Open Access Article
Open Access ArticleRing-opening-isomerization anionic polymerization via Brook rearrangement†
Asuka
Hamaguchi
,
Masaya
Terasaki
and
Kaoru
Adachi
 *
*
Faculty of Molecular Chemistry and Engineering, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan. E-mail: kadachi@kit.ac.jp
First published on 13th February 2024
Abstract
Ring-opening isomerization polymerization was developed using a combination of a ring-opening reaction of epoxides and subsequent Brook rearrangement. An epoxy monomer with a benzyltrimethylsilyl group was designed for the polymerization. Characterization of the obtained polymer by NMR and MALDI-TOF-MS indicated that polymerization proceeded exclusively via a ring-opening-isomerization anionic polymerization mechanism.
Isomerization polymerizations, which involve a rearrangement reaction in the propagation step, are unique polymerization approaches since they provide unusual polymers with unexpected repeating units due to the migration of active sites.1–3 Isomerization polymerization has a long history, e.g., hydrogen transfer polymerization of acrylamides which produces polyamides from vinyl monomers,4,5 and cationic polymerization of 3-methyl-1-butene at low temperature to produce a 1,3-type polymer.6 However, many researchers are still actively investigating the use of various polymerization modes to construct polymers with unusual backbones. For example, Ma et al. developed the isomerization polymerization of 1-cyclopropylvinylbenzene using an anion-migrated ring-opening mechanism, which involves the addition of a carbanion to a vinyl group of the monomer to form an α-substituted styryl anion and subsequent ring opening of the cyclopropyl group at an elevated temperature by anion migration.7 Gates et al. reported living anionic isomerization polymerization of mesityl(diphenylmethylene)phosphine by an intramolecular hydrogen transfer mechanism at the active chain end.8 Recently, we developed addition-isomerization anionic polymerization of an o-methyl substituted 1,1-diphenylethylene derivative, which was constituted by the addition of the monomer to the carbanion and subsequent intramolecular hydrogen transfer at the active chain end.9 By using the intramolecular isomerization mechanism, homopolymerization of the diphenylethylene derivative monomer was achieved, whereas the monomer does not have the potential for vinyl polymerization.
Separately, chemical reactions, such as desilylation, which employ the affinity of oxygen for silicon atoms, are attracting attention. We recently developed initiator systems for anionic polymerization composed of benzyltrimethylsilanes and tert-butoxide (t-BuOK).10,11
Intramolecular silyl migration from carbon to oxygen is well known as Brook rearrangement,12–14 which also occurs due to the affinity of oxygen for silicon. Brook rearrangement is widely used as a synthetic tool in organic chemistry to construct low molecular weight compounds.15,16 In addition to the conventional Brook rearrangement, a variety of similar rearrangement reactions inspired by the Brook rearrangement have recently been actively investigated by using the affinity between atoms or functional groups, such as the phospha-Brook rearrangement, which is composed of the migration of a phosphorous group from carbon to oxygen,17,18 and the aza-Brook rearrangement, which involves silyl migration from carbon to nitrogen.19
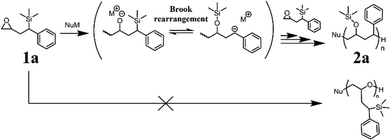
On the other hand, Brook rearrangement is frequently used in polymer chemistry. Zhukhovitskiy and coworkers applied this reaction for metamorphosis of a polymer backbone with an acylsilane moiety in the repeating unit.20 Toste and coworkers reported backbone-photodegradable polymers with an acylsilane unit in the repeating unit.21 The polymers are capable of degradation by the photo-Brook rearrangement mechanism. Brook rearrangement is predicted to have the potential to polymerize appropriate monomers by repeating the addition of the monomer and subsequent rearrangement,22 although, to the best of our knowledge, a polymerization reaction constituted by Brook rearrangement has not yet been reported. Hence, in this study, in order to polymerize a monomer by repeating the anionic ring-opening reaction of the epoxide and subsequent Brook rearrangement (ring-opening-isomerization anionic polymerization), we designed an epoxy monomer and an initiator for polymerization. We further examined the polymerization of the monomer under various reaction conditions.
In order for the monomer to polymerize via a repeating Brook rearrangement (ring-opening-isomerization anionic polymerization), we designed a monomer 1a (Fig. 1) that contained an epoxy group for the oxyanion source and a trimethylsilyl group at the benzylic position, which can be easily eliminated by appropriate oxyanions. Organolithium compounds were selected as initiating agents since they can induce the nucleophilic ring-opening of epoxides, whereas the chain polymerization reaction of epoxides does not proceed.23 Therefore, we examined the polymerization of monomer 1a to obtain polymer 2a (Scheme 1) using various organolithium reagents (Table 1, Runs 1–4). When s-BuLi was added to the monomer solution (Run 1), the solution turned orange. This color was maintained throughout the reaction, and turned colorless after the addition of methanol. Thus, it is thought that benzylanion was generated in the reaction, which was quenched with H+. A GPC measurement of the product (Fig. S1, ESI†) only shows a peak corresponding to the monomer, indicating that polymerization did not proceed. Similarly, no high molecular weight product was observed by GPC when n-BuLi was used as the initiator (Run 2). From these results, it was found that the anionic polymerization of monomer 1a is not initiated by butyllithiums. A benzyl proton abstraction instead of the ring-opening reaction of the epoxide might occur in response to the use of such strong bases. When t-BuOK was added to the monomer solution (Run 3), the solution turned orange, but the GPC result of the product showed a peak with a lower molecular weight than the monomer. In addition, a 1H-NMR spectrum of the product shows the disappearance of the trimethylsilyl proton signals at −0.04 ppm (Fig. S2, ESI†). The GPC and NMR results suggest that the trimethylsilyl group at the benzyl position of the monomer was eliminated by t-BuOK, as we reported previously.9,10 On the other hand, when benzyllithium (BnLi) was used as the initiator (Run 4), the solution turned light orange immediately after its addition, and subsequently turned light yellow after 15 minutes. The GPC result of the product showed peaks at higher molecular weights than the monomer (Mn = 530), in addition to the monomer peak. This result suggests the progressive polymerization of monomer 1a by BnLi. The peak area of the high molecular weight products, relative to monomer conversion, was 13%. The ring-opening-isomerization mechanism was suggested since the reaction solution turned orange during the polymerization reaction, suggesting the formation of the benzyl anion as the Brook rearrangement progressed after the ring-opening reaction of the epoxide. Hence, the appropriate basicity of an organolithium compound is a suitable initiator for the anionic polymerization of monomer 1avia repeating Brook rearrangements.
| Runa | Monomer | Initiator | Temp. (°C) | Solvent | Additive | Polymer peak area (%) | M n (g mol−1) | M w/Mnc |
|---|---|---|---|---|---|---|---|---|
a [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [HMPA] = 5 [HMPA] = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [M] = 0.45 mol L−1; polymerization for 3 days; initiator was added at −78 °C.
b THF 1, [M] = 0.45 mol L−1; polymerization for 3 days; initiator was added at −78 °C.
b THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene = 1 toluene = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
c Obtained from GPC analysis and calibrated against a polystyrene standard. 1.
c Obtained from GPC analysis and calibrated against a polystyrene standard.
|
||||||||
| 1 | 1a | s-BuLi | 25 | THF | — | n.d. | — | — |
| 2 | 1a | n-BuLi | 25 | THF | — | n.d. | — | — |
| 3 | 1a | t-BuOK | 25 | THF | — | n.d. | — | — |
| 4 | 1a | BnLi | 25 | THF | — | 13 | 530 | — |
| 5 | 1a | BnLi | 25 | Toluene | — | n.d. | — | — |
| 6 | 1a | BnLi | −20 | THF/Tolueneb | — | n.d. | — | — |
| 7 | 1a | BnLi | −20 | THF/Tolueneb | HMPA | 95 | 1800 | 1.61 |
| 8 | 1a | BnLi | −78 | THF/Tolueneb | HMPA | n.d. | — | — |
| 9 | 1b | BnLi | −20 | THF/Tolueneb | HMPA | n.d. | — | — |
In order to improve monomer conversion during polymerization, various reaction conditions using BnLi as an initiator were investigated (Runs 5–8). Polymerization did not proceed when only toluene was used as the solvent (Run 5). This result might be due to the nature of the Brook rearrangement, which tends to proceed in polar solvents such as THF.14 Moreover, polymerization did not proceed in a mixed solvent of THF/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) when the reaction temperature was set to −20 °C (Run 6). It is known that some additives, like HMPA, can enhance the Brook rearrangement.14,15,24 Thus, we examined the reaction in the presence of HMPA. When polymerization was carried out in the toluene/THF mixed solvent at −20 °C in the presence of HMPA using BnLi as the initiator (Run 7), anionic polymerization of 1a proceeded. GPC measurements of the product showed a peak at Mn = 1800, and the relative peak area of the polymer was 95% (Fig. 2). It is thought that the Brook rearrangement was promoted by the coordination of HMPA to Li+. However, polymerization did not proceed at −78 °C (Run 8). We examined the polymerization of the counterpart monomer 1b, which has no trimethylsilyl group, but polymerization did not proceed (Run 9). This finding offers strong support that the rearrangement of the trimethylsilyl group is involved in the polymerization mechanism.
1) when the reaction temperature was set to −20 °C (Run 6). It is known that some additives, like HMPA, can enhance the Brook rearrangement.14,15,24 Thus, we examined the reaction in the presence of HMPA. When polymerization was carried out in the toluene/THF mixed solvent at −20 °C in the presence of HMPA using BnLi as the initiator (Run 7), anionic polymerization of 1a proceeded. GPC measurements of the product showed a peak at Mn = 1800, and the relative peak area of the polymer was 95% (Fig. 2). It is thought that the Brook rearrangement was promoted by the coordination of HMPA to Li+. However, polymerization did not proceed at −78 °C (Run 8). We examined the polymerization of the counterpart monomer 1b, which has no trimethylsilyl group, but polymerization did not proceed (Run 9). This finding offers strong support that the rearrangement of the trimethylsilyl group is involved in the polymerization mechanism.
 | ||
| Fig. 2 GPC curves of monomer 1a and polymer 2a obtained in the absence (Run 4) and in the presence (Run 7) of HMPA. | ||
The obtained polymer 2a (Run 7) was purified by repeating precipitation using THF and hexane. The 1H-NMR spectrum of the obtained polymer (Fig. 3b) showed broad signals at 1.2–1.9 ppm and 2.2–3.8 ppm, and aromatic proton signals at 6.5–7.2 ppm, as well as trimethylsilyl protons at −0.3–0.0 ppm. The peak area ratio between the former two signals is about 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, and thus the obtained polymer is suggested to have a –CH2–CHR– repeating unit, corresponding to the structure after Brook rearrangement, rather than the –CH2–CHR–O– repeating unit, corresponding to ring opening polymerization. The peak area ratio of trimethylsilyl proton signals to the aromatic proton signals was smaller than that of the theoretical value, suggesting that some of the trimethylsilyl groups were hydrolyzed during the purification process. Therefore, the product was further hydrolyzed using dilute hydrochloric acid in THF for 4 hours at ambient temperature (Scheme 2). 1H-NMR measurements of the product after hydrolysis (Fig. 3c) showed a significant decrease in the signal of the trimethylsilyl group and a signal, which might correspond to the hydroxy proton, was newly observed at 4.62 ppm. Since the trimethylsilyl group at the benzyl position was hardly hydrolyzed under this condition, the result indicates that the polymer has trimethylsiloxy groups which were generated by the Brook rearrangement. The 29Si-NMR spectrum of the obtained polymer 2a (Fig. S3b, ESI†) showed a broad signal at 12–16 ppm, whereas a signal was observed at 4.0 ppm in the spectrum of the monomer 1a (Fig. S3a, ESI†). The lower field shift of the signal suggests the formation of O–Si bonds, i.e., progression of the Brook rearrangement. Such a shift by Brook rearrangement is consistent with the literature.25 The 13C-NMR spectrum of the polymer after hydrolysis (Fig. 4) showed signals at 31–33 ppm, 36–38 ppm, 42–47 ppm, and 62–73 ppm, which were assigned as benzylmethylene carbons in the initiating group (Cf), benzylmethyne carbons in the main chain (Cd), methylene carbons (Ca + Cc), and methyne carbons next to the hydroxy group (Cb), respectively, in addition to the trimethylsilyl carbon signal at −3.2 to −3.0 ppm and aromatic carbon signals at 123–130 ppm and 143–147 ppm. Thus, the polymer was obtained via Brook rearrangement. Associations between carbons and protons were observed in an HMQC NMR spectrum of the polymer after hydrolysis (Fig. S4, ESI†). No significant correlation was observed between the proton signal at 4.62 ppm and any carbon, indicating that the signal was assigned to the hydroxy proton. Other correlations between carbons and protons observed in the HMQC NMR spectrum represent obvious agreement with the polymer structure obtained via Brook rearrangement. From the above NMR characterization of the obtained polymer, polymerization indeed proceeded by a combination of three reactions: a ring-opening reaction, the Brook rearrangement, and a nucleophilic addition (Scheme 1). In other words, initially, the oxyanion was generated by a ring-opening reaction of the epoxy group with the benzyl anion initiator. Subsequently, the carbanion was produced by the intramolecular anionic migration of a silyl group from a carbon atom to an oxygen atom as the Brook rearrangement. Thereafter, the next monomer was added to the epoxy group by the nucleophilic addition of the carbanion. Repeating the sequence of ring-opening and isomerization resulted in a polymer containing a benzyltrimethylsiloxy group and a phenyl group. Of note, since racemic monomers were used in this study, the stereoregularity of the obtained polymers was not controlled.
1.1, and thus the obtained polymer is suggested to have a –CH2–CHR– repeating unit, corresponding to the structure after Brook rearrangement, rather than the –CH2–CHR–O– repeating unit, corresponding to ring opening polymerization. The peak area ratio of trimethylsilyl proton signals to the aromatic proton signals was smaller than that of the theoretical value, suggesting that some of the trimethylsilyl groups were hydrolyzed during the purification process. Therefore, the product was further hydrolyzed using dilute hydrochloric acid in THF for 4 hours at ambient temperature (Scheme 2). 1H-NMR measurements of the product after hydrolysis (Fig. 3c) showed a significant decrease in the signal of the trimethylsilyl group and a signal, which might correspond to the hydroxy proton, was newly observed at 4.62 ppm. Since the trimethylsilyl group at the benzyl position was hardly hydrolyzed under this condition, the result indicates that the polymer has trimethylsiloxy groups which were generated by the Brook rearrangement. The 29Si-NMR spectrum of the obtained polymer 2a (Fig. S3b, ESI†) showed a broad signal at 12–16 ppm, whereas a signal was observed at 4.0 ppm in the spectrum of the monomer 1a (Fig. S3a, ESI†). The lower field shift of the signal suggests the formation of O–Si bonds, i.e., progression of the Brook rearrangement. Such a shift by Brook rearrangement is consistent with the literature.25 The 13C-NMR spectrum of the polymer after hydrolysis (Fig. 4) showed signals at 31–33 ppm, 36–38 ppm, 42–47 ppm, and 62–73 ppm, which were assigned as benzylmethylene carbons in the initiating group (Cf), benzylmethyne carbons in the main chain (Cd), methylene carbons (Ca + Cc), and methyne carbons next to the hydroxy group (Cb), respectively, in addition to the trimethylsilyl carbon signal at −3.2 to −3.0 ppm and aromatic carbon signals at 123–130 ppm and 143–147 ppm. Thus, the polymer was obtained via Brook rearrangement. Associations between carbons and protons were observed in an HMQC NMR spectrum of the polymer after hydrolysis (Fig. S4, ESI†). No significant correlation was observed between the proton signal at 4.62 ppm and any carbon, indicating that the signal was assigned to the hydroxy proton. Other correlations between carbons and protons observed in the HMQC NMR spectrum represent obvious agreement with the polymer structure obtained via Brook rearrangement. From the above NMR characterization of the obtained polymer, polymerization indeed proceeded by a combination of three reactions: a ring-opening reaction, the Brook rearrangement, and a nucleophilic addition (Scheme 1). In other words, initially, the oxyanion was generated by a ring-opening reaction of the epoxy group with the benzyl anion initiator. Subsequently, the carbanion was produced by the intramolecular anionic migration of a silyl group from a carbon atom to an oxygen atom as the Brook rearrangement. Thereafter, the next monomer was added to the epoxy group by the nucleophilic addition of the carbanion. Repeating the sequence of ring-opening and isomerization resulted in a polymer containing a benzyltrimethylsiloxy group and a phenyl group. Of note, since racemic monomers were used in this study, the stereoregularity of the obtained polymers was not controlled.
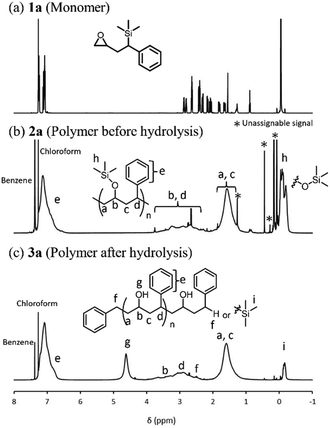 | ||
| Fig. 3 1H-NMR spectra of (a) monomer 1a, (b) obtained polymer 2a, and (c) obtained polymer 3a after hydrolysis. | ||
A MALDI-TOF-MS measurement of the obtained polymer after hydrolysis was examined to investigate the structures of the repeating unit and terminal groups (Fig. 5). The results showed two series of peaks, one major and one minor, both with peak intervals of 148.1 Da, whereas the molecular weight of the monomer 1a was 220.4 Da. Interestingly, the molecular weight difference (ca. 72 Da) is consistent with the molecular weight of the trimethylsilyl group. This result indicates that the trimethylsilyl groups in the repeating unit were completely removed by the hydrolysis reaction, i.e., benzyltrimethylsilyl groups were excluded from the polymer repeating unit. Hence, polymerization proceeded exclusively via a Brook rearrangement-based mechanism. The repeating unit of the predicted polymer structure obtained by the Brook rearrangement and subsequent hydrolysis had a molecular weight of 148.1 Da, which is in agreement with the peak intervals in the MALDI-TOF-MS result. Mn of the obtained polymer 3a calculated from the MALDI-TOF-MS spectrum was 2570 g mol−1, which is larger than the molecular weight of the polymer before hydrolysis, as observed by GPC. The difference in Mn might be due to a difference in the hydrodynamic volume between the calibration standard and polymer 2a. Additional analysis of the terminal structure of the polymer indicated, that the major series of peaks strongly corresponded to an α-benzyl-ω-hydrogen polymer, which was obtained by quenching of the benzyl anion. In contrast, the minor series corresponded to an α-benzyl-ω-trimethylsilyl polymer, which was obtained by quenching of the oxyanion. In both cases, the benzyl group in the initiating agent was attached at the α-end group of the polymer.
Repeating ring-opening of an epoxide moiety in the monomer and subsequent Brook rearrangement of an epoxide monomer having a benzyltrimethylsilyl group successfully resulted in ring-opening-isomerization anionic polymerization. The Brook rearrangement was, for a long time, predicted to be applicable for polymerization, but the present work is the first example of polymerization using Brook rearrangement. Recently, a variety of isomerization reactions related to Brook rearrangement have attracted attention. Therefore, polymerization composed of such an isomerization reaction should open up a way to polymerize appropriate monomers using a similar mechanism. Additionally, the structure of the obtained polymer is quite unique, since it only has carbon–carbon single bonding in the main chain, whereas polymerization involves the ring-opening reaction of an epoxide. This result is due to the isomerization of active species from the oxyanion to the benzyl anion. For these reasons, ring-opening-isomerization polymerization has the potential to be used in the design of a variety of unique polymers with new main chain structures.
The authors thank Professor Masato Suzuki of the Nagoya Institute of Technology for his kind advice and encouragement. This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant (JP21K05185) and a grant from the Toshiaki Ogasawara Memorial Foundation.
Conflicts of interest
The authors have no conflicts of interest to declare.Notes and references
- D. Takeuchi, J. Am. Chem. Soc., 2011, 133, 11106–11109 CrossRef CAS PubMed.
- J. Oku, T. Hasegawa, T. Kawakita, Y. Kondo and M. Takaki, Macromolecules, 1991, 24, 1253–1256 CrossRef CAS.
- S. H. Hwang and T. L. Choi, Chem. Sci., 2021, 12, 2404–2409 RSC.
- D. S. Breslow, G. E. Hulse and A. S. Matlack, J. Am. Chem. Soc., 1957, 79, 3760–3763 CrossRef CAS.
- J. P. Kennedy and T. J. Otsu, Macromol. Sci., 2006, 6, 237–283 CrossRef.
- J. P. Kennedy and R. M. Thomas, Macromol. Chem. Phys., 1962, 53, 1–235 CrossRef.
- H. Bai, L. Han, X. Wang, H. Yan, H. Leng, S. Chen and H. Ma, Macromolecules, 2022, 55, 9751–9765 CrossRef CAS.
- B. W. Rawe, A. M. Priegert, S. Wang, C. Schiller, S. Gerke and D. P. Gates, Macromolecules, 2018, 51, 2621–2629 CrossRef CAS.
- D. Tanioka and K. Adachi, Macromolecules, 2022, 55, 10933–10939 CrossRef CAS.
- M. Terasaki, Y. Hiraki and K. Adachi, Polym. J., 2023, 55, 935–943 CrossRef CAS.
- Y. Hiraki, M. Terasaki and K. Adachi, J. Polym. Sci., 2024, 62, 191–199 CrossRef CAS.
- A. G. Brook, J. Am. Chem. Soc., 1958, 80, 1886–1889 CrossRef CAS.
- A. B. Smith III and W. M. Wuesta, Chem. Commun., 2008, 5883–5895 RSC.
- A. B. Smith, M. Xian, W. S. Kim and D. S. Kim, J. Am. Chem. Soc., 2006, 128, 12368–12369 CrossRef CAS PubMed.
- W. H. Moser, Tetrahedron, 2001, 57, 2065–2084 CrossRef CAS.
- F. G. Zhang and I. Marek, J. Am. Chem. Soc., 2017, 139, 8364–8370 CrossRef CAS PubMed.
- S. J. Fitch and K. Moedritzer, J. Am. Chem. Soc., 1962, 84, 1876–1879 CrossRef CAS.
- R. Kaur and R. P. Singh, J. Org. Chem., 2023, 88, 10325–10338 CrossRef CAS PubMed.
- T. Honda and M. Mori, J. Org. Chem., 1996, 61, 1196–1197 CrossRef CAS.
- M. Ratushnyy and A. V. Zhukhovitskiy, J. Am. Chem. Soc., 2021, 143, 17931–17936 CrossRef CAS PubMed.
- B. Huang, M. Wei, E. Vargo, Y. Qian, T. Xu and F. D. Toste, J. Am. Chem. Soc., 2021, 143, 17920–17925 CrossRef CAS PubMed.
- Y. Deng and A. B. Smith III, Acc. Chem. Res., 2020, 53, 988–1000 CrossRef CAS.
- R. P. Quirk and J. J. Ma, J. Polym. Sci. A. Polym. Chem., 1988, 26, 2031–2037 CrossRef CAS.
- M. Suzuki, H. Koyama and R. Noyori, Bull. Chem. Soc. Jpn., 2004, 77, 259–268 CrossRef CAS.
- H. Stueger, B. Hasken, M. Haas, M. Rausch, R. Fischer and A. Torvisco, Organometallics, 2014, 33, 231–239 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc00144c |
| This journal is © The Royal Society of Chemistry 2024 |