Exploring O-stannyl ketyl and acyl radical cyclizations for the synthesis of γ-lactone-fused benzopyrans and benzofurans†
Abstract
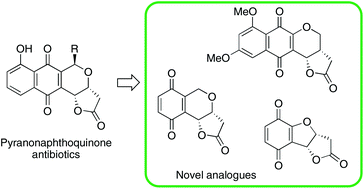
The synthesis of a series of γ-lactone-fused benzopyrans and benzofurans, analogues of the pyranonaphthoquinone antibiotics, is reported. Preparation of the heterocycles was achieved by either O-stannyl ketyl or acyl radical cyclization of benzaldehyde precursors followed by oxidation to give the pyrano- and furanobenzoquinone systems. The observed diastereoselectivity during O-stannyl ketyl radical cyclization is influenced by aromatic substitution ortho to the aldehyde, whilst acyl radical cyclization followed by stereoselective reduction of the resulting pyranones provides a complimentary approach to forming the required γ-lactone-fused benzopyran systems.

 Please wait while we load your content...
Please wait while we load your content...