reversibly reduce pyridine, either by one electron to give a new C–C bond, or by two electrons to give a pyridine-derived bridge with an unprecedented μ-η1:η3 binding mode.
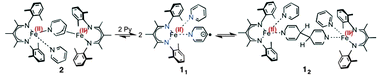
- This article is part of the themed collection: Non-Innocent Ligands

 Please wait while we load your content...
Please wait while we load your content...