A new tetraarylcyclopentadienone based low molecular weight gelator: synthesis, self-assembly properties and anion recognition†‡
Abstract
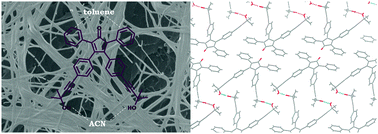
A new class of tetraarylcyclopentadienones bearing 3-hydroxy-1-propynyl substituents has been synthesized. One of them, 3,4-bis (4-(3-hydroxy-3-methylbut-1-ynyl) phenyl)-2,5-diphenylcyclopenta-2,4-dienone, exhibits pronounced aggregation properties in various organic solvents responding to thermal and ultrasound stimuli and represents the first example of a tetraarylcyclopentadienone based low molecular weight organogelator. The hydroxydimethyl group on the ethynyl substituent proved to be essential to perform the gelation process. The 1H NMR analysis and FT-IR spectroscopy suggested that the intermolecular π–π and hydrogen bonding interactions of the gelator with the solvent are the main driving forces for the supramolecular assembly. The SEM images of xerogels show the characteristic gelation morphologies of 3D fibrous network structures. Fluorescence and UV/Vis absorption studies provided more information to define the molecular packing model in the gelation state. In addition the obtained gels show selective response to the fluoride anion.

 Please wait while we load your content...
Please wait while we load your content...