Cu(ii)- and disulfide bonds-induced stabilization during the guanidine hydrochloride- and thermal-induced denaturation of NAD-glycohydrolase from the venom of Agkistrodon acutus
Abstract
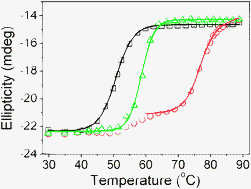
NAD-glycohydrolase (AA-NADase) from Agkistrodon acutus venom is a unique multicatalytic enzyme with both NADase and AT(D)Pase-like activities. Among all identified NADases, only AA-NADase is a disulfide-linked dimer and contains Cu2+. Cu2+ and

 Please wait while we load your content...
Please wait while we load your content...