Anion–π interactions and positive electrostatic potentials of N-heterocycles arise from the positions of the nuclei, not changes in the π-electron distribution†
Abstract
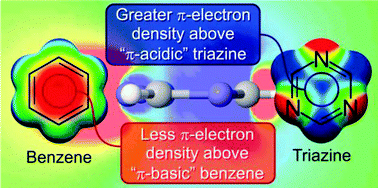
We show that the positive electrostatic potentials and molecular quadrupole moments characteristic of π-acidic azines, which underlie the ability of these rings to bind anions above their centres, arise from the position of nuclear charges, not changes in the π-electron density distribution.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions

 Please wait while we load your content...
Please wait while we load your content...