Fusion and leakage of catanionic surfactant vesicles induced by α-helical peptides: the effect of membrane charge†
Dong Wang,
Yueying Cao,
Cuixia Chen,
Meiwen Cao,
Yawei Sun,
Jiqian Wang* and
Hai Xu*
State Key Laboratory of Heavy Oil Processing & Centre for Bioengineering and Biotechnology, China University of Petroleum (East China), Qingdao, 266580, P. R. China. E-mail: jqwang@upc.edu.cn; xuh@upc.edu.cn
First published on 18th October 2016
Abstract
Leakage and fusion of vesicles have triggered great interest because they are important steps in the transportation of materials in living systems. In this paper, we have shown a process of vesicle leakage and fusion induced by a type of α-helical peptide through the adsorption of peptide molecules on or their absorption in the membrane of vesicles. The fusion process was monitored by dynamic light scattering and cryogenic transmission electron microscopy, and the leakage process was tested with fluorescence dequenching of carboxyfluorescein molecules. The mechanism of leakage and fusion was deduced through circular dichroism and ζ-potential measurements. It was found that the positively charged peptides could only interact with negatively charged vesicles and that the interaction between peptides and negative vesicles depended on the charge density of the membrane. This study could enhance the understanding of the transport of materials in vivo and promote potential applications in drug delivery systems.
Introduction
Vesicles, formed by amphiphilic molecules that tend to self-aggregate, have intriguing morphology.1 Vesicles exhibit rich structural variety and attract interest both fundamentally and practically, e.g., as the closed bilayer system for modeling cell membranes,2–6 and the encapsulation and delivery vehicle of active molecules.7–10 Vesicles usually form in two different ways, either spontaneously from mixed surfactants,11 or by the application of external energy on phospholipids.12 Spontaneous vesicles based on mixed surfactants offer a number of advantages over conventional lipid-based vesicles, namely spontaneity in formation, long-term stability, and easy modulation of size and charge. Therefore, spontaneous vesicles have triggered great interest in the last twenty years.13–15 Studies of fusion and leakage of spontaneous vesicles form an important foundation for understanding spontaneous vesicle-based drug delivery systems.The α-helix is a typical secondary structure of peptides. Positively charged α-helical peptides are well known as antimicrobial peptides through interacting with the cell membranes.16–19 Most of these works were focused on the α-helical peptide-induced vesicle fusion and leakage, and tried to elucidate the mechanism of material transportation between vesicles. For instance, Bong and co-authors used the antimicrobial peptide magainin II to induce vesicle fusion. Their findings provided a guideline for understanding more complex biomembrane systems.20 Kashiwada et al. designed high-performance membrane fusion devices based on hydrophobic α-helix interaction with membranes21 and α-helical coiled–coil interaction.22 The devices were pH-responsive, which seems to be a potential strategy for novel applications in a liposome-based delivery system. Liang et al. designed two peptide molecules and studied their interactions with binary liposomes in different phases, which elucidated their effects on lipid demixing through the interactions between peptide and membrane.23 Their work not only gave insight into the effect of the lipid demixing through the interactions between peptide and membrane, but also helped in developing drug delivery vehicles with liposomes as the carriers.
In this article, we have employed a designed α-helical peptide (GIIKKIIKKIIKKI-NH2) as a membrane modulation agent. We have previously shown that designed α-helical peptides (G(IIKK)nI-NH2, n = 2 or 3) had antibacterial activity via physically breaking the bacterial membrane.24,25 It was also found that GIIKKIIKKIIKKI-NH2 showed the highest selectivity for negatively charged bacterial membranes over normal cell membranes. In this work, we extended our work to study the vesicle fusion and leakage process induced by the cationic peptide. The model vesicles were made from mixtures of hexadecyltrimethyl ammonium bromide (CTAB) and sodium dodecyl sulfate (SDS). By varying the ratio of CTAB and SDS, their surface charge can be well regulated. We found α-helical peptides could cause fusion and leakage of negative vesicles depending on their charge density. However, positive vesicles could not undergo a fusion or leakage process. This work showed the basic rules of interaction between peptides and charged membrane, and also provided a methodology for developing drug delivery systems.
Materials and methods
Materials
The peptides (GIIKKIIKKIIKKI-NH2 (peptides), GKIKKIIKKIIKII-NH2 (peptides 2) and C16-KKKKK (K5), purity > 95%) were purchased from ChinaPeptides Co., Ltd. Hexadecyltrimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS) and carboxyfluorescein (CF) (purity > 98%) were purchased from J&K Scientific Co., Ltd and used without further purification. Water used in all experiments was processed with a Millipore Milli-Q system (conductivity ≥ 18.2 MΩ cm).Cryogenic transmission electron microscopy (Cryo-TEM)
The cryo-TEM samples were prepared in a controlled environment vitrification system (CEVS). About 2 μL sample solution was coated onto a TEM copper grid and the grid was blotted with two pieces of filter paper for about 4 seconds, leading to the formation of a thin film of solution. Then, the grid was plunged into a reservoir of liquid ethane (−165 °C, cooled by liquid nitrogen) and kept in liquid nitrogen until observation. The grid was transferred to a cryogenic sample holder (Gatan 626); then the holder was put into a JEOL JEM-1400 Plus TEM (120 kV) instrument at about −174 °C and the nanostructures were observed.Circular dichroism (CD) measurements
The CD measurements were performed on an MOS-450 spectrometer (Biologic, France) using a 0.1 mm path-length quartz cuvette. The CD spectra were recorded at room temperature with wavelengths ranging from 190 to 300 nm and a scan speed of 50 nm min−1. The bandwidth was set to 0.5 nm, and a Xe lamp was used as the light source. Using the Bio-kine software package, the solvent background was subtracted and the spectra could be smoothed. The resultant CD signals were expressed as millidegrees versus wavelength.Membrane leakage experiments
Aqueous solutions with 100 mmol L−1 CF molecules were used to prepare vesicles, and size exclusion chromatography (SEC) was used to separate vesicle-encapsulated CF molecules from free CF molecules in solution. The release of entrapped CF molecules from vesicles was studied by using the fluorescence dequenching method at 25 °C. The aqueous solution of CF-loaded vesicles was stored in a 1 mm quartz cuvette. The fluorescence emission intensity change at 517 nm (excited at 492 nm) was followed on a Hitachi F2500 fluorescence spectrophotometer as a function of time. The release of CF (%) was calculated by use of eqn (1):
 | (1) |
Other measurements
Dynamic light scattering (DLS) and ζ-potential measurements were carried out on a Malvern Zetasizer Nano ZS.Results and discussion
Vesicle preparation
Catanionic vesicles were prepared by mixing SDS and CTAB at different ratios following previously established procedures.26 The total concentration of SDS and CTAB was fixed at 20 mmol L−1. The ratio of SDS and CTAB was varied from 4 mmol L−1/16 mmol L−1 to 15 mmol L−1/5 mmol L−1. Through this variation, a micelle phase, a vesicle phase, precipitation, another vesicle phase and another micelle phase were observed in sequence (Fig. 1). The microstructures of different phases were determined by cryo-TEM. Conductivities were measured to distinguish the phase boundaries. The conductivity value decreases when vesicles form because part of the free charge is bound into vesicles which leads to a decrease of the concentration of free ions. The two micelle and the two vesicle phases were found to be differently charged. We could further adjust the charge linearly by changing the ratios of SDS and CTAB (Fig. S2†). Based on this property, we thought this system was suitable as a research model to study the influence of membrane charge on the interaction with charged α-helical peptides.Peptide interaction with negatively charged vesicles
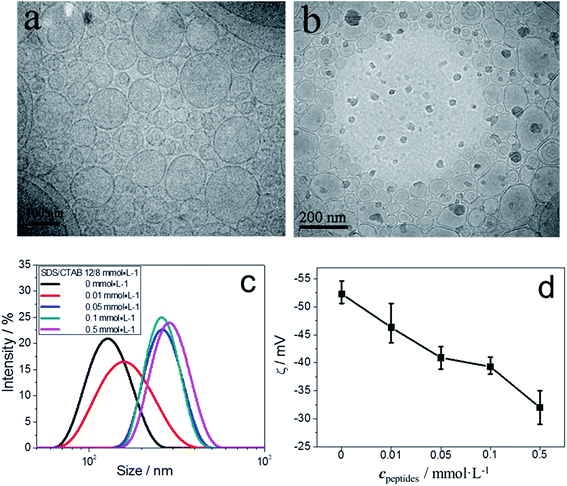
We investigated the interaction between GIIKKIIKKIIKKI-NH2 and negatively charged vesicles. We chose three different ratios of SDS/CTAB, 11/9 (cSDS/cCTAB), 12/8 and 13/7, to prepare negative vesicles.Firstly, an intermediate ratio 12/8 in the negative vesicle phase was investigated. Cryo-TEM observation and DLS measurements showed that the morphology and size of the vesicles changed after adding the peptide. The pristine vesicles were mostly round (Fig. 2a), but with the addition of peptides, the morphology of vesicles became more irregular (Fig. 2b) and some spindly vesicles even looked as though they were undergoing fusion. DLS measurements confirmed the occurrence of vesicle fusion (Fig. 2c). They showed that the average size of vesicles increased when more than 0.05 mmol L−1 of peptides was added. Vesicle size did not increase with 0.01 mmol L−1 peptide, showing that sufficient peptide molecules are a prerequisite in induced vesicle fusion.
ζ-Potential measurements of vesicles were made to monitor the membrane charge change during the addition of peptides. The ζ-potential of pure catanionic vesicles without peptides was −52 mV, as shown in Fig. 2d. With an increase of peptide, the absolute ζ-potential decreased, which indicated the adsorption or interaction of cationic peptides on the surface of negative vesicle membranes. Membrane charges are important in the stability of vesicle solutions. The 12/8 SDS/CTAB vesicles could maintain their morphology for at least 72 h (Fig. S3†). This adsorption of cationic peptides on membranes resulted in a decrease of membrane charge and the stability of the vesicles also decreased. Taken together, ζ-potential and DLS results suggested that the decrease of stability of the vesicles may lead to a higher probability of vesicle fusion.
To further understand the fusion mechanism, we regulated the membrane charges by changing the ratio of cSDS/cCTAB to 11/9 and 13/7. Cryo-TEM was used to track the fusion progress of 11/9 vesicles. Fig. 3a displays the vesicle size (about 100 nm) and morphology (round) before peptide addition. In Fig. 3b, features of vesicle fusion are indicated by the red arrows, and the primary fusion was confirmed by DLS measurements (Fig. 3c). Before peptide addition, the average size of vesicles was about 100 nm, which is in accordance with the cryo-TEM result (Fig. 3a). When 0.01 mmol L−1 peptide was added, the average size of vesicles only increased slightly. Further increase of peptide concentration to 0.05 mmol L−1 led to an increase of vesicle size to 200–300 nm, which also agreed with the cryo-TEM result in Fig. 3b. However, when the concentration of peptides was more than 0.1 mmol L−1, vesicles accumulated into large aggregates, which led to a sharp increase in size as measured by DLS.
The size evolution of vesicles could be understood through ζ-potential results. The initial ζ-potential of 11/9 vesicles without peptides was about −46 mV, slightly less than that of 12/8 vesicles without peptides. With increasing concentration of peptide, the absolute ζ-potential value decreased. When the peptides were present at more than 0.1 mmol L−1, the absolute ζ-potential values (Fig. 3d) were less than 30 mV, and precipitates were found in solutions shortly after peptide addition (the ζ-potential values were obtained before precipitation). Just like most other colloidal systems, when the absolute ζ-potential value is less than 30 mV the vesicles coagulate easily.
The 13/7 vesicle system was also investigated (Fig. 4). ζ-Potential values showed these vesicles were more negatively charged than 12/8 vesicles. Also, the ζ-potential changed from −55 mV to −47 mV when 0.5 mmol L−1 peptide was added (Fig. 4b). However, the DLS data showed that the diameters of the vesicles change little (Fig. 4a), which indicated that no fusion process occurred for 13/7 vesicles.
 | ||
| Fig. 4 DLS curves (a) and ζ-potential variation (b) of different concentrations of peptides in 13/7 (SDS/CTAB) vesicles. | ||
The dynamic fusion processes of 12/8 vesicles were also investigated by cryo-TEM and DLS. We found that vesicles with added peptide at 30 s were more irregular and smaller than those at 600 s (Fig. 5a and b). Also, a fair amount of spindle vesicles were found in the vesicles at 30 s, which indicated that they were in the process of vesicle fusion (Fig. 5a).
 | ||
| Fig. 5 Cryo-TEM images and DLS results of 0.5 mmol L−1 peptides in 12/8 SDS/CTAB vesicles at different times: (a) 30 s, (b) 600 s, (c) DLS results at 0 s, 30 s, 600 s, and 1200 s. | ||
DLS experiments also confirmed the transition process (Fig. 5c). From 0 to 30 s after adding peptide, the diameters of vesicles hardly changed. Compared with samples at 30 s, the average diameter of samples at 600 s was significantly increased. However, when the lifetime was prolonged from 600 s to 1200 s, the vesicle diameters did not increase further. This indicated that the vesicle fusion process occurred between 30 s and 600 s.
According to the above results and previous experimental and theoretical studies,6,20,27,28 we deduce that membrane fusion proceeds through at least two steps, membrane docking and actual fusion. In our system, membrane docking is induced by the peptides. When the absolute ζ-potential value of vesicles was high enough, vesicles had difficulty getting close to each other owing to electrostatic repulsion. If the positively charged peptides were adsorbed on the surfaces of the vesicles, the absolute ζ-potential value of the vesicles would be reduced. When the ζ-potential is lower than a certain value, it is difficult for vesicles to survive as individuals in the solution. Vesicles then tend to aggregate slowly, which creates an opportunity to dock. Vesicle fusion may follow docking. However, when the absolute ζ-potential value of vesicles is lower than 30 mV, much precipitation is produced fast, which is also disadvantageous for vesicle fusion.
According to our results, we found that vesicle fusion mainly occurred at ζ-potential values between −45 mV and −30 mV. However, we assume that membrane charge decrease alone would not be enough to cause vesicle fusion, because vesicle fusion would not occur in pure vesicle systems at the ζ-potential range between −45 mV and −30 mV. For instance, the 11/9 vesicles, for which ζ-potential is about −45 mV, would maintain their stability without peptide addition (Fig. S4†). Therefore, we think that the peptide molecules might act as seeds in the membranes of vesicles which break the membrane equilibrium and lead to vesicle fusion. The disequilibrium of vesicle membrane could also be proved indirectly by the leakage of vesicles.
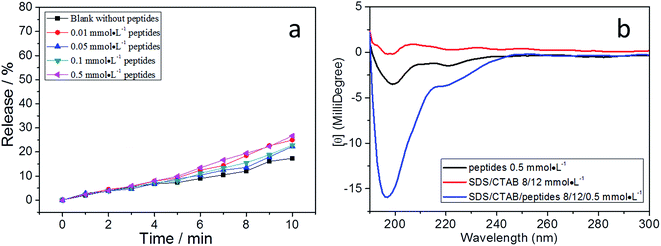
Along with the fusion process, the adsorption of peptides on vesicles also made the vesicles leak. The vesicles without peptides had a relatively low release rate (black and red lines in Fig. 6a).29 Leakage rate increased significantly after the addition of 0.5 mmol L−1 peptides. Vesicles with more negative charges (13/7) released CF faster, because the more negatively charged vesicles attracted more positive α-helical peptides. The sharp increase of CF release in the first 4 minutes indicates that the peptides were acting on the membrane of vesicles (Fig. 6a). The amount of peptides also affected the CF release rate. Fig. 6b shows 0.01 mmol L−1 peptides did not increase the CF release rate obviously in 12/8 vesicles. When the concentration of peptides increased to 0.05 mmol L−1, both the rate and amount of CF release increased sharply.
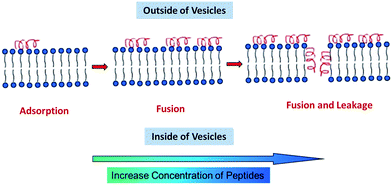
How the peptides were adsorbed on the membrane remains a question. We now discuss the possible mechanism based on our results and previous theories. Pure peptides in aqueous solution are random coils, as shown in Fig. 7a. When 5 mmol L−1 peptides was added into 12/8 vesicle solution, the peptides exhibited α-helical secondary structure. As a blank experiment, 12/8 vesicle solution was tested, and it showed no secondary structure signal from 190 nm to 300 nm. We suggest that the α-helical structure is induced by negative vesicles. First, the positive peptides are attracted by negative vesicles through electrostatic interaction. Vesicle membrane contains hydrophilic surfaces and a hydrophobic inner layer, which leads the peptide molecules to form the α-helical structure with amphipathic structures (Schiffer–Edmundson wheel in Fig. 7b). It has been proved that this kind of peptide could be induced to form amphiphilic α-helical structure by amphiphilic structures, such as SDS micelles.24,25 These α-helical amphiphilic peptides would tend to be adsorbed on the surface of the vesicles or inserted into the membrane. According to the work of Clayton, Gee and coauthors,16 α-helical peptides are first adsorbed on the surface of the membrane at low concentration of peptides, which may lead to vesicle fusion. With increasing concentration, peptides further insert into the membrane and form pores, which may lead to fusion and leakage of vesicles (Fig. 8).
To prove these assumptions, we also investigated the leakage of vesicles (11/9) using another α-helical amphiphilic peptide (GKIKKIIKKIIKII-NH2, peptides 2). We found this kind of peptide (0.5 mmol L−1) could also lead to leakage (Fig. S5†). However, when we used the cationic peptide K5, which could not form α-helical structures, we found K5 (0.5 mmol L−1) could not cause leakage (Fig. S6†). This means that K5 could not form pores in the membranes of vesicles.
Another question was, when peptides were adsorbed, what effects did this have on the membrane of vesicles except for pore formation? Some information about the effect of the peptide on the stability of the membrane comes from Helfrich's curvature energy model.30,31 The equilibrium size distribution of a population of vesicles is determined by a subtle competition between the entropy of mixing and the curvature elasticity of the bilayers. Entropy of mixing here is not the determining factor because the same surfactants and same structures were used in this system. Therefore, the size distribution could reflect the elasticity of the vesicles (eqn (2)),32,33
 | (2) |
We should go back to analyze our previous results regarding vesicle size. From Fig. 2c, we found a size distribution of 65–250 nm before the addition of peptide. When we added 0.01 mmol L−1 peptide, the size distribution was about 75–380 nm. When we further added peptide, the size distribution further increased to 150–550 nm. We deduce that adsorption of peptides causes an increase of size distribution and a decrease of the curvature elasticity of the vesicles. Fig. 3c and 4a also illustrate this trend. By cryo-TEM, we found that the vesicles became irregular after adding peptides which means that the membrane of irregular vesicles was more flexible than that of regular round ones. The change of curvature elasticity of membranes might be another important factor for vesicle fusion and leakage.
Peptide interaction with positive vesicles
The interaction between cationic vesicles and peptides was also investigated. Vesicles were prepared with 12 mmol L−1 CTAB and 8 mmol L−1 SDS. These vesicles were positively charged, as demonstrated by the ζ-potential measurement in Fig. 9d. With increase of peptide concentration, ζ-potential values were almost invariant, which indicated that peptides were hardly adsorbed on the surface of these positive vesicles at all.Cryo-TEM showed the morphology and size of vesicles before and after adding peptides (Fig. 9a and b). We found the vesicles after adding peptides retained almost the same morphology and size as before adding peptides. DLS measurements confirmed that the change of vesicle size did not have a clear trend and the vesicles have a similar distribution (Fig. 9c). When the peptide concentration was more than 1 mmol L−1, peptides might self-assemble into other big structures. This was not our focus in this article.
Leakage measurements showed the release rate remained low (Fig. 10a), which indicated that peptides cannot induce the release of CF from positive vesicles. The CD spectrum showed that the peptides present random coil secondary structure whether in pure water or in 8/12 mmol L−1 SDS and CTAB solution (Fig. 10b), indicating the peptides were not adsorbed on the membrane of vesicles.
Conclusions
The fusion and leakage processes of catanionic vesicles induced by cationic peptides have been observed and characterized. We have shown that cationic peptide interacts with negative vesicles by electrostatic and hydrophobic interactions. Adsorption of the peptide leads to a decrease of stability of vesicles and membrane curvature elasticity, which may be the main factor driving vesicle fusion and leakage. The concentration of peptide was the main factor that affected the properties of membrane owing to the charge change associated with peptide adsorption. More peptide adsorption led to easy fusion or leakage. When the ζ-potential values range from −45 mV to −30 mV, the fusion or leakage process should be promoted by adsorption of the peptide.Acknowledgements
This work was supported by National Natural Science Foundation of China (Project No. 21503276, 21573287), China Postdoctoral Science Foundation (Project No. 2014M561979), and Fundamental Research Funds for the Central Universities (Project No. 14CX 05040A).References
- M. Gradzielski, J. Phys.: Condens. Matter, 2003, 15, 655–697 CrossRef.
- B. J. Litman, Biochemistry, 1973, 12, 2545–2554 CrossRef CAS PubMed.
- F. J.-M. De Meyer, A. Benjamini, J. M. Rodgers, Y. J. Misteli and B. Smit, J. Phys. Chem. B, 2010, 114, 10451–10461 CrossRef CAS PubMed.
- J. Zimmerberg and M. M. Kozlov, Mol. Cell. Biol., 2006, 7, 9–19 CAS.
- G. Vitiello, S. Di Marino, A. M. D'Ursi and G. D'Errico, Langmuir, 2013, 29, 14239–14245 CrossRef CAS PubMed.
- R. Blumenthal, M. J. Clague, S. R. Durell and R. M. Epand, Chem. Rev., 2003, 103, 53–69 CrossRef CAS PubMed.
- M. P. Krafft, Adv. Drug Delivery Rev., 2001, 47, 209–228 CrossRef CAS PubMed.
- W. Li, T. Luo, Y. Yang, X. Tan and L. Liu, Langmuir, 2015, 31, 5141–5146 CrossRef CAS PubMed.
- E. Soussan, S. Cassel, M. Blanzat and I. Rico-Lattes, Angew. Chem., Int. Ed., 2009, 48, 274–288 CrossRef CAS PubMed.
- M. C. Branco and J. P. Schneider, Acta Biomater., 2009, 5, 817–831 CrossRef CAS PubMed.
- S. A. Safran, P. A. Pincus, D. Andelman and F. C. MacKintosh, Phys. Rev. A, 1991, 43, 1071–1078 CrossRef CAS.
- M. Antonietti and S. Förster, Adv. Mater., 2003, 15, 1323–1333 CrossRef CAS.
- E. F. Marques, O. Regev, A. Khan and B. Lindman, Adv. Colloid Interface Sci., 2003, 102, 83–104 CrossRef.
- A. Khan and E. F. Marques, Curr. Opin. Colloid Interface Sci., 2000, 4, 402–410 CrossRef.
- J. Hao and H. Hoffmann, Curr. Opin. Colloid Interface Sci., 2004, 9, 279–293 CrossRef CAS.
- Z. Ningsih, M. A. Hossain, J. D. Wade, A. H. A. Clayton and M. L. Gee, Langmuir, 2012, 28, 2217–2224 CrossRef CAS PubMed.
- L. Becucci, D. Valensin, M. Innocenti and R. Guidelli, Soft Matter, 2014, 10, 616–626 RSC.
- A. Hollmann, M. Martínez, M. E. Noguera, M. T. Augusto, A. Disalvo, N. C. Santos, L. Semorile and P. C. Maffía, Colloids Surf., B, 2016, 141, 528–536 CrossRef CAS PubMed.
- Z. L. Li, H. M. Ding and Y. Q. Ma, J. Phys.: Condens. Matter, 2016, 28, 83001 CrossRef PubMed.
- Y. Gong, M. Ma, Y. Luo and D. Bong, J. Am. Chem. Soc., 2008, 130, 6196–6250 CrossRef CAS PubMed.
- A. Kashiwada, I. Yamane, M. Tsuboi, S. Ando and K. Matsuda, Langmuir, 2012, 28, 2299–2305 CrossRef CAS PubMed.
- A. Kashiwada, M. Tsuboi and K. Matsuda, Langmuir, 2011, 27, 1403–1408 CrossRef CAS PubMed.
- Y. Xia, J. Sun and D. Liang, Langmuir, 2014, 30, 7334–7342 CrossRef CAS PubMed.
- C. Chen, J. Hu, P. Zeng, Y. Chen, H. Xu and J. R. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 16529–16536 CAS.
- C. Chen, Y. Chen, C. Yang, P. Zeng, H. Xu, F. Pan and J. R. Lu, ACS Appl. Mater. Interfaces, 2015, 7, 17346–17355 CAS.
- E. W. Kaler, A. K. Murthy, B. E. Rodriguez and J. A. N. Zasadzinski, Science, 1989, 245, 1371–1374 CAS.
- R. Blumenthal, Curr. Top. Membr. Transp., 1987, 29, 203–254 CAS.
- M. E. Haque, T. J. McIntosh and B. R. Lentz, Biochemistry, 2001, 40, 4340–4348 CrossRef CAS PubMed.
- A. Fischer, M. Hebrant and C. Tondre, J. Colloid Interface Sci., 2002, 248, 163–168 CrossRef CAS PubMed.
- H. T. Jung, B. Coldren, J. A. Zasadzinski, D. J. Iampietro and E. W. Kaler, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 1353–1357 CrossRef CAS.
- W. Xu, X. Wang, Z. Zhong, A. Song and J. Hao, J. Phys. Chem. B, 2013, 117, 242–251 CrossRef CAS PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1526–1568 RSC.
- N. D. Denkov, H. Yoshimura, T. Kouyama, J. Walz and K. Nagayama, Biophys. J., 1998, 74, 1409–1420 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22994h |
| This journal is © The Royal Society of Chemistry 2016 |