Phosphine-catalyzed [4 + 3] cycloaddition reaction of aromatic azomethine imines with allenoates†
Zhen Li,
Hao Yu,
Yalin Feng,
Zhanfeng Hou,
Lei Zhang,
Wenjun Yang,
Yang Wu,
Yumei Xiao and
Hongchao Guo*
Department of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hchguo@cau.edu.cn
First published on 8th April 2015
Abstract
An efficient phosphine-catalyzed [4 + 3] cycloaddition of aromatic azomethine imines with allenoates has been developed, providing dinitrogen-fused heterocyclic compounds in moderate to excellent yields. The reaction proceeds smoothly under mild conditions, providing an expedient access to highly valuable heterocyclic compounds with isoquinoline, quinoline and phenanthridine skeletons.
1,3-dipolar cycloadditions are one of the most powerful methods for the convergent synthesis of various heterocycles. Among 1,3-dipoles for 1,3-dipolar cycloadditions, azomethine imines have attracted much attention due to their stability and high accessibility. A variety of thermal, metal-catalyzed and organocatalytic cycloadditions including [3 + 2], [3 + 3] and [4 + 3] cycloadditions have been developed to construct dinitrogen-fused heterocyclic derivatives.1–3 Of these reactions, [4 + 3] cycloaddition reactions of azomethine imines provide a concise and efficient approach for the synthesis of dinitrogen-fused seven-membered heterocycles. However, the successful examples on metal-catalyzed and organocatalytic [4 + 3] cycloaddition of azomethine imines are quite limited. In 2007, Hayashi reported Pd-catalyzed [4 + 3] cycloadditions of azomethine imines with γ-methylidene-δ-valerolactones for the synthesis of hexahydropyrazolodiazepinone.4 Most recently, Chi disclosed the first N-heterocyclic carbene (NHC)-catalyzed [4 + 3] cycloaddition of azomethine imines and enals for the synthesis of tetrahydropyrazolodiazepinedione.5 In addition to above efforts, our group developed phosphine-catalyzed [4 + 3] cycloaddition reactions of N,N′-cyclic azomethine imines and C,N-cyclic azomethine imines with allenoates, affording biologically important dinitrogen-fused seven-membered heterocyclic compounds.6 As a continuation of our research interest in developing novel 1,3-dipolar cycloaddition reactions,7 we set out to investigate the phosphine-catalyzed [4 + 3] cycloaddition of C,N-cyclic aromatic azomethine imines including N-acetyliminoisoquinolinium betaine (1), N-acetyliminoquinolinium betaine (2), N-acetyliminophenanthridinium betaine (3) with allenoates (Scheme 1).8,9 The phosphine-catalyzed [4 + 3] cycloaddition reaction will provides efficient synthetic methods for the heterocyclic compounds with isoquinoline, quinoline and phenanthridine skeleton, which display a broad range of uses and work as key units in of many pharmaceuticals, agrochemicals, and other useful chemicals.10 Herein, we report our results on this phosphine-catalyzed [4 + 3] cycloaddition reaction of aromatic azomethine imines with allenoates.
 | ||
| Scheme 1 Phosphine-catalyzed [4 + 3] cycloaddition of C,N-cyclic aromatic azomethine imines with allenoates. | ||
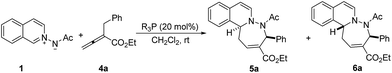
The investigation was initiated by evaluating the reaction of N-acyliminoisoquinolinium betaine 1 with allenoate 4a11 at room temperature. A control experiment indicated that no [4 + 3] cycloadduct was observed in the absence of phosphine and only the thermal [3 + 2] cycloaddition product was obtained (Table 1, entry 1).7d Using 20 mol% of Ph3P as the catalyst, a very small amount of [4 + 3] cycloadduct could be observed, and thermal cycloadduct was isolated as the major product (entry 2). When more nucleophilic MePPh2 was used, 15% yield of [4 + 3] cycloaddition product was obtained, although the competitive thermal cycloaddition reaction still dominated the reaction process (entry 3). To our delight, when strong nucleophilic Bu3P was used as the catalyst, the competitive thermal cycloaddition was effectively suppressed, and the [4 + 3] cycloaddition reaction of azomethine imine 1 with allenoate 4a proceeded efficiently to provide a seven-membered cycloadduct in 88% yield with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 4). Unfortunately, the product is a mixture of two diastereoisomers which could not be separated by flash column chromatography. Lowering the reaction temperature to 0 °C led to lower 64% yield (entry 5). The solvent screening revealed that dichloromethane is the optimal solvent (entries 6–7).
1 dr (entry 4). Unfortunately, the product is a mixture of two diastereoisomers which could not be separated by flash column chromatography. Lowering the reaction temperature to 0 °C led to lower 64% yield (entry 5). The solvent screening revealed that dichloromethane is the optimal solvent (entries 6–7).
| Entry | PR3 | Solvent | Time (h) | Yieldb (%) | dr (5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6a)c 6a)c |
|---|---|---|---|---|---|
| a Reactions of azomethine imine 1 (0.125 mmol) and the allenoate 4a (0.15 mmol) were carried out in 3 mL of dichloromethane.b Isolated yields of two products.c Based on 1HNMR analysis.d Without phosphine catalyst.e The reaction was performed at 0 °C. | |||||
| 1 | —d | CH2Cl2 | 48 | 0 | — |
| 2 | Ph3P | CH2Cl2 | 48 | <5 | — |
| 3 | MePPh2 | CH2Cl2 | 48 | 15 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Bu3P | CH2Cl2 | 40 | 88 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5e | Bu3P | CH2Cl2 | 48 | 64 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Bu3P | Toluene | 48 | 62 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Bu3P | THF | 48 | 73 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
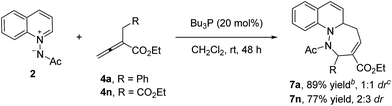
With the optimal reaction conditions in hand, we investigated the [4 + 3] cycloaddition reactions of azomethine imine 1 with various α-substituted allenoates 4.11 As shown in the Table 2, various α-benzyl allenoates bearing electron-withdrawing and electron-donating substituents at the different aromatic position underwent the reaction to give the desired cycloadducts in good to excellent yields and moderate to excellent diastereoselectivities (entries 1–11). The relative configuration was determined by the NMR spectroscopy and the single X-ray crystallographic analysis (ESI†). The α-(2-naphthylmethyl)-allenoate (4k) was also tolerated, thus producing the desired [4 + 3] cycloadduct in 69% yield with 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 11). Disappointingly, the mixture of two diastereomers could not be separated by the flash column. The α-ethylallenoate (4l) and α-n-propylallenoate (4m) underwent the reaction to give the desired product as a single diastereomer in 81% and 78% yield respectively (entries 12–13). α-Ethoxycarbonylallenoate (4n) was also a compatible substrate to provide the corresponding product in 79% yield with 5
1 dr (entry 11). Disappointingly, the mixture of two diastereomers could not be separated by the flash column. The α-ethylallenoate (4l) and α-n-propylallenoate (4m) underwent the reaction to give the desired product as a single diastereomer in 81% and 78% yield respectively (entries 12–13). α-Ethoxycarbonylallenoate (4n) was also a compatible substrate to provide the corresponding product in 79% yield with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 14).
1 dr (entry 14).
| Entry | R | Product | Yieldb (%) | dr (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)c 6)c |
|---|---|---|---|---|
| a Reactions of azomethine imine 1 (0.125 mmol) and the allenoates 4 (0.15 mmol) were carried out in 3 mL of dichloromethane at rt for 48 h.b Isolated yields of two products.c Based on 1H NMR analysis. | ||||
| 1 | Ph (4a) | 5a + 6a | 88 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 2-MeC6H4 (4b) | 5b + 6b | 80 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3-MeC6H4 (4c) | 5c + 6c | 70 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 4-MeC6H4 (4d) | 5d + 6d | 85 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 2-FC6H4 (4e) | 5e + 6e | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4-FC6H4 (4f) | 5f + 6f | 87 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3-ClC6H4 (4g) | 5g + 6g | 78 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4-ClC6H4 (4h) | 5h + 6h | 89 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3-BrC6H4 (4i) | 5i + 6i | 83 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 4-BrC6H4 (4j) | 5j + 6j | 90 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 2-Nap (4k) | 5k + 6k | 69 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | CH3 (4l) | 5l + 6l | 81 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | CH2CH3 (4m) | 5m + 6m | 78 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | CO2Et (4n) | 5n + 6n | 79 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
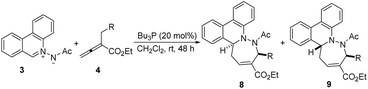
As indicated in Table 3, in the presence of 20 mol% of Bu3P, N-acetyliminoquinolinium betaine (2) could also undergo the [4 + 3] cycloaddition reaction with allenoates (4), affording the corresponding cycloadduct as a diastereoisomeric mixture in high yield, albeilt with moderate diastereoselectivity. Pitifully, the relative configuration had not been determined because the single crystal of the product could not be obtained. We next investigated the [4 + 3] cycloaddition of N-acetyliminophenanthridinium betaine (3) with a variety of allenoates 4 (Table 4). Under the optimal reaction conditions, various allenoates could be tolerated to give the desired cycloadducts in high yields. In general, α-aryl-CH2-substituted allenoates afford the corresponding products in excellent yields, albeit in moderate diastereoselectivities. Fortunately, compared with the products from azomethine imine 1 or 2, the mixture of two diastereomers from 3 could be separated by using flash column, except for the product from the reaction of 3 with 4l. The α-alkyl-substituted allenoates including α-ethyl allenoate (4l) and α-n-propyl allenoate (4m) gave the corresponding product in 81% yield and 94% yield, respectively (entry 5 and 6). Additionally, allenoate with an ester group on β′-position (4n) gave a high yield of the cycloadduct with poor diastereoselectivity (entry 7). The relative configuration of the products 8a and 9a was unambiguously confirmed by X-ray diffraction analysis (ESI†).
| Entry | R | 8, Yieldb (%) | 9, Yieldb (%) |
|---|---|---|---|
a Reactions of azomethine imine 3 (0.125 mmol) and the allenoates 4 (0.15 mmol) were carried out in 5 mL of dichloromethane.b Isolated yields.c Total yield of two diastereomers, 8l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9l = 10 9l = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
|||
| 1 | Ph (4a) | 8a, 57 | 9a, 29 |
| 2 | 2-FC6H4 (4e) | 8e, 76 | 9e, 15 |
| 3 | 4-ClC6H4 (4h) | 8h, 71 | 9h, 26 |
| 4 | 2-naphthyl (4k) | 8k, 61 | 9k, 19 |
| 5 | Me (4l) | 8l + 9l, 81c | |
| 6 | Et (4m) | 8m, 71 | 9m, 23 |
| 7 | CO2Et (4n) | 8n, 49 | 9n, 40 |
During the purification process of the cycloadducts, we observed an interesting oxidation phenomenon. In the air, the [4 + 3] cycloadducts could slowly be oxidized, but the full conversion required 10 days. Although full conversation could be accomplished, the yield was not high due to decomposition of the [4 + 3] cycloadduct in the process of oxidation. Gratefully, under oxygen atmosphere, the cycloadducts 5a and 6a were stirred in dichloromethane at 40 °C for 3 days to give the oxidation product 10 in 49% yield. With the use of 1.2 equiv. of DDQ, the cycloadduct 5f and 6f could be oxidized to the corresponding derivative 11 in 52% yield (Scheme 2). Under oxygen atmosphere, the cycloadduct 7a from N-acetyliminoquinolinium betaine 2 was oxidized in 46% yield. Treatment of the cycloadduct 8a with 20% NaOH solution resulted in the hydroxylation of ester group, giving the carboxylic acid derivative 13 in 91% yield (Scheme 3).
Experimental
Unless otherwise stated, all reagents were purchased from commercial suppliers and used without further purification. Organic solutions were concentrated under reduced pressure using a rotary evaporator or oil pump. Reactions were monitored through thin-layer chromatography (TLC). Chromatograms were visualized by fluorescence quenching under UV light at 254 nm. Flash column chromatography was performed using Qingdao Haiyang flash silica gel (200–300 mesh). Infrared spectra were recorded using a Bruker Optics TENSOR 27 instrument. 1H and 13C NMR spectra were recorded using a Bruker-300 spectrometer. Accurate mass measurements were performed using an Agilent instrument with the ESI-MS technique.General procedure for the phosphine-catalyzed [4 + 3] cycloaddition reaction: an oven-dried 15 mL of Schlenk tube was charged with azomethine imine (0.125 mmol), 3 mL or 5 mL of CH2Cl2 and the allenoate (0.15 mmol) at room temperature, then catalyst (0.025 mmol) was added to the above solution. The reaction mixture was stirred at room temperature for 48 h, and then was concentrated. The residue was purified by flash column (ethyl acetate/petroleum ether) to afford the corresponding product.
Conclusions
In summary, we have developed an efficient phosphine-catalyzed [4 + 3] cycloaddition of aromatic azomethine imines with allenoates, providing dinitrogen-fused heterocyclic compounds in moderate to excellent yields. The catalytic [4 + 3] cycloaddition reaction provided a practical synthetic method for biologically important heterocycles.Acknowledgements
The NSFC (21172253, 21372256) and Chinese Universities Scientific Fund (2014FG011) are acknowledged.Notes and references
- For reviews of the chemistry of azomethine imines, see: (a) R. Grashey, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984, vol. 1, p. 733 Search PubMed; (b) J. G. Schantl, Sci. Synth., 2004, 27, 731 CAS; (c) G. Qiu, Y. Y. Kuang and J. Wu, Adv. Synth. Catal., 2014, 356, 3483 CrossRef CAS PubMed.
- For selected thermal cycloaddition reactions of azomethine imines, see: (a) F. Chung, A. Chauveau, M. Seltki, M. Bonin and L. Micouin, Tetrahedron Lett., 2004, 45, 3127 CrossRef CAS PubMed; (b) L. Pezdirc, V. Jovanovski, D. Bevk, R. Jakše, S. Pirc, A. Meden, B. Stanovnik and J. Svete, Tetrahedron, 2005, 61, 3977 CrossRef CAS PubMed; (c) J. Gergely, J. B. Morgan and L. E. Overman, J. Org. Chem., 2006, 71, 9144 CrossRef CAS PubMed; (d) L. Pezdirc, J. Cerkovnik, S. Pirc, B. Stanovnik and J. Svete, Tetrahedron, 2007, 63, 991 CrossRef CAS PubMed; (e) S. Ogawa, T. Nishimine, E. Tokunaga and N. Shibata, Synthesis, 2010, 3274 CAS; (f) H. Kawai, Z. Yuan, E. Tokunaga and N. Shibata, Org. Lett., 2012, 14, 5330 CrossRef CAS PubMed; (g) T. Soeta, K. Tamura and Y. Ukaji, Org. Lett., 2012, 14, 1226 CrossRef CAS PubMed; (h) D. Wang, H. P. Deng, Y. Wei, Q. Xu and M. Shi, Eur. J. Org. Chem., 2013, 401 CrossRef CAS PubMed; (i) X. Q. Hu, J. R. Chen, S. Gao, B. Feng, L. Q. Lu and W. J. Xiao, Chem. Commun., 2013, 49, 7905 RSC; (j) M. Yoshida, N. Sassa, T. Kato, S. Fujinami, T. Soeta, K. Inomata and Y. Ukaji, Chem.–Eur. J., 2014, 20, 2058 CrossRef CAS PubMed.
- For selected examples on catalytic cycloadditions of azomethine imines, see: (a) R. Shintani and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 10778 CrossRef CAS PubMed; (b) A. Suárez, C. W. Downey and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 11244 CrossRef PubMed; (c) R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2006, 128, 6330 CrossRef CAS PubMed; (d) W. Chen, X. H. Yuan, R. Li, W. Du, Y. Wu, L. S. Ding and Y. C. Chen, Adv. Synth. Catal., 2006, 348, 1818 CrossRef CAS PubMed; (e) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334 CrossRef CAS PubMed; (f) H. Suga, A. Funyu and A. Kakehi, Org. Lett., 2007, 9, 97 CrossRef CAS PubMed; (g) R. Shintani, M. Murakami and T. Hayashi, J. Am. Chem. Soc., 2007, 129, 12356 CrossRef CAS PubMed; (h) W. Chen, W. Du, Y. Z. Duan, Y. Wu, S. Y. Yang and Y. C. Chen, Angew. Chem., Int. Ed., 2007, 46, 7667 CrossRef CAS PubMed; (i) M. P. Sibi, D. Rane, L. M. Stanley and T. Soeta, Org. Lett., 2008, 10, 2971 CrossRef CAS PubMed; (j) C. Perreault, S. R. Goudreau, L. E. Zimmer and A. B. Charette, Org. Lett., 2008, 10, 689 CrossRef CAS PubMed; (k) M. Keller, A. S. S. Sido, P. Pale and J. Sommer, Chem.–Eur. J., 2009, 15, 2810 CrossRef CAS PubMed; (l) N. D. Shapiro, Y. Shi and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 11654 CrossRef CAS PubMed; (m) T. Hashimoto, Y. Maeda, M. Omote, H. Nakatsu and K. Maruoka, J. Am. Chem. Soc., 2010, 132, 4076 CrossRef CAS PubMed; (n) T. Hashimoto, M. Omote and K. Maruoka, Angew. Chem., Int. Ed., 2011, 50, 3489 CrossRef CAS PubMed; (o) K. Yoshimura, T. Oishi, K. Yamaguchi and N. Mizuno, Chem.–Eur. J., 2011, 17, 3827 CrossRef CAS PubMed; (p) T. Imaizumi, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2012, 134, 20049 CrossRef CAS PubMed; (q) W. Zhou, X. X. Li, G. H. Li, Y. Wu and Z. L. Chen, Chem. Commun., 2013, 49, 3552 RSC; (r) J. T. Li, X. J. Lian, X. H. Liu, L. L. Lin and X. M. Feng, Chem.–Eur. J., 2013, 19, 5134 CrossRef CAS PubMed; (s) Y. Qian, P. J. Zavalij, W. H. Hu and M. P. Doyle, Org. Lett., 2013, 15, 1564 CrossRef CAS PubMed; (t) X. F. Xu, X. C. Xu, P. Y. Zavalij and M. P. Doyle, Chem. Commun., 2013, 49, 2762 RSC; (u) Y. Y. Zhou, J. Li, L. Ling, S. H. Liao, X. L. Sun, Y. X. Li, L. J. Wang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452 CrossRef CAS PubMed; (v) M. C. Tong, X. Chen, H. Y. Tao and C. J. Wang, Angew. Chem., Int. Ed., 2013, 52, 12377 CrossRef CAS PubMed; (w) T. Arai, Y. Ogino and T. Sato, Chem. Commun., 2013, 49, 7776 RSC; (x) T. Hashimoto, Y. Takiguchi and K. Maruoka, J. Am. Chem. Soc., 2013, 135, 11473 CrossRef CAS PubMed; (y) X. F. Xu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2013, 52, 12664 CrossRef CAS PubMed; (z) W. J. Li, Q. F. Jia, Z. Y. Du, K. Zhang and J. Wang, Chem.–Eur. J., 2014, 20, 4559 CrossRef CAS PubMed; (a a) D. Wang, Y. Lei, Y. Wei and M. Shi, Chem.–Eur. J., 2014, 20, 15325 CrossRef CAS PubMed.
- R. Shintani, M. Murakami and T. Hayashi, J. Am. Chem. Soc., 2007, 129, 12356 CrossRef CAS PubMed.
- M. Wang, Z. J. Huang, J. F. Xu and Y. G. R. Chi, J. Am. Chem. Soc., 2014, 136, 1214 CrossRef CAS PubMed.
- (a) R. S. Na, C. F. Jing, Q. H. Xu, H. Jiang, X. Wu, J. Y. Shi, J. C. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard, III, H. C. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed; (b) C. F. Jing, R. S. Na, B. Wang, H. L. Liu, L. Zhang, J. Liu, M. Wang, J. C. Zhong, O. Kwon and H. C. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed.
- (a) R. S. Na, H. L. Liu, Z. Li, B. Wang, J. Liu, M. A. Wang, M. Wang, J. C. Zhong and H. C. Guo, Tetrahedron, 2012, 68, 2349 CrossRef CAS PubMed; (b) X. Wu, R. S. Na, H. L. Liu, J. Liu, M. Wang, J. C. Zhong and H. C. Guo, Tetrahedron Lett., 2012, 53, 342 CrossRef CAS PubMed; (c) J. Liu, H. L. Liu, R. S. Na, G. Y. Wang, Z. Li, H. Yu, M. Wang, J. C. Zhong and H. C. Guo, Chem. Lett., 2012, 41, 218 CrossRef CAS; (d) L. Zhang, C. F. Jing, H. L. Liu, B. Wang, Z. Li, H. Jiang, H. Yu and H. C. Guo, Synthesis, 2013, 45, 53 CAS; (e) H. C. Guo, H. L. Liu, F. L. Zhu, R. S. Na, H. Jiang, Y. Wu, L. Zhang, Z. Li, H. Yu, B. Wang, Y. M. Xiao, X. P. Hu and M. Wang, Angew. Chem., Int. Ed., 2013, 52, 12641 CrossRef CAS PubMed; (f) Z. Li, H. Yu, L. Zhang, H. L. Liu, R. Na, Q. H. Bian, M. Wang and H. C. Guo, Lett. Org. Chem., 2014, 11, 220 CrossRef CAS; (g) H. L. Liu, Y. Wu, Y. Zhao, Z. Li, L. Zhang, W. J. Yang, H. Jiang, C. F. Jing, H. Yu, B. Wang, Y. M. Xiao and H. C. Guo, J. Am. Chem. Soc., 2014, 136, 2625 CrossRef CAS PubMed; (h) Z. Li, H. Yu, H. L. Liu, L. Zhang, H. Jiang, B. Wang and H. C. Guo, Chem.–Eur. J., 2014, 20, 1731 CrossRef CAS PubMed; (i) C. H. Yuan, H. L. Liu, Z. Z. Gao, L. J. Zhou, Y. L. Feng, Y. M. Xiao and H. C. Guo, Org. Lett., 2015, 17, 26 CrossRef CAS PubMed.
- For selected reviews on phosphine-promoted annulations, see: (a) X. Y. Lu, C. M. Zhang and Z. R. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS PubMed; (c) V. Nair, R. S. Menon, A. R. Sreekanth, N. Abhilash and A. T. Biju, Acc. Chem. Res., 2006, 39, 520 CrossRef CAS PubMed; (d) L. W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (e) C. E. Aroyan, A. Dermenci and S. J. Miller, Tetrahedron, 2009, 65, 4069 CrossRef CAS PubMed; (f) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (g) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS PubMed; (h) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (i) S. X. Wang, X. Y. Han, F. R. Zhong, Y. Q. Wang and Y. X. Lu, Synlett, 2011, 2766 CAS; (j) Q. Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (k) Z. M. Wang, X. Z. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (l) Y. M. Xiao, Z. H. Sun, H. C. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed.
- For other phosphine-catalyzed [4 + 3] annulation reactions, see: (a) K. Kumar, R. Kapoor, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 2023 CrossRef CAS; (b) S. Q. Zheng and X. Y. Lu, Org. Lett., 2009, 11, 3978 CrossRef CAS PubMed.
- (a) D. Inbar-Rozensal, A. Castiel, L. Visochek, D. Castel, F. Dantzer, S. Izraeli and M. Cohen-Armon, Breast Cancer Res., 2009, 11, R78 CrossRef PubMed; (b) T. Lu, C. Guo and P. Ni, J. China Pharm. Univ., 2004, 35, 99 CAS; (c) R. Rajagopalan, T. Lin, A. S. Karwa, A. R. Poreddy, B. Asmelash and R. B. Dorshow, ACS Med. Chem. Lett., 2012, 3, 284 CrossRef CAS PubMed; (d) D. Cappoen, J. Jacobs, N. V. Tuyen, S. Claessens, G. Diels, R. Anthonissen, T. Einarsdottir, M. Fauville, L. Verschaeve, K. Huygen and N. De Kimpe, Eur. J. Med. Chem., 2012, 48, 57 CrossRef CAS PubMed; (e) S. Tohyama, T. Choshi, K. Matsumoto, A. Yamabuki, Y. Hieda, J. Nobuhiro and S. Hibino, Heterocycles, 2010, 82, 397 CrossRef CAS; (f) C. Caballero-George, P. M. L. Vanderheyden, S. Apers, H. Van den Heuvel, P. N. Solis, M. P. Gupta, M. Claeys, L. Pieters, G. Vauquelin and A. J. Vlietinck, Planta Med., 2002, 68, 770 CrossRef CAS PubMed; (g) C. J. Liu, D. Y. Liu and L. Xiang, Acta Pharmacol. Sin., 2010, 45, 9 CAS; (h) J. Y. Blay, Eur. J. Clin. Med. Oncol., 2010, 2, 1 CAS; (i) B. Vincenzi, A. Napolitano, A. M. Frezza, G. Schiavon, D. Santini and G. Tonini, Pharmacogenomics, 2010, 11, 865 CrossRef CAS PubMed; (j) N. J. Carter and S. J. Keam, Drugs, 2010, 70, 355 CAS; (k) N. Ramkumar and R. Nagarajan, J. Org. Chem., 2013, 78, 2802 CrossRef CAS PubMed.
- For preparation of allenoates, see ref. 6a.
Footnote |
| † Electronic supplementary information (ESI) available: [DETAILS]. CCDC 1049475–1049479. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra04374c |
| This journal is © The Royal Society of Chemistry 2015 |