Donor-stabilised cations, phosphinamide anions, and unusual oxidative cyclisation products from halogenated phosphoranimines and phosphinimines with a bulky 2,4,6-tri-tert-butylphenyl substituent at nitrogen†
Abstract
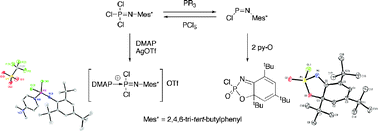
New aspects of the chemistry of the phosphoranimine Cl3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMes* (Mes* = 2,4,6-tri-tert-butylphenyl) (7) and the phosphinimine ClP
NMes* (Mes* = 2,4,6-tri-tert-butylphenyl) (7) and the phosphinimine ClP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMes* (2) have been explored. A cationic derivative of 7 was prepared from the reaction between this species and
NMes* (2) have been explored. A cationic derivative of 7 was prepared from the reaction between this species and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMes*]+ ([9]+). When treated with tertiary phosphinesnBu3P or Ph3P, 7 was found to undergo a reductive dechlorination reaction to yield 2 and dichlorophosphoranes R3PCl2 (R = nBu (13a), Ph (13b)). The phosphinimine 2 reacts with Cl− sources to form the novel dichlorophosphinamide anion [Cl2PNMes*]− ([14]−) which was characterized in solution. Treatment of [Ph4P][14], generated in situ, with GaCl3 or MeOTf regenerated 2 and provided further evidence for the formation of the anion [14]−. In addition, phosphoranimine 2 was found to undergo an unexpected oxidative cyclization reaction when treated with the oxygen transfer agent
NMes*]+ ([9]+). When treated with tertiary phosphinesnBu3P or Ph3P, 7 was found to undergo a reductive dechlorination reaction to yield 2 and dichlorophosphoranes R3PCl2 (R = nBu (13a), Ph (13b)). The phosphinimine 2 reacts with Cl− sources to form the novel dichlorophosphinamide anion [Cl2PNMes*]− ([14]−) which was characterized in solution. Treatment of [Ph4P][14], generated in situ, with GaCl3 or MeOTf regenerated 2 and provided further evidence for the formation of the anion [14]−. In addition, phosphoranimine 2 was found to undergo an unexpected oxidative cyclization reaction when treated with the oxygen transfer agent
- This article is part of the themed collection: Dalton Transactions 40th Anniversary

 Please wait while we load your content...
Please wait while we load your content...