Phase transformation, morphology control, and luminescence evolution of cesium lead halide nanocrystals in the anion exchange process
Meng Lia,
Xiao Zhangb,
Simin Lua and
Ping Yang*a
aSchool of Material Science and Engineering, University of Jinan, Jinan, 250022, P. R. China. E-mail: mse_yangp@ujn.edu.cn; Fax: +86 531 87974453; Tel: +86 531 89736225
bSchool of Chemistry, University of New South Wales, Sydney 2052, Australia
First published on 18th October 2016
Abstract
Colloidal fully inorganic cesium lead halide (CsPbX3, X = Cl, Br, I) perovskite nanocrystals (NCs) have emerged as next generation candidates for photonics and optoelectronic applications. In this paper, deliberately partial or complete anion exchange reactions in cesium lead halide perovskites have been carried out at room temperature to create homogeneous solid solutions. Through adjusting chemical composition, the photoluminescence properties of the pre-synthesized cesium lead halide perovskite NCs can be tuned to cover the whole visible spectrum through controlling the ratio of parent cesium lead halide NCs and the halide precursors. By exploring the process of anion exchange, it can be concluded that the anion exchange reaction undergoes an inhomogeneous exchange process firstly and the added halide source in the solution is completely exhausted. Finally, the incoming halide will shuttle in the solution phase to reach the steady state and form the homogeneous resulting NCs. When the Br anions in the CsPbBr3 NCs were replaced by I anions, it is found that the phase composition changed gradually from a cubic to orthorhombic phase with increasing the amount of I anions. It is found that the effective anion exchange process in CsPbX3 NCs just takes place in the cubic phase rather than others.
Introduction
The halide perovskites have been identified as halide ion conductors and exhibit high ionic conductivities because of the anion migration. As a result, lead halide perovskites have been used as important optoelectronic materials with excellent efficiencies in photovoltaic and light-emitting applications such as solar cells, light-emitting diodes, and lasers.1–3 Hybrid organic–inorganic CH3NH3PbX3 (X = I, Br, Cl) as a kind of metal halide perovskite has emerged as a solution-deposited absorbing layer in solar cells because of the power conversion efficiencies.4 Compared with hybrid organic–inorganic perovskites, the study of their pure inorganic counterparts, like cesium lead halides (CsPbX3), lags far behind. However, the hybrid organic–inorganic halide perovskites are unstable; as a result, this stability issue restricts their application in many fields.5 Kovalenko and co-workers fabricated all-inorganic CsPbX3 colloidal nanocrystals (NCs) which exhibited excellent optical properties, especially a tunable and high photoluminescence (PL) efficiency.6 Compared with hybrid organic–inorganic halides, all-inorganic perovskites can be stored for more than two months with higher stability as well as huge potential in various optoelectronics.7 Except for II–VI semiconductor quantum dots, halide perovskite NCs have also attracted enormous attention recently because of their precise size and compositional control during synthesis, offering very bright, tunable, and narrow band luminescence over the whole visible wavelength range.1Other than the single kind of CsPbX3 NCs, hybrid perovskite semiconductors (CsPb(Br/I)3 or CsPb(Br/Cl)3) as an active material have outstanding performance in solar cells. Based on these semiconductors, the device efficiencies are more than 20%.1,8,9 Generally, there are two protocols to synthesize hybrid perovskite NCs. It has been proposed that through a mixture of the different cesium lead halide perovskite NCs (except for mixing CsPbI3 with CsPbCl3) in colloidal solutions, homogeneous CsPb(Br/I)3 or CsPb(Br/Cl)3 solid solutions can be synthesized with a narrow PL spectral region and are intermediate between the two parent nanoparticles.10,11 Another protocol is to combine PbX2 salts with appropriate ratios in the process of the reaction. However, these two protocols were still complicated, and it is not easy to effectively control the degree of reaction. In contrast, the anion exchange reaction is a particularly versatile tool for the synthesis of nanomaterials. Meanwhile, cation exchange reactions provide a promising avenue to prepare a variety of optoelectronic materials with perovskite structures.
It has been reported that a cation exchange reaction is particularly powerful; it keeps an uninterrupted anionic sublattice and maintains the pre-existing shape with a partial or complete replacement of cations.12 Compared with cation exchange reactions, anion exchange reactions have disadvantages, for example, substantial reconstruction or fracture of the NCs may occur during anion exchange processing. Generally, the reaction demanded the necessary reaction conditions such as the proper reaction temperature.10,13 In addition, it has been reported that the process sometimes is incomplete, partial or limited to only a few surface atomic layers.14,15 It is worth pointing out that there are few reports focused on the mechanism of anion exchange.
Here, we focus on the synthesis of all-inorganic cesium lead halide perovskite (CsPbX3, X = Cl, Br, I) NCs at room temperature. Considering the limitations of anion exchange mentioned above, we developed a fast and deliberately partial or complete anion exchange reaction to prepare homogeneous solid solutions. In this regard, we explored the exchange process and supposed a mechanism about anion exchange reactions. It is found that when the Br anions in CsPbBr3 NCs were replaced by I anions, the phase would be changed gradually from a cubic to orthorhombic phase (which is the same as that in directly synthesized CsPbI3 NCs) with increasing the inserted amount of I anions. This is different from the report in the literature.10 Except for the Br/I exchange, the initial phase of the parent perovskites is always maintained for other exchange processes.
Experimental section
Chemicals
PbCl2 (99.5%), PbBr2 (99%), PbI2 (99%), Cs2CO3 (99%), and oleylamine (OAm, 90%) were purchased from Aladdin. Octadecene (ODE, technical grade of 90%) and oleic acid (OA) were obtained from Sigma-Aldrich. Hexane, toluene and ethanol were purchased from Tianjin Chemical Reagent Company. All chemicals were used as received without any further purification.Synthesis of samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and centrifuged at 8000 rpm for 4 min. The resulting sample was re-dispersed in non-polar solvents (hexanes or toluene).
10 and centrifuged at 8000 rpm for 4 min. The resulting sample was re-dispersed in non-polar solvents (hexanes or toluene).Characterisation
The absorption spectra of the samples were taken using a Hitachi U-4100 spectrometer. The PL spectra of the samples were recorded on a Hitachi F-4600 spectrometer. The PL quantum yield (PLQY) of the samples in solutions was estimated by comparing with the standard solution, such as a rhodamine 6G (efficiency of ∼95%) ethanol solution. The emission intensity P0 (in units of the number of photons) is expressed as P0 ≈ K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η0
η0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a0
a0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × 10−0.5a0 (K is the apparatus function, and a0 is the absorbance at the 365 nm excitation wavelength). The absorbance a and PL intensity P of the sample were measured when the same apparatus parameters were kept. The PLQY of the sample is calculated by comparing with the PL intensity P0 of the rhodamine 6G solution. PL lifetime measurements were measured using the time-correlated single-photon-counting spectrofluorometer system (λex = 370 nm, Fluorocube-01, JY-IBH, Horiba). The powder X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 X-ray powder diffractometer using a Cu Kα target. Transmission electron microscopy (TEM) images were recorded using a JEM-2010 microscope operated at 100 kV. X-ray photoelectron spectroscopy (XPS) analysis was carried out using a Thermo Scientific K-Alpha Al-Kα X-ray source (hν = 1486.6 eV) with a 400 μm spot size and 20 eV pass energy. The high-resolution transmission electron microscopy (HRTEM) observation of the samples was carried out using FEI Tecnai G2 F20.
× 10−0.5a0 (K is the apparatus function, and a0 is the absorbance at the 365 nm excitation wavelength). The absorbance a and PL intensity P of the sample were measured when the same apparatus parameters were kept. The PLQY of the sample is calculated by comparing with the PL intensity P0 of the rhodamine 6G solution. PL lifetime measurements were measured using the time-correlated single-photon-counting spectrofluorometer system (λex = 370 nm, Fluorocube-01, JY-IBH, Horiba). The powder X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker D8 X-ray powder diffractometer using a Cu Kα target. Transmission electron microscopy (TEM) images were recorded using a JEM-2010 microscope operated at 100 kV. X-ray photoelectron spectroscopy (XPS) analysis was carried out using a Thermo Scientific K-Alpha Al-Kα X-ray source (hν = 1486.6 eV) with a 400 μm spot size and 20 eV pass energy. The high-resolution transmission electron microscopy (HRTEM) observation of the samples was carried out using FEI Tecnai G2 F20.
Results and discussion
We prepared CsPbX3 NCs via a typical synthesis method by hot-injecting Cs-OA into a PbX2 solution at 180 °C. It has been indicated that the nucleation and growth processes are very fast for CsPbX3.6 CsPbBr3 crystallized as orthorhombic, tetragonal, and cubic crystal phases. However, the cubic phase is formed at 160–200 °C. This is ascribed to the high synthesis temperature and surface energy.16 Furthermore, the cubic phase can be retained when the temperature was decreased to room temperature.17 This property provides the conditions for CsPbBr3 NCs to conduct anion exchange. During synthesis, dry ODE is used as a solvent. OAm and OA serve as surfactants which are densely packed on the surfaces of the NCs.6 It has been proven that none of neat ODE, OAm, or OA can dissolve PbBr2. Equal proportions of OAm and OA into a solvent (ODE) were added to solubilize PbBr2 and the NCs. We adjusted the amount of OAm and OA to 1 mL. Compared with long chain acids, it was proven that amines play a pivotal role. The perovskite formation reaction is written formally as follows:18| 2Cs-OA + 3PbBr2 → 2CsPbBr3 + Pb-OA2 | (1) |
CsPbBr3 has a high crystallinity and regular morphology, and is easy to centrifuge; on account of this property, we used CsPbBr3 for anion exchange to explore this process. Before anion exchange processing, the NC solution has to be purified. We added a small amount of ethanol into the CsPbBr3 NC solution due to it being sufficiently polar to initiate NC aggregation and precipitation upon centrifugation. In addition, the amount of ethanol is key to prevent PL degradation and maintain the high PLQY. An anion exchange source was prepared by adding OAm and OA into hexane or toluene to completely dissolve the PbX2 salt. The formation reaction is listed below:19
| PbBr2 + xHOOCR + xRNH2 → PbBr(2−x)(OOCR)x + xRNH3Br, x = 1, 2 | (2) |
HOOCR and RNH2 are derived from OA and OAm, respectively. There are some thermodynamic factors such as the difference in anion solubility and kinetic factors such as the interactions between anions and cations that influence the anion exchange process. After the synthesis of CsPbBr3 NCs, the synthesis mixture contained oleylammonium bromide, which formed by the oleylammonium cation binding with the surface bromide and stabilizing the CsPbBr3 NCs.18 Oleylammonium bromide is in the fast exchange process between a free and bound state.20 This shuttles anions from NC to NC through the solution phase to homogeneous NCs. Because of the highly dynamic stabilization mechanism with oleylammonium bromide, anion exchange reactions took place. We conduct anion exchange reactions at room temperature and in non-polar or moderately polar solvents such as toluene or hexane, since the NCs were generally unstable at high temperature or in polar solvents.21 Because of slow anion exchange reactions at room temperature, the resulting samples remained in regular morphologies.
Table 1 illustrates the preparation conditions of samples from the CsPbBr3 NCs. The value of [X]incoming/[X]parent means the composition of anion exchanged NCs, which is the molecular ratio of the added halide sources to prepare the CsPbBr3 NCs. The concentration of CsPbBr3 solutions is approximately 0.3 mM. The concentration of PbI2 and PbCl2 solutions is about 5 and 5.04 mM, respectively. Sample A0 is directly synthesized CsPbBr3 NCs without the anion exchange process. A1, A2, A3, and A4 were prepared by adding different amounts of PbI2 solution into CsPbBr3 solution. Similarly, A5, A6, A7, and A8 were prepared by adding different amounts of PbCl2 solution into CsPbBr3 solution.
| Sample | CsPbBr3 (mL) | PbI2 (μL) | PbCl2 (μL) | [X]incoming/[X]parent |
|---|---|---|---|---|
| A0 | 2 | 0 | 0 | 0 |
| A1 | 2 | 20 | 0 | 0.16 |
| A2 | 2 | 35 | 0 | 0.28 |
| A3 | 2 | 45 | 0 | 0.38 |
| A4 | 2 | 75 | 0 | 0.63 |
| A5 | 2 | 0 | 10 | 0.08 |
| A6 | 2 | 0 | 20 | 0.17 |
| A7 | 2 | 0 | 53 | 0.44 |
| A8 | 2 | 0 | 80 | 0.67 |
The emission colour of samples A1 to A8 in hexane is in the range from blue to red under 365 nm light irradiation as shown in Fig. 1(a). The PL properties can be tuned by varying the composition. When I or Cl anions were introduced into the reaction system, CsPb(Br/I)3 or CsPb(Br/Cl)3 NCs were created. The PL peak wavelength of the CsPbBr3 NCs (sample A0) is about 516 nm as shown in the middle in Fig. 1(a). The PL peak was red-shifted with increasing the amount of I ions. In contrast, on introducing Cl ions, the PL peak was blue-shifted to the short wavelength direction (Fig. 1(c)). Meanwhile, the continuous change of the PL peak wavelength corresponds to the change of energy (Fig. 1(d)). The PL spectra shown in Fig. 1(c) indicate that the PL of the samples can be effectively tuned to cover the whole visible region from blue emission of 450 nm with Cl− ions, and 516 nm with Br− ions, to red emission of 640 nm with I− ions.
Fig. 2(a) shows the absorption and PL spectra of the samples prepared by anion exchange and the as-synthesized CsPbBr3 NCs. The anion exchange reactions discussed here resulted in the PL spectra either blue-shifting when Cl− replaces Br−, or red-shifting when Br− is replaced by I−, proving the incoming of new anions. The result indicates the continuous formation of homogeneous CsPb(Br/X)3 (X = I, Cl) solid solutions from the gradual shift of the absorption and PL spectra. The full width at half maximum (fwhm) of the PL spectra is illustrated in Fig. 2(b). Sample A8, in which the great majority of Br anions in CsPbBr3 are replaced by Cl anions, holds a minimal fwhm value. In contrast, the PL spectra became broad when the maximum amount of I anions was introduced. To some degree, the fwhm of the PL spectra is relative to the size distribution of the NCs. In other words, the incoming of Cl anions makes the particles more uniform while the incorporation of I anions tends to give a broad size distribution. We assume that the Cl anion addition does not influence the crystallinity, monodispersity, and morphology of the NCs. We describe this in more detail subsequently.
 | ||
| Fig. 2 (a) Absorption and PL spectra of samples. (b) Fwhm of PL spectra of samples. (c) Time-resolved PL decay curves of samples. (d) PLQY and PL peak wavelength of samples. | ||
The time-resolved PL decay curves of the prepared samples are shown in Fig. 2(c). The fitting parameters in the fluorescence decay curves and average lifetimes are illustrated in Table 2. A bi-exponential model described by F(t) = A + B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + B2
exp(−t/τ1) + B2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) was used to fit the decay curves. In this equation, τ1 and τ2 represent the time constants, and B1 and B2 represent the amplitudes of the fast and slow components, respectively. The short average lifetime τ is calculated using τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2). τ1 is in the range of 3.5–18.5 ns, and τ2 is within 40.5–128.5 ns. The average lifetimes are in the range of 40.02–127.03 ns. The fast component of the PL decay is associated with exciton recombination. The slow component is considered to originate from the surface-related emission of the CsPbX3 NCs. In general, the fast component (B1) of the PL decay decreased with the increase of the slow component (B2).22 The value τ2 of sample A8 with the greatest amount of Cl anions is the smallest; unlike this, the more I there is, the bigger the τ2 value is. The surface states such as defects and impurities form defect levels have some effect on the slow components (Table 2).
exp(−t/τ2) was used to fit the decay curves. In this equation, τ1 and τ2 represent the time constants, and B1 and B2 represent the amplitudes of the fast and slow components, respectively. The short average lifetime τ is calculated using τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2). τ1 is in the range of 3.5–18.5 ns, and τ2 is within 40.5–128.5 ns. The average lifetimes are in the range of 40.02–127.03 ns. The fast component of the PL decay is associated with exciton recombination. The slow component is considered to originate from the surface-related emission of the CsPbX3 NCs. In general, the fast component (B1) of the PL decay decreased with the increase of the slow component (B2).22 The value τ2 of sample A8 with the greatest amount of Cl anions is the smallest; unlike this, the more I there is, the bigger the τ2 value is. The surface states such as defects and impurities form defect levels have some effect on the slow components (Table 2).
| Sample | B1 | τ1 (ns) | B2 | τ2 (ns) | τ (ns) |
|---|---|---|---|---|---|
| A8 | 37.69 | 3.76 | 62.32 | 40.58 | 40.02 |
| A7 | 41.82 | 7.03 | 58.18 | 43.82 | 40.36 |
| A6 | 43.38 | 9.89 | 56.62 | 48.85 | 43.62 |
| A5 | 40.76 | 9.88 | 59.24 | 49.57 | 44.79 |
| A0 | 32.51 | 9.66 | 67.49 | 52.29 | 48.81 |
| A1 | 26.47 | 7.99 | 73.53 | 51.28 | 48.98 |
| A2 | 19.74 | 12.74 | 80.26 | 69.85 | 67.4 |
| A3 | 15.76 | 17.35 | 84.24 | 89.91 | 87.28 |
| A4 | 8.57 | 18.46 | 91.43 | 128.49 | 127.03 |
The lifetime of luminescent NCs is related to the composition, size, morphology, microstructure, and surface state. It has been reported that the average PL decay lifetimes of CsPbBr3 nanocubes were 1–22 ns.23 However, there are few reports about the fluorescence lifetimes of mixed CsPb(Br/X)3 perovskite NCs. In our experiment, the average lifetimes of CsPbBr3 and exchanged samples are in the range of 40–127 ns, which is long compared with that reported in the literature.6 The average PL lifetime of colloidal CsPb(Br/I)3 NCs was greatly increased with increasing the ratio of [I]incoming/[Br]parent in the reaction system. In the case of I ions instead of Br ions (samples A1 to A4), the lifetime increased. In contrast, the lifetime decreased when Br ions were replaced by Cl ions. This phenomenon is firstly ascribed to the component changing. Secondly, an ion exchange process resulted in the difference of morphology and surface state such as surface defects.5 Fig. 2(d) shows the PLQY of the samples. The value of the PLQY is a function of the energy of the emitted photons.11 The as-synthesized CsPbBr3 NCs possess the highest PLQY up to 90%. The PLQY of the NCs also decreased gradually with an increasing amount of ion replacement. The maximum exchange amount of both Cl and I ions resulted in a minimum PLQY. This is ascribed to the increase of surface defects during ion exchange. This phenomenon is different from the change of the PL lifetime of the NCs. This lifetime is ascribed to several factors including the components, size, and microstructure, and the PLQY is mainly related to surface defects which affect the crystallinity.
The TEM images in Fig. 3 indicate that the fast halide exchange reaction did not change the shape and crystal structure of the initial CsPbBr3 NCs. And no remarkable defects are observed (Fig. 3(a)–(e)). The lattice fringes of the synthesized CsPbBr3 NCs are 0.58 nm, corresponding to the (001) surface of the CsPbBr3 cubic phase (Fig. 3(f)). The average particle size of the NCs decreased slightly after exchange with Cl anions, from 18 ± 1.0 nm to 14 ± 1.0 nm, whereas the exchange with I− led to a slight increase in size to 27 ± 1.0 nm (Fig. 4(a)). As is shown in Fig. 4(c), we find that the directly synthesized CsPbBr3 and CsPbCl3 NCs crystallize in the cubic phase, as well as the pure CsPbI3 crystallizing in an orthorhombic structure. CsPb(Br/I)3 is the transition state from the cubic phase to orthorhombic phase. As expected, the incorporation of Cl− resulted in cell shrinkage; as a result, all the XRD peaks shifted toward the large angle direction. The incorporation of I− ions expanded the cell and the XRD peaks shifted to the small angle direction. The continuous changes of the XRD peaks also demonstrated the formation of homogeneous solid solutions. It has been reported that Cl anions can form a strong and directional bond to lead. As a result, the energetic costs of a transition from the cubic phase to orthorhombic phase were increased.24,25 Additionally, it has been indicated that chloride doping increases the bulk modulus and Goldschmidt tolerance factor, thus perovskites show greater stability with the smaller diameter chlorine.26 Furthermore, CsPbI3 NCs are not stable and are easily affected by ambient conditions, but solid solutions formed by bromide and iodide such as CsSnX3,27 CsPbX3,6 and MAPbX3 recently have been shown to be more stable than the pure iodide phase.28 We assume that the change of the structure has some connection with the bandgap and lattice parameters; in our following research, we intend to study its structure.
 | ||
| Fig. 3 TEM images of samples A8 (a), A6 (b), A0 (c), A2 (d), and A4 (e). Scale bar is 50 nm. (f) HRTEM micrograph depicting atomic resolution of as-prepared CsPbBr3 NCs (sample A0). | ||
The results from the TEM images and XRD patterns indicate that the addition of Cl anions has no influence on the crystallinity, monodispersity, and morphology of the parent NCs, whereas the addition of I anions changes the phase structure of the NCs from cubic to orthorhombic. This change of phase structure resulted in a broad PL spectrum for sample A4 (Fig. 2(b)). The samples prepared by anion exchange from CsPbBr3 NCs exhibit very saturated and pure colours, as demonstrated by the Commission International de l’Eclairage (CIE) chromaticity diagram as shown in Fig. 4(b). Each point in the chromaticity diagram corresponds to a different halide composition of the NCs.
XPS analysis of the samples was performed to further investigate the elemental composition of the samples after ion exchange. Fig. 5(a)–(c) show the survey spectrum and high-resolution spectra of Br 3d and I 3d. The Br 3d peaks of CsPb(Br/I)3 can be divided into two peaks with binding energies of 66.5 and 67.6 eV, which are similar to the reported values (67.7 and 68.7 eV).29,30 There are two I 3d peaks at 617.1 and 628.7 eV, corresponding to the I 3d5/2 peaks (620.1 and 624.6 eV).31 Fig. 5(d)–(f) show the survey X-ray photoelectron spectrum and high-resolution X-ray photoelectron spectra of Br 3d and Cl 2p.
 | ||
| Fig. 5 XPS spectra of NC samples. (a) Survey of CsPb(Br/I)3. (b) Br 3d. (c) I 3d. (d) Survey of CsPb(Br/Cl)3. (e) Br 3d. (f) Cl 2p. | ||
Similar to Fig. 5(b), the Br 3d spectrum consists of two peaks at approximately 66.7 and 67.8 eV (Fig. 5(e)). Fig. 5(f) shows two Cl 2p peaks around 196 and 198 eV, corresponding to the Cl 2p3/2 and Cl 2p1/2 peaks.32,33 The XPS spectrum can clearly demonstrate the incoming of the halide source after the anion exchange reaction.
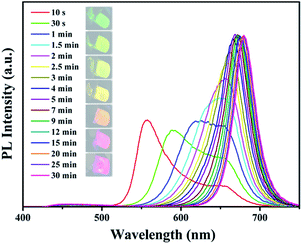
For the purpose of investigating the process of anion exchange, we explored the PL properties of the NCs during the Br to I anion exchange process. Fig. 6 shows the evolution of the PL spectra with time. It illustrates that the anion exchange in the NCs is initiated immediately while the halide sources were added to the purified NCs solution. The reaction started quickly during the first three minutes, and after that, the reaction proceeded very slowly and gradually reached the steady state. It is interesting to observe two peaks in the tenth second corresponding to the wavelengths of 557 nm and 658 nm, and the first peak quickly red-shifted and gradually disappeared. Meanwhile, the second peak increased quickly and shifted toward the longer wavelength direction slowly. The anion exchange reactions are mainly in the first five minutes. It has been demonstrated that the halides have high conductivity.34 We assume that in the first stage of this reaction, the CsPbBr3 NCs were exchanged inhomogeneously by I anions.
This means that in some cubic nanoparticles, a large amount of Br anions was replaced by I anions, whereas in some other nanoparticles few Br anions were replaced. The exchange process between NCs in the solution takes place quickly. When the halide resources are exhausted (the second stage), the I anions shuttle from NC to NC through the solution phase to reach a steady state as well as forming homogeneous NCs in the end (the third stage) due to the high conductivity of I anions. This stage is likely to be the formation of homogeneous CsPb(Br/Cl)3 or CsPb(Br/I)3 solution by mixing the different kinds of CsPbX3 solution.11
We also investigate the exchange process of Br anions to Cl anions as shown in Fig. 7(a). The value of [Cl]incoming/[Br]parent is 0.46 in the reaction system. Fig. 7(b) is the enlarged image of the dotted portion in Fig. 7(a). It can be observed that there are two obscure wavelength peaks for 30 s, 1 min, 1.5 min, and 2 min. During the reaction, a peak disappeared and the other peak increased. This is ascribed to the formation of inhomogeneous CsPb(Br/Cl)3 and the shuttling of Cl anions subsequently. It is worth noting that there is only one wavelength peak in the first 10 seconds due to the exchange reactions not having been initiated. The result indicates that the CsPbCl3 NCs have good crystallization as shown in Fig. 8(a). Then we replace the Cl anions by adding a Br anion source. Fig. 8(a) and (b) show the TEM images of CsPbCl3 and exchanged CsPbBr3 NCs. It can be demonstrated that the CsPbCl3 morphology was maintained very well.
 | ||
| Fig. 7 (a) In situ PL evolution of CsPbCl3 instead of CsPbBr3 in the ion exchange process. (b) Enlarged image of the dotted portion in (a). | ||
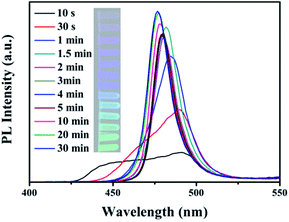
Similarly, we investigate the in situ PL evolution of the NCs during the ion exchange process below. Fig. 9 shows the in situ PL evolution of a NC sample in an anion exchange process from CsPbCl3 to CsPbBr3. The appearance of two PL peaks in the 10th second demonstrates the inhomogeneous ion exchange by Br anions until a PL peak disappeared. The value of [Br]incoming/[Cl]parent is 0.85 in the reaction system. This phenomenon proved our proposal above again. For comparison, we synthesized CsPbI3 NCs which crystallize in an orthorhombic phase (Fig. 8(c)), and replaced I with Br anions. However, different from the exchange process mentioned before, the exchanged samples can’t keep the morphology of the initial CsPbI3 NCs (Fig. 8(d)). Therefore, the effective anion exchange process can’t proceed while the directly synthesized CsPbX3 NCs crystallized in an orthorhombic phase, rather than in a cubic phase. The PL spectrum shift in Fig. 9 is different from that in Fig. 7. The PL peak of the sample obtained after ion exchange is around 475 nm in Fig. 9 while the PL peak wavelength after the exchanging process in Fig. 7 is about 440 nm. This is mainly ascribed to the difference of the [Br]incoming/[Cl]parent ratios. In addition, this phenomenon indicates that the process of ion exchange affects the formation of the resulting samples.
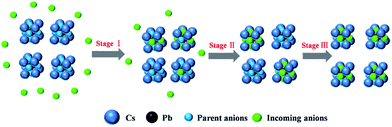
It has been reported that the easily exchanged reactions mainly occurred for three reasons: firstly, the high diffusion/migration ability of halide anions which have a single ionic charge in the perovskite lattice; secondly, the cation framework has a rigid nature and is highly defect-tolerant; and finally, the effective vacancy-assisted diffusion mechanism.10,35 In addition, the distribution of two halide anions in the solution and the NCs was controlled by the balance of the crystal energies and the solvation energies. For all halides, the crystal energies are similar, as well as the lack of a strong preference toward the formation of one kind of perovskite. As a result, the balance was maintained between the crystal and the solution.10 Fig. 10 shows a schematic illustration of the anion exchange process. In stage I, the parent anions in the CsPbX3 NCs are exchanged inhomogeneously by the incoming anions from the solution. This means that in some cubic nanoparticles, most of the parent anions are replaced by incoming anions, whereas in some other nanoparticles, fewer are replaced. In stage II, the incoming anions are used, as well as exchanged particles forming with a different amount of incoming anions. Finally in stage III, the I anions shuttle from NC to NC through the solution phase to reach a steady state as well as forming homogeneous NCs in the end due to the high conductivity of I anions.
Conclusions
In summary, we have demonstrated the remarkably fast anion exchange approach to study a wide variety of CsPbX3 perovskite NCs. The chemical composition and the optical properties of the pre-synthesized CsPbBr3 perovskite NCs can be tuned to cover the whole visible spectrum via controlling the ratio of parent cesium lead halide NCs and the halide precursors. It is found that the directly synthesized CsPbI3 NCs crystallized in an orthorhombic phase, and the exchanged samples can’t keep the morphology of the initial CsPbI3 NCs. Thus, the effective anion exchange process can’t proceed as the directly synthesized CsPbX3 NCs are not crystallized in the cubic phase. The shape and crystal structure of the starting CsPbBr3 sample can be preserved while undergoing the Br− to Cl− exchange process. However, we discovered that when the Br anions in the CsPbBr3 NCs were replaced by I anions, the phase would change gradually from the cubic to orthorhombic phase (which is the same phase as that in directly synthesized CsPbI3 NCs) with the increasing inserted amount of I anions. In addition, by exploring the process of anion exchange, we discovered that the anion exchange reaction undergoes an inhomogeneous exchange process firstly, and while the added halide source in the solution is used, the incoming halide will shuttle from NC to NC through the solution phase to reach the steady state and form homogeneous NCs. The effective anion exchange process is mainly because of the high ion mobility. However, the high ion mobility may be one of the possible reasons for anomalous hysteresis in the current–voltage curves of perovskite-based solar cells.Acknowledgements
This work was supported by the program for Taishan Scholars, the projects from National Natural Science Foundation of China (grant no. 51572109, 51501071, 51302106, 51402123, and 51402124).Notes and references
- G. E. Eperon, Energy Environ. Sci., 2014, 7, 982–988 CAS.
- F. Palazon, F. D. Stasio, Q. A. Akkerman, R. Krahne, M. Prato and L. Manna, Chem. Mater., 2016, 28, 2902–2906 CrossRef CAS PubMed.
- K. Young-Hoon, C. Himchan, H. J. Hyuck, K. Tae-Sik, M. Nosoung, L. Chang-Lyoul, I. S. Hyuk and L. Tae-Woo, Adv. Mater., 2015, 27, 1248–1254 CrossRef PubMed.
- N. G. Park, J. Phys. Chem. Lett., 2013, 4, 2423–2429 CrossRef CAS.
- F. Zhang, H. Zhong, C. Chen, X. G. Wu, X. Hu and H. Huang, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7161 CrossRef CAS.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu and J. Seo, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu and J. Seo, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D’Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza and M. Prato, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D’Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza and M. Prato, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- H. Li, M. Zanella, A. Genovese, M. Povia, A. Falqui and C. Giannini, Nano Lett., 2011, 11, 4964–4970 CrossRef CAS PubMed.
- J. Park, H. Zheng, Y. Jun and A. P. Alivisatos, J. Am. Chem. Soc., 2009, 131, 13943–13945 CrossRef CAS PubMed.
- M. Saruyama, Y. G. So, K. Kimoto, S. Taguchi, Y. Kanemitsu and T. Teranishi, J. Am. Chem. Soc., 2011, 133, 17598–17601 CrossRef CAS PubMed.
- D. Choi, S. Lee, J. Lee, K. S. Cho and S. W. Kim, Chem. Commun., 2015, 51, 899–902 RSC.
- C. C. Stoumpos, C. D. Malliakas, J. A. Peters, Z. Liu, M. Sebastian and J. Im, Cryst. Growth Des., 2013, 13, 2722–2727 CAS.
- G. Rainò, G. Nedelcu, L. Protesescu, M. I. Bodnarchuk, M. V. Kovalenko and R. F. Mahrt, ACS Nano, 2016, 10, 2485–2490 CrossRef PubMed.
- R. J. De, M. Ibáñez, P. Geiregat, G. Nedelcu, W. Walravens and J. Maes, ACS Nano, 2016, 10, 2071–2081 CrossRef PubMed.
- N. C. Anderson, M. P. Hendricks, J. J. Choi and J. S. Owen, J. Am. Chem. Soc., 2013, 135, 18536–18548 CrossRef CAS PubMed.
- Z. Hens and J. C. Martins, Chem. Mater., 2013, 25, 1211–1221 CrossRef CAS.
- L. C. Schmidt, O. Malinkiewicz, S. Agouram, H. J. Bolink and R. E. Galian, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- C. D. M. Donegá, M. Bode and A. Meijerink, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 085320 CrossRef.
- S. Sun, D. Yuan, Y. Xu, A. Wang and Z. Deng, ACS Nano, 2016, 10, 3648–3657 CrossRef CAS PubMed.
- S. Sun, Y. Fang, G. Kieslich, T. J. White and A. K. Cheetham, J. Mater. Chem. A, 2015, 3, 18450–18455 CAS.
- S. Dastidar, D. A. Egger, L. Z. Tan, S. B. Cromer, A. D. Dillon, S. Liu, L. Kronik, A. M. Rappe and A. T. Fafarman, Nano Lett., 2016, 16, 3563–3570 CrossRef CAS PubMed.
- V. M. Goldschmidt, Naturwissenschaften, 1926, 14, 477–485 CrossRef CAS.
- D. Sabba, H. K. Mulmudi, R. R. Prabhakar, T. Krishnamoorthy, T. Baikie, P. P. Boix, S. Mhaisalkar and N. Mathews, J. Phys. Chem. C, 2015, 119, 1763–1767 CAS.
- R. Raffael, B. Felix, B. Felix, S. Martina and S. Derck, ChemPhysChem, 2016, 17, 1505–1511 CrossRef PubMed.
- F. Zhang, H. Zhong, C. Chen, X. G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. P. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- F. Palazon, Q. A. Akkerman, M. Prato and L. Manna, ACS Nano, 2016, 10, 1224–1230 CrossRef CAS PubMed.
- P. Y. Chuang, W. L. Lee, T. F. Teng, Y. H. Lai and W. H. Hung, J. Phys. Chem. C, 2009, 113, 17447–17454 CAS.
- F. Gao and A. V. Teplyakov, J. Phys. Chem. C, 2016, 120, 5539–5548 CAS.
- T. P. Chopra, R. C. Longo, K. Cho, M. D. Halls, P. Thissen and Y. J. Chabal, Chem. Mater., 2015, 27, 6268–6281 CrossRef CAS.
- J. Mizusaki, K. Arai and K. Fueki, Solid State Ionics, 1983, 11, 203–211 CrossRef CAS.
- G. Li, Y. L. Ho, W. Man and H. S. Kwok, J. Phys. Chem. C, 2015, 119, 26883–26888 CAS.
| This journal is © The Royal Society of Chemistry 2016 |