The conformation and orientational order of a 1,2-disubstituted ethane nematogenic molecule (I22) in liquid crystalline and isotropic phases studied by NMR spectroscopy†
Abstract
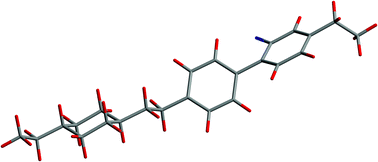
The structure, conformation and orientational order of the mesogen I22 have been studied by

 Please wait while we load your content...
Please wait while we load your content...