The importance of adding EDTA for the nanopore analysis of proteins
Abstract
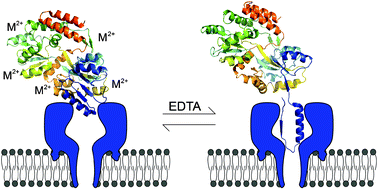
Nanopore analysis is a promising technique for studying the conformation of
Maintenance work is planned from 09:00 BST to 12:00 BST on Saturday 28th September 2024.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
Department of Biochemistry, 107 Wiggins Road, University of Saskatchewan, Saskatoon, SK, Canada S7N 5E5
E-mail:
Jeremy.lee@usask.ca
Nanopore analysis is a promising technique for studying the conformation of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
