Hong-Qiang Shen, Cong Liu, Ji Zhou and Yong-Gui Zhou
Org. Chem. Front., 2018,5, 611-614
DOI:
10.1039/C7QO00815E,
Research Article
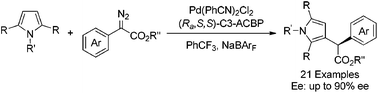
An enantioselective C–H functionalization of pyrrole derivatives with diazo compounds has been successfully realized with up to 90% ee by employing dichlorobis(benzonitrile)palladium with an axially chiral bipyridine ligand C3-ACBP as the catalyst. Asymmetric C–H functionalization at the gram scale was also conducted smoothly with good reactivity and enantioselectivity.