A steric control on the reductive capacity of ytterbocenes towards iminopyridine ligands is described. The reaction of (η5-C9H7)2Yb(THF)2 with a series of 6-organyl-2-(aldimino)pyridyl ligands (IPy) takes place with the replacement of two THF molecules by one IPy unit. In contrast to the rich reductive ytterbocene chemistry described in the presence of the unsubstituted (aldimino)pyridyl ligand, all 6-aryl substituted IPys scrutinized hereafter are involved into the metal coordination as neutral bidentate {N,N} or tridentate {N,N,S; N,N,O} ligands, with no changes of the metal oxidation state in the final complexes. A series of YbII metallocene complexes of general formula (η5-C9H7)2YbII(η2 or η3)[2,6-iPr2(C6H3)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(C5H3N)-6-R)] have been isolated and completely characterized. The stereo-electronic role of the aryl substituents in the IPy ligands on the ytterbocene redox chemistry has also been addressed.
CH(C5H3N)-6-R)] have been isolated and completely characterized. The stereo-electronic role of the aryl substituents in the IPy ligands on the ytterbocene redox chemistry has also been addressed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(C5H3N)-6-R)] have been isolated and completely characterized. The stereo-electronic role of the
CH(C5H3N)-6-R)] have been isolated and completely characterized. The stereo-electronic role of the 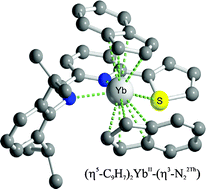

 Please wait while we load your content...
Please wait while we load your content...