Efficiency optimization of BaY2Al2−yScyGa2SiO12:xCr3+ garnet phosphors with sustained anti-thermal quenching behavior†
Haiyan
Shi
,
Yan
Yuan
,
Desheng
Yin
 ,
Xiaohong
Zhang
,
Pengbo
Lyu
,
Xiaohong
Zhang
,
Pengbo
Lyu
 *,
Changfu
Xu
* and
Lizhong
Sun
*,
Changfu
Xu
* and
Lizhong
Sun
 *
*
Hunan Provincial Key Laboratory of Thin Film Materials and Devices, School of Materials Science and Engineering, Xiangtan University, Xiangtan, 411105, People's Republic of China. E-mail: pengbo.lyu@xtu.edu.cn; xcf@xtu.edu.cn; lzsun@xtu.edu.cn
First published on 10th April 2025
Abstract
Systematic studies on optimizing the efficiency of garnet phosphors while preserving their anti-thermal quenching behavior remain limited. In this study, we explore the garnet-structured BaY2Al2−yScyGa2SiO12:xCr3+ phosphor system, emphasizing the critical role of crystal field modulation in achieving this balance. For y = 0, the optimized BaY2Al1.95Ga2SiO12:0.05Cr3+ phosphor exhibits broadband deep-red and near-infrared (NIR) emission, with an internal quantum efficiency (IQE) of 73%, an external quantum efficiency (EQE) of 13%, and a luminescence intensity that reaches 108% of its room-temperature value at 150 °C, demonstrating a pronounced anti-thermal quenching behavior. This desired property arises from the thermal population shift of the dominant excited states from the 2E to 4T2 state with increasing temperature. With y > 0, the systematic substitution of Al3+ with Sc3+ induces greater structural distortion around Cr3+ ions, optimizing the crystal field environment, broadening NIR emission, and further enhancing luminescence efficiency. The optimized composition, BaY2Al1.5Sc0.5Ga2SiO12:0.05Cr3+, achieves an impressive IQE of 82%, an EQE of 25% and maintains 104% of its luminescence intensity at 150 °C. A NIR pc-LED with a remarkable output power of 241 mW@300 mA was fabricated using this phosphor, enabling the capture of high-quality finger vein images. This demonstration confirms the feasibility of these phosphors for biometric authentication and other advanced NIR applications.
Introduction
Lighting accounts for about 20% of global electricity consumption,1,2 making energy-efficient solutions critical. Phosphor-converted light-emitting diodes (pc-LEDs) have emerged as the leading technology for energy-efficient lighting.3,4 However, their luminous efficacy decreases by 0.2–1% for every 1 °C rise in temperature,5–7 a challenge that becomes more pronounced under high-current operation due to thermal accumulation. This performance degradation, driven by non-radiative transitions in the phosphors,8 leads to a phenomenon known as thermal quenching, where the luminescence decreases as the operating temperature exceeds 150 °C.9,10 Thermal quenching not only reduces emission efficiency but also destabilizes the emission center, compromising the color rendering quality of pc-LEDs.11,12 Addressing this limitation requires the development of thermally robust phosphors with zero- or anti-thermal quenching behavior, enabling improved performance in practical applications.Thermal quenching originates from non-radiative relaxation, where excitation energy dissipates as heat through lattice vibrations.13 This process involves several deactivation pathways, including crossover, multiphonon relaxation and thermal ionization, all of which contribute to luminescence loss.14,15 Various strategies have been developed to mitigate thermal quenching, such as defect engineering, energy transfer-based approaches and structural modulations.16–18 Defect engineering leverages defect energy levels within the bandgap to transfer energy to the emission center, compensating for light emissions. This approach has been successfully employed in phosphor doped with ions such as Eu2+, Ce3+, Cr3+, and Bi3+.19–22 However, designing precise defect energy levels remains a significant challenge, limiting its widespread applicability. Energy transfer strategies, commonly observed between active center pairs, such as Bi3+/Eu3+, Eu2+/Mn2+, and Cr3+/Yb3+,22–25 aim to prevent thermal quenching of the main luminescence center. While effective, these strategies often sacrifice the luminescence of the donor ions, leading to overall efficiency loss.26 In contrast, structural modulation offers a more comprehensive approach, by not only influencing defect energy levels and energy transfer processes but also tailoring structural rigidity, crystallinity, symmetry, and particularly the crystal field environment of the materials.27,28 By improving these properties, structural modulation significantly enhances the luminescence thermal stability of phosphors, making it a more promising strategy for addressing thermal quenching and advancing high-performance materials.
Cr3+-doped deep-red and near-infrared phosphors are ideal candidates to achieve highly efficient NIR emission. These materials find extensive applications in advanced lighting systems, night vision, non-destructive analysis, plant growth, bioimaging, and biometrics,29,30 owing to their high penetrability, rapid response, and low chemical damage potential.31–34 Notably, the 3d3 electronic configuration of Cr3+ allows for broad-band emission spanning deep-red to NIR regions.35 However, Cr3+-doped phosphors are also susceptible to thermal quenching, limiting their practical utility. Structural modulation has proven effective in addressing this limitation by regulating the local crystal environment to enhance luminescence thermal stability.36,37 For example, the incorporation of Li+ within Ca3Sc2Si3O12:Cr3+ makes it retain 97.4% of NIR emission at 150 °C,38 while co-substitution of Zn2+–Zr4+ in Ca3Y2Ge3O12:Cr3+ also enhances the luminescence thermal stability.39 Specifically, the structural modulation of garnet phosphors can induce a change in the luminescence mechanism from the spin-forbidden 2E → 4A2 to the spin-allowed 4T2 → 4A2 transition,37,40 which is closely linked to the anti-thermal quenching.41 However, systematic studies on optimizing the efficiency of garnet phosphors while preserving their anti-thermal quenching behavior remain limited.
In this work, we developed a series of garnet-structured BaY2Al2−x−yScyGa2SiO12:xCr3+ phosphors, leveraging the crystal field modulation approach to achieve enhanced NIR emission while maintaining the sustained anti-thermal quenching properties. For y = 0, the optimal composition BaY2Al2Ga2SiO12:0.05Cr3+ already demonstrated anti-thermal quenching behavior attributed to the thermal population shift of the dominant excited states from the 2E to 4T2 state with increasing temperature. Substituting Al3+ with Sc3+, which has a larger ionic radius and weakens the crystal field, induced atomic disorder and lattice distortion. This structural modulation broke the d–d forbidden transition nature of Cr3+, significantly boosting the emission efficiency. The optimized composition, BaY2Al1.5Sc0.5Ga2SiO12:0.05Cr3+ (y = 0.5), achieved a remarkable IQE of 82% and retained 104% of its luminescence intensity at 150 °C. To demonstrate its application potential, a pc-LED integrating a blue LED chip with a BaY2Al1.5Sc0.5Ga2SiO12:0.05Cr3+ phosphor was fabricated, successfully enabling finger vein imaging, night vision and non-destructive testing. These findings highlight the critical role of crystal field modulation in optimizing luminescence efficiency while maintaining sustained anti-thermal quenching behavior.
Results and discussion
Structure characterization and phase analysis
BaY2Al2Ga2SiO12 (AG) adopts a typical garnet structure with a space group of Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d. The crystal structure and coordination polyhedra are depicted in Fig. 1a.42,43 In this structure, each Al3+ and Ga3+ center coordinates with 6 oxygen atoms, forming octahedra that provide two distinct octahedral coordination environments in the phosphor. Additionally, Al3+, Ga3+ and Si4+ centers coordinate with four oxygen atoms each, forming three distinct tetrahedra: [AlO4], [GaO4] and [SiO4]. Meanwhile, Ba2+ or Y3+ centers coordinate with eight oxygen atoms to form [BaO8] and [YO8] dodecahedra, respectively. The [Y/BaO8] dodecahedra connect to the [Al/GaO6] octahedra through shared edges, while the [Al/Ga/SiO4] tetrahedra link to the [Al/GaO6] octahedra via shared corners. With a similar ionic radius (R = 0.615 Å, CN = 6) and valence state to Al3+ (R = 0.535 Å, CN = 6) and Ga3+ (R = 0.62 Å, CN = 6), Cr3+ preferentially occupies octahedral sites, replacing either Al3+ or Ga3+.
d. The crystal structure and coordination polyhedra are depicted in Fig. 1a.42,43 In this structure, each Al3+ and Ga3+ center coordinates with 6 oxygen atoms, forming octahedra that provide two distinct octahedral coordination environments in the phosphor. Additionally, Al3+, Ga3+ and Si4+ centers coordinate with four oxygen atoms each, forming three distinct tetrahedra: [AlO4], [GaO4] and [SiO4]. Meanwhile, Ba2+ or Y3+ centers coordinate with eight oxygen atoms to form [BaO8] and [YO8] dodecahedra, respectively. The [Y/BaO8] dodecahedra connect to the [Al/GaO6] octahedra through shared edges, while the [Al/Ga/SiO4] tetrahedra link to the [Al/GaO6] octahedra via shared corners. With a similar ionic radius (R = 0.615 Å, CN = 6) and valence state to Al3+ (R = 0.535 Å, CN = 6) and Ga3+ (R = 0.62 Å, CN = 6), Cr3+ preferentially occupies octahedral sites, replacing either Al3+ or Ga3+.
Fig. 1b presents the XRD patterns of AG:xCr3+ phosphors over the 2θ range of 10–90°. The diffraction peaks match the standard reference card (PDF#89-6660), confirming the successful synthesis of the garnet-structured AG:xCr3+ phosphors. The impurity phase peak observed at 2θ = 28° is attributed to trace amounts of BaAl2O4 which has a minimal impact on the luminescence performance due to its negligible content. To further analyze the crystal structure of AG:xCr3+ phosphors, Rietveld refinement was conducted on the XRD data using Y3Al2Ga3O12 (PDF#89-6660) and BaAl2O4 (PDF#82-2001) as starting models. Fullprof software was utilized to refine both the primary and impurity phases with the results presented in Fig. S1(a–f) (ESI†). The refinement factors Rp, Rwp and Rexp, all below 6.70, 9.30 and 5.20, respectively, indicate a strong correlation between experimental and simulated data, validating the reliability of the refinements. Furthermore, as the Cr3+ doping concentration increases, the unit cell constants (a/b/c) and volume (V) show a consistent upward trend (Fig. S1g and h, ESI†), confirming the substitution of Al3+ by Cr3+ ions, which possess a slightly larger ionic radius.
To enhance the phosphor performance, Sc3+ was introduced to replace Al3+ in a series of BaY2Al2−yScyGa2SiO12:0.05Cr3+ (denoted as A2−ySyG:0.05Cr3+) phosphors. XRD patterns (Fig. 1c) reveal that at Sc3+ content below y = 0.9, an impurity phase peak corresponding to trace amount of BaAl2O4 is present at 2θ = 28°. EDS elemental mapping (Fig. 1d) confirms uniform elemental distribution and successful incorporation of Sc3+ into the lattice. At y = 0.9, the impurity phase peak of BaAl2O4 disappears, forming a pure A1.1S0.9G:0.05Cr3+ phase. However, a trace of Ba9Sc2(SiO4)6 emerge at y = 1.7. An enlarged XRD view at 31–34° shows peaks shift to lower angles with increasing Sc3+ content, indicating lattice expansion due to Sc3+ substitution for smaller Al3+ ions.44,45 Rietveld refinement (Fig. S2a–g, ESI†) yields Rp, Rwp, and Rexp within an acceptable range, confirming reliable fitting. Increasing the Sc3+ content also causes a consistent rise in cell constants (a/b/c) and unit cell volume (V), further validating the successful substitution (Fig. S2h, ESI†).
Photoluminescence properties
To evaluate the impact of Cr3+ doping on the luminescence performance of the AG matrix and identify the optimal Cr3+ doping concentration, the absorption, excitation, and emission spectra of AG:xCr3+ phosphors were systematically analyzed. The absorption spectra, shown in Fig. 2a, reveal a host absorption band near 280 nm, while the absorption peaks near 370, 450 and 600 nm are attributed to the 4A2 → 4T1 (4P), 4A2 → 4T1 (4F), and 4A2 → 4T2 (4F) electron transitions of Cr3+, respectively. Notably, the absorption band at around 450 nm aligns with the emission wavelength of the commercially available blue LED chips, enabling their efficient and cost-efficient excitation by a 450 nm source. The excitation spectra, illustrated in Fig. 2b, display two prominent excitation peaks near 445 and 600 nm when monitored at 708 nm. These peaks are attributed to the 4A2 → 4T1 and 4A2 → 4T2 electron transitions of Cr3+, respectively. The emission spectra presented in Fig. 2c confirm the presence of NIR emission spanning 650–1100 nm under 445 nm excitation. A strong emission band centered at 690 nm is observed, attributed to the spin-forbidden 2E → 4A2 transition of Cr3+.46,47 As the Cr3+ content increases, the emission intensity initially rises and then declines, peaking at x = 0.05, corresponding to AG:0.05Cr3+. The reduction in luminous efficiency at Cr3+ concentrations above 0.05 is attributed to concentration quenching. As the Cr3+ content increases, the probability of energy transfer between adjacent Cr3+ ions also increases. This energy transfer mechanism is typically assessed by the critical distance (Rc). When Rc is less than 5 Å, energy transfer occurs primarily via exchange interactions; when Rc exceeds 5 Å, multipole–multipole interactions become dominant. Rc can be estimated using the following equation:21,48,49 | (1) |
The decay curves of AG:xCr3+ phosphors, as shown in Fig. S4 (ESI†), could not be adequately described by a single exponential function. Instead, they were well-fitted using a double exponential decay function provided below:50,51
| It = I0 + A1e−(t/τ1) + A2e−(t/τ2) | (2) |
 | (3) |
To further enhance the luminescence efficiency of the optimal AG:0.05Cr3+ phosphor, Sc3+ was introduced to modulate the crystal field at a fixed Cr3+ content, resulting in a series of A2−ySyG:0.05Cr3+ phosphors. The absorption spectra (Fig. 2d) reveal a consistent enhancement in absorption intensity with the incorporation of Sc3+. This improvement arises from the substitution of Al3+ with Sc3+, a cation with a larger radius, which disrupts the inversion symmetry of the octahedral sites occupied by Cr3+. Such disruption enhances the electric dipole transition of Cr3+, as indicated by our density functional theory (DFT) calculations shown in Fig. S5 (ESI†), thereby increasing both the absorptivity and luminescence efficiency of the phosphors. Additionally, the incorporation of Sc3+ decreases the crystal field strength of AG:0.05Cr3+ phosphors, resulting in a luminescence mechanism shift from the spin-forbidden 2E → 4A2 transition to the spin-allowed 4T2 → 4A2 transition, thus enhancing the luminescence intensity. The normalized excitation spectra (Fig. 2e) reveal the 4A2 → 4T1 transition within the range of 350–500 nm and the 4A2 → 4T2 transition in the range of 500–675 nm. The corresponding emission spectra (Fig. 2f) demonstrate that the luminescence in the range of 650–1100 nm arises from the spin-allowed transition 4T2 → 4A2 and the partially spin-forbidden transition 2E → 4A2, indicating an intermediate crystal field environment. In addition, a very sharp peak at ∼690 nm is observed, which is typically attributed to the ZPL emission of the 2E → 4A2 transitions. As the Sc3+ content increases, the integrated emission intensity of A2−ySyG:0.05Cr3+ phosphors initially increases, peaking at y = 0.5, before declining with further doping. Furthermore, the relative intensity ratio of emission from 4T2 → 4A2 and 2E → 4A2 transitions progressively increased with higher Sc3+ content, indicating a reduction in the crystal field strength of A2−ySyG:0.05Cr3+ phosphors.
The above conclusion is further supported by calculations of the crystal field strength for A2−ySyG:0.05Cr3+ phosphors, which can be determined using the following equations:52–54
| 10Dq = E(4A2 → 4T2) − ΔS/2 | (4) |
 | (5) |
 | (6) |
Fig. 2h presents the decay curves of A2−ySyG:0.05Cr3+ phosphors monitored at their strongest emission peaks under 445 nm excitation. The average photoluminescence values calculated using eqn (2) and (3) are 325.68, 260.61, 202.54, 164.50, 143.54, 120.40, 109.40, and 104.34 μs, for increasing Sc3+ content, as summarized in Table S2 (ESI†). This decreasing trend corresponds to a luminescence mechanism shift from 2E → 4A2 to 4T2 → 4A2 as the crystal field strength decreases. Since the 4T2 → 4A2 transition has a lower lifetime compared to the 2E → 4A2 transition,59–61 the weakening crystal field strength induced by Sc3+ substitution promotes the dominance of the spin-allowed 4T2 → 4A2 transition, leading to a significant reduction in the photoluminescence lifetimes.
The calculated AE, IQE, and EQE values for all the samples are plotted in Fig. 2i. The results show a consistent trend of first increasing and then decreasing as the Sc3+ content increases. The optimal quantum efficiency is achieved when the Sc3+ doping content reaches y = 0.5. Specifically, compared to the unsubstituted AG:0.05Cr3+ phosphor, the Sc3+-substituted sample shows significant improvements, with an IQE increase from 73% to 82%, AE from 18% to 31%, and EQE from 13% to 25%. This enhancement can be attributed to the increased atomic disorder and modulation of the crystal field strength of A2−ySyG:0.05Cr3+ phosphors by incorporating Sc3+. These changes optimize the local coordination environment, thereby improving the luminescence efficiency of the phosphors.18
Anti-thermal quenching
The operating temperature of the blue LED chip often exceeds 100 °C, highlighting the importance of luminescence thermal stability of the phosphors in determining the luminescence performance of pc-LEDs. For Cr3+ doped phosphors, the unshielded nature of 3d3 electrons leads to strong interactions between the lattice vibration and the crystal field. Significantly influencing their photoluminescence properties, including thermal stability. Upon excitation, the valence electrons of Cr3+ transition from the ground state to the excited state and subsequently return to the ground state, and release energy either as photons through radiative transitions or as thermal energy via non-radiative transitions. As the temperature increases, enhanced lattice vibrations amplify non-radiative relaxation processes, thereby reducing the luminescence efficiency of Cr3+.19,62 Notably, materials with higher structural rigidity tend to exhibit higher-energy phonon modes, minimizing the probability of non-radiative transitions. Consequently, such materials demonstrate superior luminescence thermal stability.14,15,63,64 Regarding anti-thermal quenching, Adachi65–67 established a theoretical model that combines phonon-assisted luminescence enhancement effects with thermal quenching effects, and they validated the model with experimental data. In their framework, increased phonon populations at elevated temperatures actively facilitates radiative transitions by breaking the parity-forbidden selection rules, thereby explaining the anti-thermal quenching phenomenon. Their model acknowledges that thermal quenching is an inherent process in all phosphor materials. In contrast, our interpretation focuses how structural rigidity reduces non-radiative losses, thereby mitigating (not eliminating) thermal quenching. Thus, while enhanced structural rigidity can improve luminescence thermal stability by limiting non-radiative decay, it alone cannot fully account for the anti-thermal quenching behavior observed; a comprehensive understanding must also incorporate the phonon-assisted radiative processes described by Adachi et al.Remarkably, as illustrated in Fig. 3a, AG:0.05Cr3+ demonstrates stable anti-thermal quenching behavior, with the integrated emission intensity increasing as the temperature rises. Specifically, at 423 K, the total emission intensity reaches 108% of its room temperature value and remains constant until 473 K. Similarly, as shown in Fig. 3b, A1.5S0.5G:0.05Cr3+ also demonstrates anti-thermal quenching behavior, achieving 104% of its room temperature emission intensity at 423 K and maintaining a value higher than room temperature until 473 K. Moreover, Fig. 3a and b reveal a clear trend of spectral broadening and red-shift of the emission center as the temperature rises. This behavior is further analyzed in Fig. 3c and d, where the emission intensity of the zero-phonon line (ZPL) from the 2E → 4A2 transition gradually decreases, while the intensity of the 4T2 → 4A2 transition increases significantly as the temperature rises. To quantify these changes, the photoluminescence spectra were deconvoluted into sharp line (2E → 4A2) and broadband (4T2 → 4A2) components, where their integrated emission intensities were labeled as I(2E) and I(4A2), respectively. As shown in Fig. 3e and f, the ratio I(4A2)/I(2E) increases progressively with temperature, resulting in a noticeable broadening of the FWHM and the red-shifts in the emission peaks. Although the anti-thermal quenching behavior of A1.5S0.5G:0.05Cr3+ is slightly less pronounced than that of AG:0.05Cr3+, the incorporation of Sc3+ significantly enhances its luminescence efficiency while retaining a satisfactory level of anti-thermal quenching. This highlights the potential of structural modulation as an effective strategy for optimizing the performance of Cr3+-doped phosphors.
To explore the mechanism of the anti-thermal quenching behavior observed in these phosphors, XRD patterns of the optimal AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ samples were measured at 423 K to evaluate lattice expansion. As shown in Fig. 4a, the diffraction peaks at 423 K remain consistent with those at room temperature, with negligible peak shifts as the temperature increases. This consistency suggests that both AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ exhibit excellent structural rigidity, a crucial characteristic in minimizing non-radiative transitions.20,62 To further assess lattice parameter variations, Rietveld refinement of the XRD patterns for AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ was conducted at both room temperature and 423 K using Fullprof software. The refinement results, presented in Fig. S6 and Table S3 (ESI†), show only minimal increases in lattice constants with temperature elevation. This slight lattice expansion reflects the robust structural stability of these phosphors. Additionally, the minor increase in lattice parameters leads to a corresponding elongation of the ligand bond lengths for the octahedral central atom, as illustrated in Fig. 4b. Specifically, the bond lengths increase from 1.946 Å and 1.984 Å at room temperature to 1.955 Å and 1.998 Å at 423 K for AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+, respectively. Since the crystal field constant (Dq) is inversely proportional to the ligand bond length, these changes slightly weaken the crystal field strength. This relationship is described by the following equation:68
 | (7) |
To explore the relationship between octahedral distortion and luminescence properties, we performed DFT calculations to evaluate transition dipole moments (TDMs) for both undistorted and distorted octahedral environments (Fig. S7a and b, ESI†). Since the square of the TDM correlates with radiative transition probability, these calculations provide insight into luminescence efficiency. For AG:0.05Cr3+, the TDM increases from 0.07 in the undistorted structure to 0.60 in the distorted one, while in A1.5S0.5G:0.05Cr3+, it increases from 0.11 to 0.78. These results suggest that local distortions enhance radiative transitions by relaxing parity-forbidden selection rules. However, experimentally, the luminescence intensity increases at higher temperatures despite reduced octahedral distortion. This discrepancy can be reconciled by considering that experimentally refined structures represent statistical averages over thermally fluctuating configurations. At elevated temperatures, local distortions persist but become more uniformly distributed, making the average structure appear closer to an undistorted octahedron while still allowing enhanced transitions. Thus, while DFT results indicate that increased local distortion enhances radiative transitions, the experimental trend is influenced by the dynamic nature of thermal vibrations, which must be considered when interpreting structure–property relationships in luminescent materials.
Debye temperature (ΘD) is commonly employed to evaluate the structural rigidity of phosphors. In general, a higher Debye temperature corresponds to a superior structural rigidity. The ΘD values of AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ phosphors are thus calculated as follows:64,69
 | (8) |
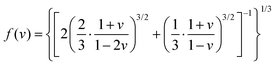 | (9) |
To further evaluate the impact of phonon vibrations on photoluminescence quenching, temperature-dependent Raman spectra of AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ were measured from room temperature to 473 K, as shown in Fig. 4c. Notably, the positions of the vibrational peaks remain almost unchanged across the temperature range, reflecting the high structural rigidity of AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ from another perspective. Notably, the vibrational peaks exhibit negligible shifts in the position across the temperature range, underscoring the high structural rigidity of these phosphors. A closer examination reveals slight leftward shifts of the vibrational peaks at 473 K, attributable to phonon softening, which suggests a minor reduction in polyhedral distortion.70 Additionally, the Raman spectra indicate a max phonon energy of approximately 760 cm−1, significantly lower than the transition energy (∼13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1) between the 4T2 and 4A2 states. This substantial energy gap implies that non-radiative transitions are unlikely to occur, thereby reinforcing the exceptional thermal stability and luminescence efficiency of AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ phosphors.
300 cm−1) between the 4T2 and 4A2 states. This substantial energy gap implies that non-radiative transitions are unlikely to occur, thereby reinforcing the exceptional thermal stability and luminescence efficiency of AG:0.05Cr3+ and A1.5S0.5G:0.05Cr3+ phosphors.
While structural rigidity, high phonon energy, and a wide bandgap are generally recognized as key factors for achieving high thermal stability, they do not fully account for the observed anti-thermal quenching behavior in A2−ySyG:0.05Cr3+ phosphors, highlighting the need for a more comprehensive understanding. To address this, the thermal quenching processes at both room temperature and elevated temperature (423 K) are further elucidated using the configurational coordinate diagram of Cr3+ (Fig. 4d). At low Cr3+ concentrations, AG:xCr3+ phosphors exhibit photoluminescence characteristic of strong crystal fields, as shown in Fig. 2c. As illustrated in the left panel of Fig. 4d, electrons excited by 445 nm light populate the 2E energy level and subsequently return to the ground state 4A2via radiative transition (Process I). As Cr3+ concentration increases, the weakening of the crystal field strength enables partial thermal activation of electrons from 2E to 4T2 (Process II), requiring an activation energy ΔE1. Radiative transitions from 4T2 to 4A2 (Process III) then contribute to the emission spectrum, introducing a spectral shoulder due to the relatively low thermal activation energy ΔE1. Additionally, electrons in the 4T2 level may also reach the intersection of the 4T2 and 4A2 potential curves (Process IV), which requires a higher activation energy ΔE2. From this intersection, non-radiative transitions to the ground state 4A2 (Process V) depend on the magnitude of ΔE2. In AG:0.05Cr3+ phosphors, the high structural rigidity results in a significantly larger ΔE2 compared to ΔE1 (ΔE2 ≫ ΔE1), suppressing non-radiative quenching via Process V. Consequently, as the temperature increases, the emission intensity from the 4T2 → 4A2 transition increases, compensating for potential non-radiative losses and giving rise to anti-thermal quenching behavior. When Al3+ is partially substituted with Sc3+, the crystal field strength weakens further, transitioning from strong to intermediate and ultimately to weak fields. This reduction in crystal field strength lowers ΔE1, facilitating thermal activation of electrons from 2E to 4T2. Consequently, broadband emission from 4T2 → 4A2 becomes dominant, while the spin-forbidden 2E → 4A2 transition is further suppressed. Thermal population from 2E to 4T2 enhances the 4T2 → 4A2 emission, compensating for non-radiative transitions and sustaining anti-thermal quenching. However, as the Sc3+ content increases further, ΔE2 begins to decrease, making non-radiative quenching through Process V more likely. This explains the slightly reduced anti-thermal quenching effect in A1.5S0.5G:0.05Cr3+ compared to AG:0.05Cr3+. Importantly, because spin-allowed transitions (4T2 → 4A2) are far more probable than spin-forbidden transitions (2E → 4A2), the integrated emission intensity from 4T2 → 4A2 transitions increases with temperature between room temperature and 423 K. These findings illustrate the critical interplay between structural rigidity, crystal field strength, and thermal population processes in governing the anti-thermal quenching behavior of Cr3+-doped phosphors, offering valuable insights for designing high-performance phosphors with exceptional thermal stability.
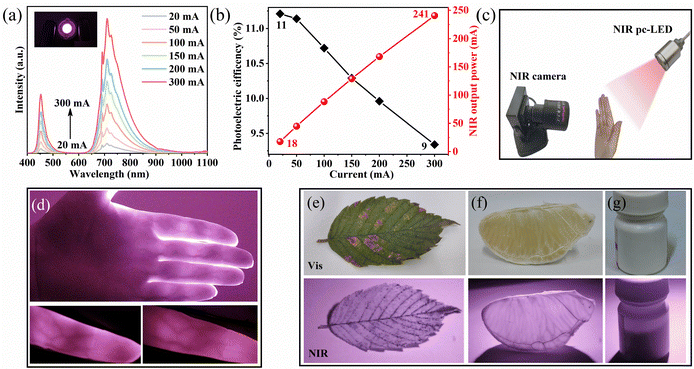
Photoelectric efficiency and pc-LED application
A pc-LED was prepared by combining a A1.5S0.5G:0.05Cr3+ phosphor with a commercial 450 nm blue chip to evaluate its photoelectric conversion performance. Fig. 5a presents the emission spectra of the devices under different driving currents. As the driving current increases, the luminous intensity also increases while the shape of the emission spectrum remains unchanged. Fig. 5b shows the NIR output power and photoelectric efficiency profiles across varying drive currents. At a driving current of 300 mA, the pc-LED device achieves an NIR output power of 241 mW, demonstrating its capability of high NIR output. However, the photoelectric efficiency decreases from 11 to 9% as the driving current increases from 50 mA to 300 mA, primarily due to the efficiency loss caused by the temperature rise of the blue LED chip at high power levels.Intravenous blood vessels are rich in deoxyhemoglobin and oxyhemoglobin, which are known to effectively absorb NIR light within the 700–1000 nm range.71 Leveraging this characteristic, we fabricated an NIR pc-LED by packaging the A1.5S0.5G:0.05Cr3+ phosphor onto a 450 nm blue LED chip, which serves as the light source of a vein image acquisition system, as schematically shown in Fig. 5c. As shown in Fig. 5(d), the captured images clearly differentiate veins (dark areas) from other tissues (light area). Each finger exhibits a unique vein distribution, emphasizing the individuality of vein patterns across different fingers. These distinct and clear finger vein images acquired using the NIR pc-LED device highlight its potential application in biometric recognition systems. Additionally, the technology can serve as a core component for portable and integrated NIR spectroscopy devices with expanded functionalities. Furthermore, we explored other potential application scenarios, as illustrated in Fig. 5(e–g). Under NIR pc-LED irradiation, the leaf and grapefruit veins are distinctly visible, and the content of the drug in the opaque bottle can be observed. This demonstrates the potential application of the NIR pc-LED device in night vision and non-destructive testing.
Conclusion
In conclusion, we successfully synthesized a series of garnet-structured BaY2Al2−yScyGa2SiO12:xCr3+ phosphors using a high-temperature solid-state reaction method. These phosphors exhibit tunable NIR emission spanning 650–1100 nm under 445 nm excitation, with emission characteristics strongly influenced by the crystal field environment. For y = 0, the optimal composition, BaY2Al2Ga2SiO12:0.05Cr3+, demonstrated an IQE of 73%, an EQE of 13%, and remarkable anti-thermal quenching, retaining 108% luminescence intensity at 150 °C. Substituting Al3+ with Sc3+ introduced significant structural modulation, inducing lattice distortion and red-shifting of the emission center from 708 nm to 752 nm while broadening the FWHM from 75 nm to 138 nm. At an optimal Sc3+ content (y = 0.5), the BaY2Al1.5Sc0.5Ga2SiO12:0.05Cr3+ phosphor achieved enhanced quantum efficiencies, with the IQE increasing to 82% and the EQE to 25%, while retaining 104% of its luminescence intensity at 423 K. DFT calculations and experimental analyses revealed that octahedral distortion plays a crucial role in enhancing TDMs, contributing to the superior luminescence efficiency. Structural rigidity, low phonon energy, and the broad bandgap of the host material explain their outstanding thermal stability. The sustained anti-thermal quenching behavior is attributed to the well-preserved thermal repopulation from the 2E to 4T2 state during structural modulation. To demonstrate the application potential of the optimized A1.5S0.5G:0.05Cr3+ phosphor, an NIR pc-LED was fabricated by integrating it with a commercially available 450 nm blue LED chip. The device achieved an impressive NIR output power of 241 mW at a driving current of 300 mA. Clear finger vein images were successfully captured using the device as an irradiation source, demonstrating its potential for biometric identification and other NIR applications. These findings underscore the pivotal role of crystal field modulation in optimizing luminescence efficiency while maintaining sustained anti-thermal quenching behavior. Moreover, they highlight the potential of Cr3+-doped garnet phosphors as advanced materials for high-performance NIR pc-LEDs in next-generation optoelectronic applications.Experimental
Materials synthesis
BaY2Al2Ga2SiO12:xCr3+ and BaY2Al2−yScyGa2SiO12:0.05Cr3+ (x = 0, 0.01, 0.02, 0.04, 0.05, and 0.06; y = 0, 0.3, 0.5, 0.7, 0.9, 1.2, 1.7, and 2) phosphors were synthesized via a high-temperature solid-state reaction. The raw materials included BaCO3 (Aladdin, 99.95%), Y2O3 (Aladdin, 99.90%), Al2O3 (Aladdin, 99.90%), Ga2O3 (Aladdin, 99.99%), SiO2 (Aladdin, 99.90%), Sc2O3 (Aladdin, 99.99%), and Cr2O3 (Rhawn, 99.99%). All compounds were weighed based on their stoichiometric ratios. 1 wt% Li2CO3 was added as a flux. The weighed materials were mixed and ground for 40 minutes before being transferred into corundum crucibles. The samples were sintered at 1350 °C for 6 hours in a furnace. After the sintering process, the samples were allowed to cool to room temperature before collection.For clarity, BaY2Al2Ga2SiO12:xCr3+ and BaY2Al2−yScyGa2SiO12:0.05Cr3+ are referred to as AG:xCr3+ and A2−ySyG:0.05Cr3+, respectively, where “A” represents Al, “S” denotes Sc, and G represents the garnet structure.
Materials characterization
The phase structures of the synthesized materials were analyzed using an X-ray powder diffractometer Cu Kα1 radiation (λ = 0.15406 Å), operating at 40 kV and 40 mA. Diffraction patterns were collected over a 2θ range of 10–90° at a scanning speed of 10° min−1 with a step size of 0.02°. Morphological and compositional analyses were conducted using a scanning electron microscope (SEM, EVO-MA10) equipped with an energy dispersive spectrometer (EDS). UV-vis absorption spectra were measured using a UV-2550 spectrophotometer, covering a wavelength range of 200–800 nm. Photoluminescence spectra, excitation spectra, and decay curves were obtained using an FLS1000 (Edinburgh Instruments) transient steady-state fluorescence spectrometer. Absolute photoluminescence quantum yield and absorption rates were measured with a Hamastu C9920 Quanturus-QY spectrometer. Raman spectra were recorded using an alpha300R-Raman Imaging Microscope.NIR pc-LED fabrication
To fabricate the NIR pc-LED device, the optimized near-infrared phosphor (A1.5S0.5G:0.05Cr3+) was integrated with commercially available 450 nm blue LED chips. The phosphor was thoroughly mixed with epoxy resin and stirred for 10 minutes to ensure homogeneity. A precise amount of the mixture was carefully applied onto the LED chip using a plastic dropper, left to settle for 20 minutes, and then cured in a drying oven at 100 °C for 2 hours. The emission spectra, near-infrared output power, and photoelectric conversion efficiency of the device were evaluated using a high-precision fast spectroradiometer (HASS 2000).Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the Hunan Provincial Natural Science Foundation of China (2021JJ30686 and 2023JJ40621) and the Education Department of Hunan Province (24A0096).References
- P. Wang, Q. Ren, N. Zhang, G. Zhou, S. L. Li and X. M. Zhang, ACS Mater. Lett., 2024, 6(12), 5242–5247 CrossRef CAS.
- J. Chen, H. Xiang, J. Wang, R. Wang, Y. Li, Q. Shan, X. Xu, Y. Dong, C. Wei and H. Zeng, ACS Nano, 2021, 15(11), 17150–17174 CrossRef CAS PubMed.
- G. Zheng, C. Lou, Z. Yuan, W. Xiao, L. Shang, J. Zhong, M. Tang and J. Qiu, Adv. Mater., 2025, 37(4), 2415791 CrossRef CAS.
- C. Zhong, Y. Xu, X. Wu, S. Yin, X. Zhang, L. Zhou and H. You, Adv. Mater., 2024, 36(9), 2309500 CrossRef CAS PubMed.
- S. Wang, R. Pang, T. Tan, H. Wu, Q. Wang, C. Li, S. Zhang, T. Tan, H. You and H. Zhang, Adv. Mater., 2023, 35(22), 2300124 CrossRef CAS.
- Y. Zhang, S. Miao, Y. Liang, C. Liang, D. Chen, X. Shan, K. Sun and X.-J. Wang, Light: Sci. Appl., 2022, 11(1), 136 CrossRef CAS.
- J. Qiao, D. Li, Q. Shi, H. Guo, P. Huang and L. Wang, Light: Sci. Appl., 2024, 13(1), 1–10 CrossRef.
- Z. Tang, X. Wang, T. Bai, S. Liu, S. Wang, Q. Wei, B. Fan and J. Chen, Adv. Opt. Mater., 2024, 13(5), 2402409 CrossRef.
- X. Fan, W. Chen, S. Xin, Z. Liu, M. Zhou, X. Yu, D. Zhou, X. Xu and J. Qiu, J. Mater. Chem. C, 2018, 6(12), 2978–2982 RSC.
- F. Jahanbazi, N. Dimakis and Y. Mao, Adv. Opt. Mater., 2024, 12(1), 2301219 CrossRef CAS.
- M. Liu, Q. Wan, H. Wang, F. Carulli, X. Sun, W. Zheng, L. Kong, Q. Zhang, C. Zhang, Q. Zhang, S. Brovelli and L. Li, Nat. Photonics, 2021, 15(5), 379–385 CrossRef CAS.
- K. Chen, S. Jia, X. Zhang, Z. Shao, Y. Zhou, T. Fan, T. Yu and T. Deng, Inorg. Chem., 2023, 62(20), 7964–7975 CrossRef CAS PubMed.
- Y. Wu, Y. Zhu, A. Ahmed, M. Imran, S. Qiu, Y. Liu, X. Hu, Y. Hassan, Z. Sun, R. Deng and X. Li, Angew. Chem., Int. Ed., 2025, 64(5), e202417018 CrossRef CAS PubMed.
- H. Liu, H. Liang, W. Zhang, Q. Zeng and D. Wen, Chem. Eng. J., 2021, 410, 128367 CrossRef CAS.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10(6), 2102287 CrossRef CAS.
- Y. Wei, H. Yang, Z. Gao, X. Yun, G. Xing, C. Zhou and G. Li, Laser Photonics Rev., 2021, 15(1), 2000048 CrossRef CAS.
- H. Li, J. Jiao, X. Xiang, J. Wu, W. Hu, J. Xie, S. Huang, H. Zhang and J. Zhu, Adv. Opt. Mater., 2024, 12(11), 2302391 CrossRef CAS.
- F. He, E. Song, C. Zhang, H. Chang, G. Dong, Z. Xia, W. Wang and Q. Zhang, Laser Photonics Rev., 2024, 18(3), 2300668 CrossRef CAS.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, Nat. Mater., 2017, 16(5), 543–550 CrossRef CAS.
- Q. Wei, J. Ding and Y. Wang, Chem. Eng. J., 2020, 386, 124004 CrossRef CAS.
- J. X. Lim, H. Wang, X. Kang, W. Lü, C. S. Lim, Z. Zhu, Q. Pan, N. Sakundarini and Y. D. Chuah, Laser Photonics Rev., 2024, 18(12), 2400750 CrossRef CAS.
- X. Chen, R. Pang, S. Wang, T. Tan, J. Su, W. Yuan, S. Zhang and H. Zhang, Laser Photonics Rev., 2024, 18(11), 2400283 CrossRef CAS.
- M. U. Dumesso, W. Xiao, G. Zheng, E. T. Basore, M. Tang, X. Liu and J. Qiu, Adv. Opt. Mater., 2022, 10(16), 2200676 CrossRef CAS.
- W. Yang, P. Dang, G. Zhang, H. Lian, Z. Cheng, G. Li and J. Lin, Adv. Opt. Mater., 2023, 11(20), 2300468 CrossRef CAS.
- J. Tang, X. Zhang, S. Liao, Y. Zhu, Y. Han, H. Su, Z. Qiu, S. Lian and J. Zhang, Adv. Opt. Mater., 2024, 12(35), 2401811 CrossRef CAS.
- H. H. Li, Y. K. Wang and L. S. Liao, Adv. Mater., 2024, 36(30), 2403076 CrossRef CAS.
- M. Zhao, Q. Zhang and Z. Xia, Acc. Mater. Res., 2020, 1(2), 137–145 CrossRef CAS.
- P. Dang, D. Liu, G. Li, A. A. Al Kheraif and J. Lin, Adv. Opt. Mater., 2020, 8(16), 1901993 CrossRef CAS.
- M. H. Huang, K. C. Chen, N. Majewska, M. Kaminski, G. Leniec, E. Mijowska, W. Kong Pang, V. K. Peterson, D. H. Cherng, K. M. Lu, S. Mahlik and R. S. Liu, Angew. Chem., Int. Ed., 2024, 136(47), e202412815 CrossRef.
- F. Zhao, Z. Song and Q. Liu, Laser Photonics Rev., 2022, 16(11), 2200380 CrossRef CAS.
- R. J. Xie, Light: Sci. Appl., 2020, 9, 155 CrossRef.
- L. Zhou, Z. Lyu, D. Sun, S. Shen, T. Tan, L. Wang, H. Zhao and H. You, Adv. Opt. Mater., 2022, 10(22), 2201308 CrossRef CAS.
- S. Jin, R. Li, H. Huang, N. Jiang, J. Lin, S. Wang, Y. Zheng, X. Chen and D. Chen, Light: Sci. Appl., 2022, 11(1), 52 CrossRef CAS PubMed.
- S. Miao, R. Shi, Y. Zhang, D. Chen and Y. Liang, Adv. Mater. Technol., 2023, 8(13), 2202103 CrossRef CAS.
- N. Majewska, M. H. Fang and S. Mahlik, J. Am. Chem. Soc., 2024, 146(32), 22807–22817 CrossRef CAS PubMed.
- Y. Yan, M. Shang, S. Huang, Y. Wang, Y. Sun, P. Dang and J. Lin, ACS Appl. Mater. Interfaces, 2022, 14(6), 8179–8190 CrossRef CAS.
- J. Zhou, T. Ye, Q. Zhu, J. Huo and Q. Zhang, Inorg. Chem., 2024, 63(31), 14665–14672 CrossRef CAS PubMed.
- Z. Jia, C. Yuan, Y. Liu, X.-J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9(1), 86 CrossRef CAS PubMed.
- D. Liu, G. Li, P. Dang, Q. Zhang, Y. Wei, L. Qiu, H. Lian, M. Shang and J. Lin, Light: Sci. Appl., 2023, 12(1), 248 CrossRef CAS.
- A. Balhara, S. K. Gupta, B. Modak, M. Abraham, A. K. Yadav, H. V. Annadata, S. Das, N. S. Rawat and K. Sudarshan, J. Mater. Chem. C, 2024, 12(26), 9716–9732 RSC.
- Q. Zhang, X. Wei, J. Zhou, B. Milićević, L. Lin, J. Huo, J. Li, H. Ni and Z. Xia, Adv. Opt. Mater., 2023, 11(14), 2300310 CrossRef CAS.
- Y. Qiang, Y. Liu, J. Chen, S. Liu, L. Zhang, H. Kang, F. Xu, Z. Xiao, W. You, L. Han and X. Ye, J. Lumin., 2020, 224, 117293 CrossRef CAS.
- W. Yuan, R. Pang, S. Wang, T. Tan, J. Su, X. Chen, R. Liu, C. Li, S. Zhang and H. Zhang, Inorg. Chem. Front., 2023, 10(4), 1203–1214 RSC.
- C. Wang, Y. Zhang, X. Han, D. Hu, D. He, X. Wang and H. Jiao, J. Mater. Chem. C, 2021, 9(13), 4583–4590 RSC.
- F. Zhu, Y. Gao, B. Zhu, L. Huang and J. Qiu, Chem. Eng. J., 2024, 479, 147568 CrossRef CAS.
- S. Wu, B. Xiao, Y. Xiao, P. Shao, Y. Wang and P. Xiong, Nano Energy, 2023, 116, 108811 CrossRef CAS.
- C. Dou, C. Cai, Z. Song and Q. Liu, Adv. Opt. Mater., 2024, 12(4), 2301579 CrossRef CAS.
- L. Li, H. Yang, Y. Wang, F. Ling, X. Zhou, G. Xiang, Z. Cao, S. Jiang, Z. Yang and Y. Hua, Ceram. Int., 2024, 50(6), 9753–9761 CrossRef CAS.
- D. Xu, X. Wu, Q. Zhang, W. Li, T. Wang, L. Cao and J. Meng, J. Alloys Compd., 2018, 731, 156–161 CrossRef CAS.
- X. Ding, G. Zhu, W. Geng, Q. Wang and Y. Wang, Inorg. Chem., 2016, 55(1), 154–162 CrossRef CAS PubMed.
- Y. Zhuo, F. Wu, Y. Niu, Y. Wang, Q. zhang, Y. Teng, H. Dong and Z. Mu, Laser Photonics Rev., 2024, 18(8), 2400105 CrossRef CAS.
- X. Xu, Q. Shao, L. Yao, Y. Dong and J. Jiang, Chem. Eng. J., 2020, 383, 123108 CrossRef CAS.
- S. Zhao, L. Lou, S. Yuan, D. Zhu, F. Wu and Z. Mu, J. Lumin., 2022, 251, 119188 CrossRef CAS.
- S. Guo, L. Ma, M. Abudureyimu, R. Wei, F. Lu, F. Hu and H. Guo, Inorg. Chem. Front., 2023, 10(7), 2197–2205 RSC.
- S. S. Yukito Tanabe, J. Phys. Soc. Jpn., 1954, 9(5), 766–779 CrossRef.
- S. Adachi, ECS J. Solid State Sci. Technol., 2020, 9(4), 046004 CrossRef CAS.
- S. Adachi, ECS J. Solid State Sci. Technol., 2021, 10(2), 026001 CrossRef CAS.
- S. Adachi, J. Lumin., 2024, 273, 120628 CrossRef CAS.
- J. Yuan, Y. Zhang, J. Xu, T. Tian, K. Luo and L. Huang, J. Alloys Compd., 2020, 815, 152656 CrossRef CAS.
- X. Meng, Z. Wang, K. Qiu, Y. Li, J. Liu, Z. Wang, S. Liu, X. Li, Z. Yang and P. Li, Cryst. Growth Des., 2018, 18(8), 4691–4700 CrossRef CAS.
- J. Li, P. Li, Y. Wang, Y. Shi, S. He, Y. Yang, R. Li, G. Wei, H. Suo and Z. Wang, Mater. Today Chem., 2022, 26, 101102 CrossRef CAS.
- J. Lu, Q. Liu, X. Chen, K. Li, W. Chen, Y. Feng, S. Liu, X. Qian, B. Wei and L. Zhang, Ceram. Int., 2023, 49(11), 19062–19071 CrossRef CAS.
- X. Qin, X. W. Liu, W. Huang, M. Bettinelli and X. G. Liu, Chem. Rev., 2017, 117(5), 4488–4527 CrossRef CAS.
- J. Qiao, J. Zhao, Q. Liu and Z. Xia, J. Rare Earths, 2019, 37(6), 565–572 CrossRef CAS.
- S. Adachi, ECS J. Solid State Sci. Technol., 2022, 11(6), 066001 CrossRef CAS.
- S. Adachi, ECS J. Solid State Sci. Technol., 2022, 11(9), 096002 CrossRef CAS.
- S. Adachi, ECS J. Solid State Sci. Technol., 2022, 11(10), 106002 CrossRef CAS.
- V. Rajendran, H. Chang and R.-S. Liu, Opt. Mater.: X, 2019, 1, 100011 CAS.
- E. Francisco, M. A. Blanco and G. Sanjurjo, Phys. Rev., 2001, 63(9), 094107 Search PubMed.
- N. Chaban, M. Weber, S. Pignard and J. Kreisel, Appl. Phys. Lett., 2010, 97(3), 031915 CrossRef.
- L. Zhang, D. Wang, Z. Hao, X. Zhang, G. h Pan, H. Wu and J. Zhang, Adv. Opt. Mater., 2019, 7(12), 1900185 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc00698h |
| This journal is © The Royal Society of Chemistry 2025 |