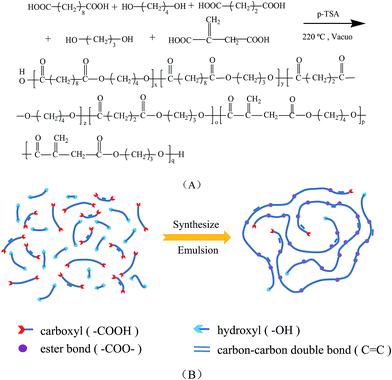
Bio-based elastomer nanoparticles with controllable biodegradability
Qingguo Wang*ab,
Yingying Zaia,
Dejing Yanga,
Liyan Qiua and
Chengqun Niua
aKey Laboratory of Rubber-Plastics of Ministry of Education, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: qwang@qust.edu.cn; Tel: +86-532-84022418
bShandong Provincial Key Laboratory of Rubber-Plastics, Qingdao 266042, China
First published on 18th October 2016
Abstract
Recently, biodegradable polymers and nanoscale polymers have become hot topics in advanced polymer research. In this manuscript, we report novel electron beam irradiation vulcanized soft polyester nanoparticles through a three-step method. Some dicarboxylic acids (e.g., succinic acid, sebacic acid, itaconic acid) and diols (e.g., 1,3-propanediol and 1,4-butanediol), which can also be derived from biomass resources, were firstly synthesized into the aliphatic unsaturated polyester by using melt polycondensation, which was followed with polyester emulsification and radiation crosslinking to obtain the elastic polyester nanoparticles. Interestingly, the crosslinked soft polyester nanoparticles with controllable gel content, controllable biodegradability (exhibiting a weight loss ratio of 52.3% within 5 days in the presence of lipase) and low glass transition temperature of about −55 °C are potential candidates to meet the requirements not only for drug delivery carriers but also for modifying some biodegradable brittle polymers which are widely used in pharmaceutical sciences, biotechnology, and biomedicine.
1 Introduction
Recently, research into nanoscale particles has become a hot topic in the field of advanced materials.1–4 The small size, large specific surface area (defined as the total surface area of a solid material per unit of mass) and special structure of these nanoscale particles play an important role in pharmaceutical sciences, biotechnology, and biomedicine.5–8 Different types of polymer carriers, such as polymeric micelles,9 polymersomes,10 polymer capsules,11 and polymer replica particles,12 are expected to revolutionize diagnostics and the delivery of therapeutics.13–16Generally, polymer nano- and microspheres with intricate internal domain structures have been prepared from emulsion copolymerization,17 self-assembly of block copolymers,18 and the sol–gel process.19,20 Discher and co-workers also found that cross-linking nanopolymer particles with resultant increased stiffness led to rapid phagocytic clearance via the immune system.21
Using some synthetic rubber latex (nitrile–butadiene latex, styrene–butadiene latex) and electron beam irradiation technology, our previous work has prepared some nano- and micro elastomer particles with controllable crosslinking density. The irradiation-vulcanized elastomer particles has the effects on toughening some brittle polymer while improving the crystallization behavior and heat resistance of some polymer.22–24 However, the conventional synthetic rubber latex derived from fossil resource are not biodegradable, thus the resultant crosslinked elastomer particles are not biodegradable. Therefore, how to fabricate the crosslinked nanopolymer particles which can be biodegradable is more significative.
In this manuscript, we report on the fabrication of a novel biodegradable elastomer nanoparticles (BEN) with controllable biodegradability. Firstly, biodegradable unsaturated polyester (BUP) was firstly synthesized by melt polycondensation using some dicarboxylic acids (e.g., succinic acid, sebacic acid, itaconic acid) and diols (e.g., 1,3-propanediol and 1,4-butanediol) which can be selected from bio-derived monomers. Secondly, the BUP was emulsified into biodegradable unsaturated polyester emulsion (BUPE), followed with radiation crosslinking to obtain the BEN. This three-step strategy has advantages to control the final products with well-defined particle size, controllable gel content and biodegradability.
On the one hand, the resultant biodegradable crosslinked polyester elastomer nanoparticles is the potential candidate to meet the requirements for certain biomedical applications, such as drug delivery systems. On the other hand, the biodegradable elastomer particles are suitable for the modification of biodegradable brittle polymers, such as polylactic acid (PLA)25–28 and polyhydroxyalkanoate (PHA),29,30 which are widely used as tissue engineering scaffolds, sutures and clips.
2 Experimental section
2.1 Materials
Itaconic acid (IA) with a molecular weight of 130.10 g mol−1 and a concentration of 98% was supplied by Qingdao Langyatai Biochemical Technology Co., Ltd., China; 1,3-propanediol (PDO) with a molecular weight of 76.09 g mol−1 and a concentration of 98% was supplied by Shanghai Aladdin Biochemical Technology Co., Ltd., China; 1,4-butanediol (BDO) with a molecular weight of 90.12 g mol−1 and a concentration of not less than 99.8% was supplied by Tianjin Bodi Chemical Industry Co., Ltd., China; succinic acid (SU) with a molecular weight of 118.09 g mol−1 and a concentration of not less than 99.5% was supplied by Tianjin Bodi Chemical Industry Co., Ltd., China; sebacic acid (SA) with a molecular weight of 202.25 g mol−1 and a concentration of not less than 99% was supplied by Tianjin Basifu Chemical Co., Ltd., China; para-toluenesulfonic acid with a molecular weight of 202.25 g mol−1 and a concentration of not less than 99.0% was supplied by Sinopharm Chemical Reagent Co., Ltd., China; polyethylene glycol monooleate was supplied by Jiangsu Haian Petrochemical Company, China. Above chemical reagents except for IA and PDO were of analytically pure grade. Lipase, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U, was supplied by Xinyu Food Additives Co., Ltd., China.
000 U, was supplied by Xinyu Food Additives Co., Ltd., China.
2.2 Fabrication of the BEN
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:2
2:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.75
2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.75) were placed in a reactor which was blanketed with nitrogen gas for protection, and slowly heated to 180 °C until the monomers melted. The unsaturated polyester prepolymer was prepared by the polycondensation reaction for 1 h, and the water produced during the preparation of prepolymer was removed with help of nitrogen gas. Then the catalyst (para-toluenesulfonic acid, with weight ratio of 0.05%), polymerization inhibitor (hydroquinone, with weight ratio of 0.01%), and stabilizer (phosphorous acid, with weight ratio of 0.01%) were subsequently added into above polyester prepolymer. After decompressing the pressure of the reactor below 5000 Pa and increasing the temperature of polyester prepolymer from 180 °C to 220 °C, the esterification reaction retained for 4 h, and the finished product (BUP) was obtained. Molecular weight and molecular weight distribution of the BUP was measured by a gel permeation chromatograph (GPC, HLC-8320, manufactured by Tosoh Corp., Japan).
2.75) were placed in a reactor which was blanketed with nitrogen gas for protection, and slowly heated to 180 °C until the monomers melted. The unsaturated polyester prepolymer was prepared by the polycondensation reaction for 1 h, and the water produced during the preparation of prepolymer was removed with help of nitrogen gas. Then the catalyst (para-toluenesulfonic acid, with weight ratio of 0.05%), polymerization inhibitor (hydroquinone, with weight ratio of 0.01%), and stabilizer (phosphorous acid, with weight ratio of 0.01%) were subsequently added into above polyester prepolymer. After decompressing the pressure of the reactor below 5000 Pa and increasing the temperature of polyester prepolymer from 180 °C to 220 °C, the esterification reaction retained for 4 h, and the finished product (BUP) was obtained. Molecular weight and molecular weight distribution of the BUP was measured by a gel permeation chromatograph (GPC, HLC-8320, manufactured by Tosoh Corp., Japan).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm in 30 min and ultrasonic processing in 5 min with frequency of 32 kHz.
000 rpm in 30 min and ultrasonic processing in 5 min with frequency of 32 kHz.2.3 Characterization and testing methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixed solution was titrated by the ethanol solution of potassium hydroxide until the indicator, thymol blue, turned blue. Thus, the acid value can be calculated from the results of titration.
1. The mixed solution was titrated by the ethanol solution of potassium hydroxide until the indicator, thymol blue, turned blue. Thus, the acid value can be calculated from the results of titration.1H-NMR characterization of purified BUP was performed by a nuclear magnetic resonance (NMR) spectrometer (AVANCE, bought from Bruker Corp., Germany) at ambient temperature with a testing frequency of 300 Hz. Deuterated chloroform and tetramethylsilane (TMS) were used as the solvent and internal standard, respectively.
IR spectra of purified BUP was performed by a ATR-IR analyser (VERTEX 70, from the Bruker Company, Germany) at room temperature, and the scanning wavenumbers were ranged from 4000 cm−1 to 600 cm−1.
| Gel content (100%) = [(Wa − Wb)/Ws] × 100% | (1) |
| Weight loss ratio (100%) = [(W0 − Wt)/Wp] × 100% | (2) |
About 0.01 g of lipase and a specific amount of mixed phosphate buffer solution with pH = 6.8 were weighed and added into the sampling tube to repeat the tests, which ensures that similar biodegradation conditions is supplied for biodegradation tests of remainder BENs in following biodegradation tests.
3 Results and discussion
3.1 Structural analysis of the BUP macromolecule
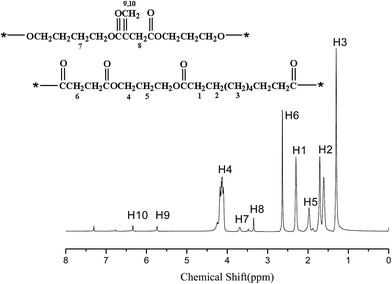
Fig. 2 shows the 1H-NMR spectrum of the BUP with the number-average molecular weight of 2300 and the weight-average molecular weight of 5736 (Table 1). The peaks at 5.74 and 6.34 ppm are the resonance absorption peaks of H in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 bond of itaconic acid, thereby suggesting the existence of double-bond in polyester. The peaks at 1.30, 1.71, and 2.30 ppm are the resonance absorption peaks of H in H3, H2, and H1 of methylene in sebacic acid, whereas the peaks at 2.63, 4.13, 1.97, and 3.69 ppm are the resonance absorption peaks of H6, H4, H5, and H7 of methylene in succinate, propanediol alginate, and butanediol, respectively. The 1H-NMR spectrum indicates that BUP was produced using itaconic acid, succinic acid and sebacic acid.
CH2 bond of itaconic acid, thereby suggesting the existence of double-bond in polyester. The peaks at 1.30, 1.71, and 2.30 ppm are the resonance absorption peaks of H in H3, H2, and H1 of methylene in sebacic acid, whereas the peaks at 2.63, 4.13, 1.97, and 3.69 ppm are the resonance absorption peaks of H6, H4, H5, and H7 of methylene in succinate, propanediol alginate, and butanediol, respectively. The 1H-NMR spectrum indicates that BUP was produced using itaconic acid, succinic acid and sebacic acid.
| Sample | Ratio of IA/SA/SU (mol%) | Mn | Mw | Mz + 1 | PDI | [η] (g mL−1) |
|---|---|---|---|---|---|---|
| BUP | 0.2/0.4/0.4 | 2300 | 5736 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738 738 |
2.49 | 2.069 |
3.2 Effects of BUP weight concentration on the colloid size distribution of BUPE
Fig. 3 presents the relationship between the BUP weight concentration and the emulsion colloid sizes in BUPE (with the wt/wt of emulsifiers to BUP is 18%). With increased weight concentration of BUP, the viscosity of the emulsion increases and a good interface layer of the surfactant can hardly be formed. Moreover, the probability of collision and aggregation of the oil phases in the emulsion increases, thereby leading to a large and non-uniformly distributed colloid size.To sum up, the appropriate BUP weight concentration and the amount of surfactant in BUPE are significant in producing the polyester emulsions with small and uniformly distributed colloid sizes.
3.3 Formation of the electron beam irradiated BEN
During the electron beam irradiation process of the BUPE, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of BUP macromolecules will be broken and the polyester macromolecules free radicals occurred, thus leading to the crosslinking of BUP macromolecules in the colloid particles (Fig. 4), and the vulcanized BEN is obtained. Fig. 5 shows TEM images of some BENs. The particle sizes of BENs can be controlled with uniform distribution, and the particle sizes also can be controlled within the range of 100 nm.
C bonds of BUP macromolecules will be broken and the polyester macromolecules free radicals occurred, thus leading to the crosslinking of BUP macromolecules in the colloid particles (Fig. 4), and the vulcanized BEN is obtained. Fig. 5 shows TEM images of some BENs. The particle sizes of BENs can be controlled with uniform distribution, and the particle sizes also can be controlled within the range of 100 nm.
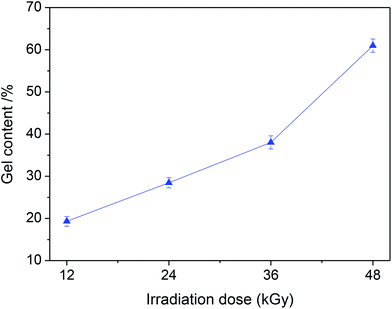
Fig. 6 is some DSC curves of various BENs irradiated with different radiation doses. With the increasing of radiation dose, the glass transition temperature (Tg) of various BENs increases from −55.2 °C to −53.4 °C within the dose range of 48 kGy, and the corresponding gel content of BEN increases to 62% (Fig. 7) because the electron beam irradiation induces the generation of polyester macromolecular free radicals that are easily cross-linked with each other to form the three-dimensional network. Therefore, with the radiation dose increasing, the cross-linking density and gel content of the BEN increase.
3.4 Biodegradation performance of the BEN
In the lipase buffer solution, the bio-degradation of polyester is mainly introduced by the breaking of the ester bond.31 In this study, Fig. 8 illustrates the biodegradation schematic of the vulcanized BEN, and we focused on the biodegradation mechanism of the BEN and the relevant factors.Fig. 9(a) presents the degradation curves of BEN samples with different gel contents, and the degree of degradation increases steadily with increasing degradation time. The BEN with the lowest cross-linking density exhibits a weight loss ratio of 52.3% within 5 d in the presence of lipase. With increasing cross-linking density of the BEN, the degradation rate gradually decreases. By controlling the number of double-bonds in the BUP molecules and the radiation dose, we can control the cross-linking density of the polyester elastomer as well as the degradation rate. Fig. 9(b) presents the acid values of the centrifugal supernatant liquid of various BEN degraded for 1 and 5 d. With increasing degradation time, the acid value of the degraded supernatant liquid of BEN increases. However, acid value of the degraded supernatant liquid decreases with increasing gel content of the polyester elastomer. These results suggest that the amount of terminal hydroxylic groups in the polyester degradation system increases with increasing degradation rate. In other words, with the catalytic action of lipase, the ester bond in the polyester macromolecular chain is easily broken, thereby leading to the generation of new acids and alcohols.
 | ||
| Fig. 9 Biodegradation of BEN samples. (a) Weight loss ratios of various BENs; (b) acid values of the centrifugal supernatant liquid of various degraded BENs. | ||
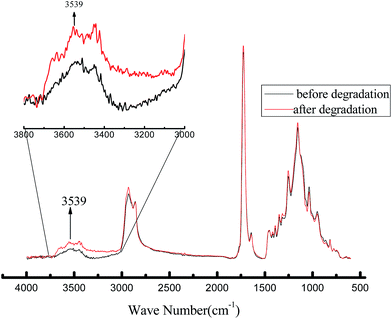
The infrared spectra of the BEN before and after the degradation are shown in Fig. 10. Using the absorption peak of –CH2 (2858 cm−1) as the internal standard, it is obvious that the absorption peak area of hydroxylic group (around 3500 cm−1) significantly increases after the polyester is degraded. The polyester molecular chains vary in length after degradation and exhibit a broad absorption peak. The results indicate that the number of the terminal hydroxylic groups of the polyester increases after the degradation, which is accompanied by the generation of hydroxy and carboxyl groups.
4 Conclusions
We successfully prepared the BEN with controllable particle sizes and degradation performance by melting polycondensation, polyester emulsification and radiation vulcanization. The BEN exhibited controllable degradability to a particular extent, which decreased with increasing cross-linking density. It is worth noting that the resulted BEN is a potential candidate to meet the requirements for both biomedical applications in drug delivery carrier and modifying brittle biodegradable polymers.Acknowledgements
This research is supported by National Basic Research Program of China (973 Program) (Grant No. 2015CB654706), National Natural Science Foundation of China (Grant No. 51373085) and Collaborative Innovation Center of Green Tyres & Rubber (2014GTR0006).References
- H. Hou, M. Jing, Y. Yang, Y. Zhang, W. Song, X. Yang, J. Chen, Q. Chen and X. Ji, Antimony nanoparticles anchored on interconnected carbon nanofibers networks as advanced anode material for sodium-ion batteries, J. Power Sources, 2015, 284, 227–235 CrossRef CAS.
- F. Tang, L. Li and D. Chen, Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery, Adv. Mater., 2012, 24(12), 1504–1534 CrossRef CAS PubMed.
- M. Lee, J. Hong, H. Kim, H.-D. Lim, S. B. Cho, K. Kang and C. B. Park, Organic nanohybrids for fast and sustainable energy storage, Adv. Mater., 2014, 26(16), 2558–2565 CrossRef CAS PubMed.
- C. Kuemin, L. Nowack, L. Bozano, N. D. Spencer and H. Wolf, Oriented Assembly of Gold Nanorods on the Single-Particle Level, Adv. Funct. Mater., 2012, 22(4), 702–708 CrossRef CAS.
- A. Z. Wang, R. Langer and O. C. Farokhzad, Nanoparticle delivery of cancer drugs, Annu. Rev. Med., 2012, 63(1), 185–198 CrossRef CAS PubMed.
- K. Fox, P. A. Tran and N. Tran, Recent Advances in Research Applications of Nanophase Hydroxyapatite, ChemPhysChem, 2012, 13(10), 2495–2506 CrossRef CAS PubMed.
- B. V. Parakhonskiy, A. M. Yashchenok, M. Konrad and A. G. Skirtach, Colloidal micro- and nano-particles as templates for polyelectrolyte multilayer capsules, Adv. Colloid Interface Sci., 2014, 207(1), 253–264 CrossRef CAS PubMed.
- D. A. LaVan, T. McGuire and R. Langer, Small-scale systems for in vivo drug delivery, Nat. Biotechnol., 2003, 21(10), 1184–1191 CrossRef CAS PubMed.
- K. Kataoka, A. Harada and Y. Nagasaki, Block copolymer micelles for drug delivery: design, characterization and biological significance, Adv. Drug Delivery Rev., 2001, 47(1), 113–131 CrossRef CAS PubMed.
- D. E. Discher and F. Ahmed, Polymersomes, Annu. Rev. Biomed. Eng., 2006, 8(1), 323–341 CrossRef CAS PubMed.
- J. Cui, M. P. van Koeverden, M. Mullner, K. Kempe and F. Caruso, Emerging methods for the fabrication of polymer capsules, Adv. Colloid Interface Sci., 2014, 207(1), 14–31 CrossRef CAS PubMed.
- Y. Wang, A. D. Price and F. Caruso, Nanoporous colloids: building blocks for a new generation of structured materials, J. Mater. Chem., 2009, 19(36), 6451–6464 RSC.
- S. T. Gunawan, K. Kempe, G. K. Such, J. Cui, K. Liang, J. J. Richardson, A. P. R. Johnston and F. Caruso, Tuning Particle Biodegradation Through Polymer–Peptide Blend Composition, Biomacromolecules, 2014, 15(12), 4429–4438 CrossRef CAS PubMed.
- J. P. Best, J. Cui, M. Müllner and F. Caruso, Tuning the Mechanical Properties of Nanoporous Hydrogel Particles via Polymer Cross-Linking, Langmuir, 2013, 29(31), 9824–9831 CrossRef CAS PubMed.
- O. C. Farokhzad and R. Langer, Impact of nanotechnology on drug delivery, ACS Nano, 2009, 3(3), 16–20 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Strategies in the design of nanoparticles for therapeutic applications, Nat. Rev. Drug Discovery, 2010, 9(9), 615–627 CrossRef CAS PubMed.
- Y. C. Chen, V. L. Dimonie and M. S. El-Aasser, Effect of interfacial phenomena on the development of particle morphology in a polymer latex system, Macromolecules, 1991, 24(13), 3779–3787 CrossRef CAS.
- Z. H. Lu, G. J. Liu, H. Phillips, J. M. Hill, J. Chang and R. A. Kydd, Palladium Nanoparticle Catalyst Prepared in Poly(Acrylic Acid)-Lined Channels of Diblock Copolymer Microspheres, Nano Lett., 2001, 1(12), 683–687 CrossRef CAS.
- Y. Lu, H. Fan, A. Stump, T. L. Ward, T. Rieker and C. J. Brinker, Aerosol-assisted self-assembly of mesostructured spherical nanoparticles, Nature, 1999, 398(398), 223–226 CAS.
- R. Zheng, G. Liu and X. Yan, Polymer Nano- and Microspheres With Bumpy and Chain-Segregated Surfaces, J. Am. Chem. Soc., 2005, 127(44), 15358–15359 CrossRef CAS PubMed.
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Shape effects of filaments versus spherical particles in flow and drug delivery, Nat. Nanotechnol., 2007, 2(4), 249–255 CrossRef CAS PubMed.
- Q. Wang, X. Zhang, S. Liu, H. Gui, J. Lai, Y. Liu, J. Gao, F. Huang, Z. Song, B. H. Tan and J. Qiao, Ultrafine full-vulcanized powdered rubbers/PVC compounds with higher toughness and higher heat resistance, Polymer, 2005, 46(24), 10614–10617 CrossRef CAS.
- Q. Wang, X. Zhang, Y. Jin, H. Gui, W. Dong, J. Lai, Y. Liu, J. Gao, F. Huang, Z. Song and J. Qiao, Preparation and properties of PVC ternary nanocomposites containing elastomeric nanoscale particles and exfoliated sodium-montmorillonite, Macromol. Mater. Eng., 2006, 291(6), 655–660 CrossRef CAS.
- Q. Wang, X. Zhang, W. Dong, H. Gui, J. Lai, J. Gao, Y. Liu, F. Huang, Z. Song and J. Qiao, Novel Rigid Poly(vinyl chloride) Ternary Nanocomposite Containing Ultrafine Full-Vulcanized Powdered Rubber and Nano-Sized Calcium Carbonate, Mater. Lett., 2007, 61(4–5), 1174–1177 CrossRef CAS.
- T. Serra, M. Navarro, M. A. Mateos-Timoneda, J. C. Rodriguez-Cabell and J. A. Planell, Biofunctionalization of rapid prototyping PLA scaffolds for tissue engineering, J. Tissue Eng. Regener. Med., 2012, 6, 373 Search PubMed.
- Y. Wang, D. E. Noga, K. Yoon, A. M. Wojtowicz, A. S. P. Lin, A. J. García, D. M. Collard and M. Weck, Highly Porous Crosslinkable PLA–PNB Block Copolymer Scaffolds, Adv. Funct. Mater., 2008, 18(22), 3638–3644 CrossRef CAS.
- S. Tawfick, D. M. Volder, D. Copic, S. J. Park, C. R. Oliver, E. S. Polsen, M. J. Roberts and A. John Hart, Engineering of Micro- and Nanostructured Surfaces With Anisotropic Geometries and Properties, Adv. Mater., 2012, 24(13), 1628–1674 CrossRef CAS PubMed.
- Z. Tang, X. Chen, X. Pang, Y. Yang, X. Zhang and X. Jing, Stereoselective polymerization of rac-lactide using a monoethylaluminum Schiff Base Complex, Biomacromolecules, 2004, 5(3), 965–970 CrossRef CAS PubMed.
- T. Volova, D. Goncharov, A. Sukovatyi, A. Shabanov, E. Nikolaeva and E. Shishatskaya, Electrospinning of polyhydroxyalkanoate fibrous scaffolds: effects on electrospinning parameters on structure and properties, J. Biomater. Sci., Polym. Ed., 2014, 25(4), 370–393 CrossRef CAS PubMed.
- E. Masaeli, P. A. Wieringa, M. Morshed, M. H. Nasr-Esfahan, S. Sadri, C. A. van Blitterswijk and L. Moroni, Peptide functionalized polyhydroxyalkanoate nanofibrous scaffolds enhance Schwann cells activity, Nanomedicine, 2014, 10(7), 1559–1569 CAS.
- M. Ding, M. Zhang, J. Yang and J.-H. Qiu, Study on the enzymatic degradation of aliphatic polyester–PBS and its copolymers, J. Appl. Polym. Sci., 2012, 124(124), 2902–2907 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |