Zn induced structural aggregation patterns of β-amyloid peptides by first-principle simulations and XAS measurements
Abstract
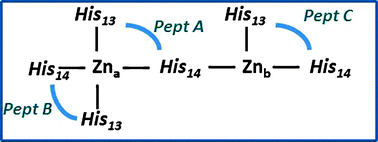
We show in this paper that in the presence of Zn ions a peculiar structural aggregation pattern of β-amyloid
Maintenance work is planned from 09:00 BST to 12:00 BST on Saturday 28th September 2024.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a University of Udine, Department of Chemistry, Physics and Environment, via del Cotonificio 108, 33100 Udine, Italy
b John von Neumann Institute for Computing—DESY Zeuthen, Platanen allee 6, 15738 Zeuthen, Germany
c
National Research Council, Institute for Chemistry of Organo-metallic Compounds, via Madonna del Piano 10, 50019 Sesto fiorentino (Firenze), Italy
E-mail:
glapenna@iccom.cnr.it
Fax: +39 0555225203
Tel: +39 0555225264
d Department of Physics, University of Roma “Tor Vergata” and National Institute of Nuclear Physics, Section of Roma “Tor Vergata”, via della Ricerca Scientifica, 00133 Roma, Italy
e Centre for Free-Electron Laser Science—DESY, Notkestrasse 85, 22607 Hamburg, Germany
We show in this paper that in the presence of Zn ions a peculiar structural aggregation pattern of β-amyloid

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
