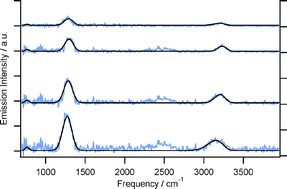
The ν4 + ν5 combination band, which appears relatively weak in the IR absorption spectrum, has been identified with exceptionally high intensity in the IR emission spectra from highly vibrationally excited acetylene, which is produced with ∼71 kcal mol−1 of vibrational energy from the 193 nm photolysis of vinyl bromide. The ‘fundamental’ transition of this combination band, from the (0,0,0,11,1−1) level to the zero point, occurs at 1328 cm−1. The intensity and frequency of this band as well as the ν3 and ν5 bands, IR active but with lower emission intensity, as a function of the acetylene energy can be modeled accurately using the normal mode harmonic oscillator model with frequency anharmonicity corrections. Good fitting results are achieved even though the normal mode quantum numbers are no longer good for levels in the high energy region and the combination band is forbidden in the harmonic oscillator model. The identification of this intense combination band in emission, compared to its weak intensity in the absorption spectrum, highlights the necessity to include in consideration the combination bands for assignment of emission spectra in general and in particular emission from vibrationally hot acetylene which is ample in combustion, atmospheric, and interstellar environments.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...