DOI:
10.1039/C5RA03317A
(Paper)
RSC Adv., 2015,
5, 34327-34334
Removal of methyl violet dye by adsorption onto N-benzyltriazole derivatized dextran†
Received
23rd February 2015
, Accepted 8th April 2015
First published on 8th April 2015
Abstract
In this work, N-benzyltriazole derivatized dextran was evaluated for its potential as a novel carbohydrate-based adsorbent for the removal of methyl violet dye from water. The modified dextran was synthesized by a click reaction of pentynyl dextran and benzyl azide, and the structure was characterized by nuclear magnetic resonance spectroscopy, elemental analysis, and scanning electron microscopy. Dextran was substituted with a triazole-linked benzyl group. For decolorization of the dye effluent, adsorption is a very effective treatment; here, the driving force is based on hydrogen bonding, pi stacking, and electrostatic interaction between the methyl violet dye and the N-benzyltriazole derivatized dextran. Batch experiments were carried out to investigate the required contact time and the effects of pH, initial dye concentrations, and temperature. The experimental data were analyzed with equilibrium isotherms including the Langmuir, Freundlich, and Temkin models. Based on the Langmuir isotherm, the maximum adsorption capacity was determined to be 95.24 mg of dye per gram of the adsorbent. The adsorption obeyed pseudo-second order kinetics, and a negative ΔG0 value indicated adsorption spontaneous in nature.
1. Introduction
Dyes are widely used in the textile and paper industries; however, their effluents cause environmental pollution.1 Since dyes partially block transmission of sunlight into water, they can inhibit the photosynthesis of aquatic plants and the growth of bacteria.2 Therefore, color is considered to be one obvious indicator of water pollution. Due to their high solubility in water, the removal of dyes is difficult to achieve by conventional physicochemical and biological treatment methods. Methyl violet is a member of a group of basic dyes, are highly visible even at very low concentrations. Since it is also mutagen and mitotic poison, much attention has been paid to developing remediation procedures for methyl violet pollution.3,4 Although various dye removal methods have been reported in the literature, such as chemical- and photo-oxidation,5 biological treatment,6 coagulation,7 and adsorption, adsorption has particular advantages: no remaining toxic fragments are left behind, it has flexibility and simplicity of design, and it is a highly effective, easy, and low-cost method. Activated carbon is the most common material used to remove dye by adsorption but it is expensive and requires reactivation of the carbon;8,9 inorganic compounds such as alum, polyaluminum chloride, and silica gel present their own environmental problems.10 Therefore, the development of eco-friendly polysaccharide-based adsorbents would be of great importance. Although chitosan and its derivatives have been investigated for use in the removal of several acidic dyes,11,12 there has been no report on the use of modified dextran for the removal of toxic dyes from water.
Dextrans are extracellular polysaccharides of varying lengths (Mr 3–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), synthesized from sucrose by Leuconostoc mesenteroides.13 The general structure of dextrans consists of a linear backbone of repeating α-1,6-linked D-glucopyranosyl units branched with α-1,2, α-1,3, and α-1,4 linkages, and is viscoelastic in water.14 Since dextrans are non-toxic, biocompatible, and inert in physiological conditions, they have been targeted for use in some industrial fields, such as food, cosmetics, and pharmaceuticals.15,16 To further the potential applications of dextrans, it is possible to modify their hydroxyl groups with specific substituents; for instance, cross-linked and carboxymethyl- and diethylaminoethyl-derivatized dextrans have been utilized as chromatographic separation resins.17
000), synthesized from sucrose by Leuconostoc mesenteroides.13 The general structure of dextrans consists of a linear backbone of repeating α-1,6-linked D-glucopyranosyl units branched with α-1,2, α-1,3, and α-1,4 linkages, and is viscoelastic in water.14 Since dextrans are non-toxic, biocompatible, and inert in physiological conditions, they have been targeted for use in some industrial fields, such as food, cosmetics, and pharmaceuticals.15,16 To further the potential applications of dextrans, it is possible to modify their hydroxyl groups with specific substituents; for instance, cross-linked and carboxymethyl- and diethylaminoethyl-derivatized dextrans have been utilized as chromatographic separation resins.17
In the present study, dextrans were modified with a benzyltriazole moiety and explored as functional biopolymers for the methyl violet dye removal. In biomedical science, click chemistry has been used for DNA conjugation and the synthesis of microcapsules and glycopolymers.18 In this case, the triazole formed is stable against reduction, hydrolysis, and oxidation. Given the physicochemical properties of the hydrophobic-functionalized dextran, we investigated it for use in the adsorption of methyl violet. The driving force of the adsorption process is considered as non-covalent bonds including hydrogen bonds, hydrophobic effects, π-stacking interactions, and electrostatic interactions as well as the backbone structure of the polymer. Furthermore, the effects of the contact time, initial dye concentration, initial solution pH, and reaction temperature are presented and discussed. Equilibrium isotherms were analyzed according to the Langmuir, Freundlich, and Temkin equations. A pseudo-second order kinetic model and thermodynamic parameters have been determined.
2. Experimental
2.1. Chemicals
Methyl violet 2B and dextran (Mr ∼ 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, from Leuconostoc sp.) were purchased from Fluka analytical and Sigma-Aldrich Co, respectively. Diethyl ether (99.5%) from J.T.Baker and dimethyl sulfoxide (DMSO, 99.9%) from Sigma-Aldrich Co. were used as organic solvents. All other chemicals used were of analytical reagent grade.
000, from Leuconostoc sp.) were purchased from Fluka analytical and Sigma-Aldrich Co, respectively. Diethyl ether (99.5%) from J.T.Baker and dimethyl sulfoxide (DMSO, 99.9%) from Sigma-Aldrich Co. were used as organic solvents. All other chemicals used were of analytical reagent grade.
2.2. Synthesis of dimsyl lithium
A basic agent, dimsyl lithium (CH3SOCH2−Li+) introduced by Hakomori,19 was prepared as follows. In a round bottom flask dried by heating under nitrogen, 1.6 M methyllithium in diethyl ether was added to an equal volume of DMSO. The mixture was stirred under nitrogen for 90 min, and the freshly prepared dimsyl lithium was used immediately.
2.3. Synthesis of O-(N-benzyl-[1,2,3]-triazoyl)-propyl dextran via a click reaction of pentynyl dextran with benzyl azide
Pentynyl dextran was synthesized using dimsyl lithium, 5-chloro-1-pentyne, and dextran as previously described.20 Pentynyl dextran (5 g) was dissolved in DMSO/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 150 mL). After dissolution, benzyl azide (2 equiv. per alkynyl group) was added, followed by a freshly prepared 1 M aqueous solution of sodium L-ascorbate (20 mol% per alkynyl group) and CuSO4·5H2O (5 mol% per alkynyl group). The reaction mixture was stirred at room temperature for 96 h.21 The product was dialyzed (molecular weight cutoff (MWCO) > 10
1 v/v, 150 mL). After dissolution, benzyl azide (2 equiv. per alkynyl group) was added, followed by a freshly prepared 1 M aqueous solution of sodium L-ascorbate (20 mol% per alkynyl group) and CuSO4·5H2O (5 mol% per alkynyl group). The reaction mixture was stirred at room temperature for 96 h.21 The product was dialyzed (molecular weight cutoff (MWCO) > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against deionized water and the final solution was lyophilized.
000) against deionized water and the final solution was lyophilized.
2.4. Preparation of succinoglycan and N-benzyltriazole derivatized succinoglycan
As an acidic polysaccharide, succinoglycan was isolated and purified from Sinorhizobium meliloti.22 N-Benzyltriazole derivatized succinoglycan was synthesized by the same protocol described in section 2.3.23
2.5. Nuclear magnetic resonance (NMR) spectroscopy
A Bruker Avance 500 spectrometer was used to record 1H-NMR spectra. The samples were dissolved in d6-DMSO at room temperature.
2.6. Scanning electron microscopy (SEM)
To fix the samples on a brass stub, double-sided adhesive carbon tape was used. The samples were coated with a thin gold layer at 30 W for 30 s in a vacuum. SEM images were acquired using a 20 kV accelerating voltage on a JSM-6380 (Jeol, Japan) scanning electron microscope.
2.7. Fourier-transform infrared (FT-IR) spectroscopy
FT-IR Spectra were obtained in potassium bromide matrix by using Bruker IFS-66 spectrometer (AMX, Germany). The spectra were recorded in the scanning range was 400–4000 cm−1.
2.8. Intraparticle diffusion study
The possibility of intra-particle diffusion resistance affecting adsorption was explored by using the intraparticle diffusion model as:where kid (mg g−1 min−1/2) is the intraparticle diffusion rate constant and C (mg g−1) is the boundary layer thickness.24
2.9. Activation energy and thermodynamics of adsorption
Using an Arrhenius plot,25 the Arrhenius energy of activation (Ea) can be calculated as follows:| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 = ln k2 = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT A − Ea/RT
| (2) |
where A and R refer to the Arrhenius frequency factor and the gas constant respectively.
Using Eyring's eqn (3), the enthalpy (ΔH#) and entropy (ΔS#) of activation were calculated from the slope and intercept, respectively, of a plot of ln(k2/T) versus 1/T:
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2/T = ln(kB/h) + (ΔS#/R) − (ΔH#/RT) k2/T = ln(kB/h) + (ΔS#/R) − (ΔH#/RT)
| (3) |
where
kB and
h are the Boltzmann and Planck constants, respectively.
The free energy of activation (ΔG#) was obtained as follows:
The spontaneity of the adsorption process was evaluated by measuring changes in the thermodynamic parameters, namely the free energy change (ΔG0, kJ mol−1), the enthalpy change (ΔH0, kJ mol−1), and the entropy change (ΔS0, J mol−1 K−1). The values of ΔG0, ΔH0, and ΔS0 were calculated by eqn (5) and (6). A plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc vs. 1/T yields a straight line with −ΔH0/R and ΔS0/R as its slope and intercept, respectively.
Kc vs. 1/T yields a straight line with −ΔH0/R and ΔS0/R as its slope and intercept, respectively.
| |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (5) |
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc = ΔS0/R − ΔH0/RT Kc = ΔS0/R − ΔH0/RT
| (6) |
where
Kc, the distribution coefficient of the adsorbate, is equal to
qe/
Ce.
3. Results and discussion
3.1. N-Benzyltriazole derivatized dextran and its structural analysis
A structural analysis of polysaccharides and information about substituent distribution in their derivatives may offer the most fundamental understanding about the functions and properties of polysaccharides. Dextran and its derivatives have been investigated as the critical polysaccharides for medical and industrial applications.26 In the present study, we evaluated the prepared O-(N-benzyl-[1,2,3]-triazoyl)-propyl dextran as a carbohydrate-based adsorbent for the dye removal. N-Benzyltriazole derivatized dextran was synthesized via a click reaction of pentynyl dextran and benzyl azide. The primary dextran derivative, pentynyl dextran, allowed the introduction of aromaticity in the second reaction step (a copper-catalyzed azide-alkyne cycloaddition). The resulting N-benzyltriazole derivatized dextran was evaluated for the adsorption of methyl violet which has aromatic ring structures with heteroatoms.
N-Benzyltriazole derivatized dextran was characterized by NMR, elemental analysis (EA), and SEM. As shown in the 1H NMR spectrum (Fig. 1A), aromatic protons and protons at the 2′-position of the benzyl group had peaks located at 7.29 and 5.49 ppm, respectively. Glucose ring protons appear in the range of 3.00–4.00 ppm, and the hydroxyl protons are visible at 4.46 ppm (OH-2), 4.83 ppm (OH-3), and 4.90 ppm (OH-4). Some signals between 1.00 and 3.00 ppm are due to the resonance from residual pentynyl groups.23 The EA results of N-benzyltriazole derivatized dextran and the parent unsubstituted dextran are summarized in the ESI, in Table S1.† Chemical modifications led to a significant increase in the carbon and nitrogen contents of N-benzyltriazole derivatized dextran compared with those of parent dextran. Based on the nitrogen contents, the degree of substitution (DS) value was determined to be 0.56. The spherical morphology of the parent dextran was also changed into an uneven state after modification (Fig. 2A and S2†). These structural analyses indicated that the novel adsorbent of dye, N-benzyltriazole derivatized dextran was successfully synthesized.
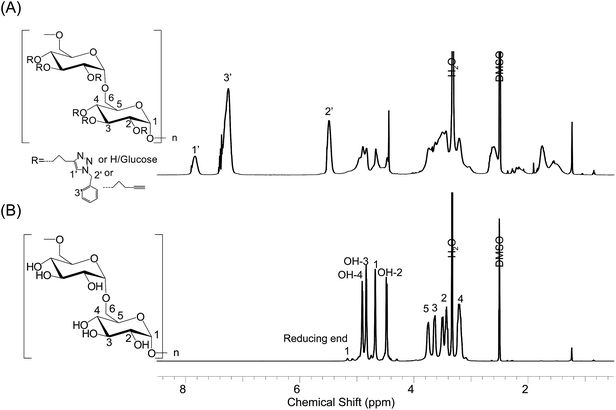 |
| | Fig. 1 1H-NMR spectra of (A) O-(N-benzyl-[1,2,3]-triazoyl)-propyl dextran and (B) dextran. The left insets show the chemical structures of N-benzyltriazole derivatized dextran and dextran. | |
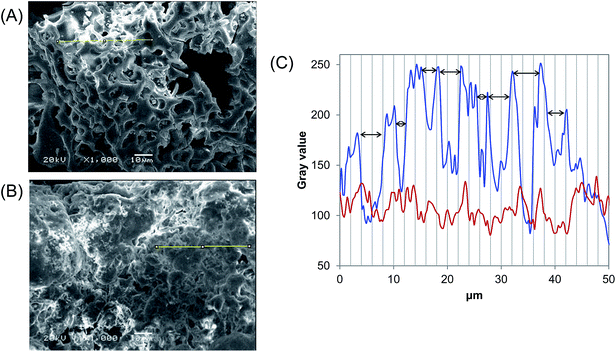 |
| | Fig. 2 SEM images of (A) N-benzyltriazole derivatized dextran and (B) N-benzyltriazole derivatized dextran with dye adsorbed. (C) Plot profiles of the selected yellow line (blue line: N-benzyltriazole derivatized dextran; red line: N-benzyltriazole derivatized dextran with dye adsorbed). Black arrows indicate the gap width. | |
3.2. Adsorption isotherm analysis
Table 1 summarizes the Langmuir, Freundlich, and Temkin isotherm constants for the adsorption of methyl violet 2B onto N-benzyltriazole derivatized dextran. The applicability of the isotherm equation was evaluated through the correlation coefficients, r2 (Table 1 and Fig. S3†). The best fit, with an r2 value of 0.9815, was obtained from the Langmuir isotherm model. Langmuir adsorption quantitatively describes the formation of an adsorbate monolayer on the outer surface of the adsorbent, after which no further adsorption takes place.27 The maximum amount of the dye adsorbed from the aqueous solution, qm, was 95.24 mg g−1; the constant associated with the binding energy of the sorption system, KL, was 0.16 L mg−1. Other natural adsorbents such as bagasse fly ash, sugarcane dust, and sunflower seed hull have shown qm values in the range of 25–93 mg g−1 for methyl violet adsorption (Table S2†).3,4,28 These agricultural materials are localized, and their structural information in the literature is deficient. In these respects, modified polysaccharides can be developed with advantage, and applied for the conventional methods of dye removal from water. The separation factor, RL, calculated from the Langmuir constant, indicates the nature of the isotherm. The values of RL in the present investigation were found to range from 0.286 to 0.032 for initial dye concentrations ranging from 10 to 200 ppm (Fig. S4†). Higher RL values at lower dye concentrations showed that the adsorption was more favorable at lower initial adsorbate concentrations; however, all of the RL values indicate favorable adsorptions, as they all lie in the range of 0 < RL < 1. This favorable adsorption may be attributed to van der Waals forces and hydrogen bonding between methyl violet 2B and N-benzyltriazole derivatized dextran, as well as π–π interactions between the aromatic residues of the dye and the adsorbent.
Table 1 Isotherm constants for methyl violet adsorption onto N-benzyltriazole derivatized dextran at 25 °C (adsorbent dose: 5 g L−1)
| Langmuir isotherm constant |
| KL (L mg−1) |
qm (mg g−1) |
r2 |
| 0.16 |
95.24 |
0.9815 |
| Freundlich isotherm constant |
| Kf (mg g−1) |
n |
r2 |
| 16.04 |
0.36 |
0.9787 |
| Temkin isotherm constant |
| B (J mol−1) |
AT (L g−1) |
r2 |
| 22.73 |
1.37 |
0.9433 |
Freundlich adsorption isotherms are commonly used to describe the adsorption characteristics of heterogeneous surfaces. The extent of adsorption varies directly depending on the pressure until the saturation pressure is reached. The constant Kf and n are the empirical parameters which must be determined by data fitting.29 The constant Kf is a rough indicator of the adsorption capacity, and 1/n is the adsorption intensity. Chemical adsorption is expected if 1/n < 1, while if n = 1, the partition between the two phases is independent of the concentration, and 1/n > 1 indicates that the adsorption is a favorable physical process.30 Therefore, the 1/n value of 2.77 suggests that the adsorption of methyl violet 2B onto N-benzyltriazole derivatized dextran is a favorable physical process.
Temkin's adsorption isotherm model takes into account the adsorbate/adsorbent interaction, and assumes that the heat of adsorption of all molecules in the layer will decrease linearly with increased surface coverage.31 It predicts a uniform distribution of binding energies over the whole population of surface binding sites. Theoretically, such a uniform distribution of binding energies would arise from a truly random arrangement of surface binding sites. According to the Temkin plot of adsorption data collected in this experiment (r2 = 0.9433), the constants were calculated as AT = 1.37 L g−1 and B = 22.73 J mol−1. Since the heat of sorption lower than 20 kJ mol−1 is characteristics for physisorption.32
3.3. Effect of initial dye concentration on methyl violet 2B removal
The effect of the initial dye concentration on its subsequent removal was studied (Fig. 3). The experiments were performed at different initial dye concentrations (12.5, 25, and 50 ppm) with N-benzyltriazole derivatized dextran acting as the adsorbent (1 g L−1). In the case of the initial concentration of 12.5 ppm, 89% of the dye was removed from water at 25 °C within 30 min. At a 50 ppm initial dye concentration, 57% was removed after 2 h. Using the graph shown in Fig. 3B, the rate constants of the pseudo-second order, k2 (g mg−1 min−1), and amounts of dye adsorbed at equilibrium, qe (mg g−1), were determined to be: k2 = 0.0515 (12.5 ppm), 0.0271 (25 ppm), and 0.0042 (50 ppm); qe = 11.05 (12.5 ppm), 23.47 (25 ppm), 30.21 (50 ppm). At a low initial concentration, the adsorption of dye by the adsorbent is very intense and reaches equilibrium very quickly. For the diffusion mechanism, the kinetic results were further analyzed by the intraparticle diffusion model. Fig. 3C shows plots of qt vs. t1/2 at different initial dye concentration, and they are presented as at least two linear graphs. The results indicate that two or more steps simultaneously operate the adsorption process.33 In addition, the presence of intercept implies that the intraparticle diffusion is not the only rate-limiting step.34 The first portion represents boundary layer diffusion through the bulk liquid to the external surface of the adsorbent. The second linear phase is a gradual adsorption stage through the intraparticle diffusion. The intraparticle diffusion rate constants were calculated as: kid (mg g−1 min−1/2) = 0.1274 (12.5 ppm), 0.1372 (25 ppm), and 1.0389 (50 ppm). Therefore, we suggest that both surface adsorption and intraparticle diffusion affect the present adsorption mechanism.
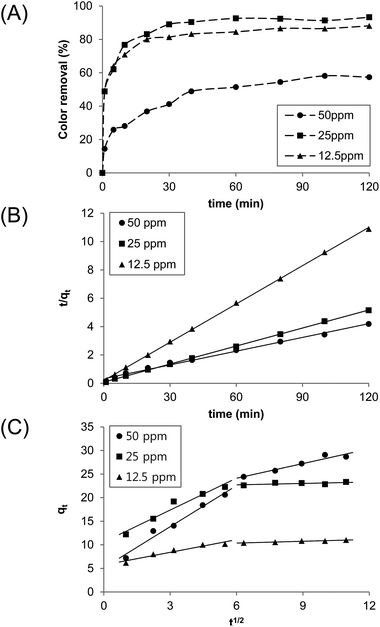 |
| | Fig. 3 (A) The effect of initial dye concentration. (B) Pseudo-second order kinetic plots on the removal of methyl violet 2B by adsorption onto N-benzyltriazole derivatized dextran. (C) Intraparticle diffusion plots for the adsorption of methyl violet onto N-benzyltriazole derivatized dextran (25 °C; 1 g L−1 adsorbent). | |
3.4. Comparison with N-benzyltriazole derivatized succinoglycan
Considering that methyl violet 2B is a basic dye (Fig. 4A), it is meaningful to investigate the contribution of acidic groups to its adsorption. Since succinoglycan is an anionic glucan containing succinyl and pyruvyl substituents,22 N-benzyltriazole derivatized succinoglycan was prepared via a click reaction for the comparison with N-benzyltriazole derivatized dextran. Fig. 4B and C show the unmistakable effectiveness of N-benzyltriazole derivatized dextran as an adsorbent for the removal of methyl violet, with residual color less than 5%. Parent dextran showed a better result than the unmodified succinoglycan, and N-benzyltriazole derivatized succinoglycan gave a removal improvement of 30% over unmodified succinoglycan. In a previous study, the removal of another class of organic contaminant, polycyclic aromatic hydrocarbons (PAH), was attributed to the exposed aromatic cores in the modified pine bark.35 The aromatic cores resulted in a stronger specific π–π interaction between the PAH and the adsorbent. Based on the EA of the tested polysaccharides (Table S1†), the values of (N + O)/C indicating polarity, decreased after modification from 0.96 and 0.92 to 0.50 and 0.50 for succinoglycan and dextran, respectively. The aliphatic character (H/C) of each also decreased from 2.06 and 1.98 to 1.46 and 1.37, respectively. Thus, aromaticity is not the only deciding factor for adsorption; the polysaccharide backbone structure is also important.
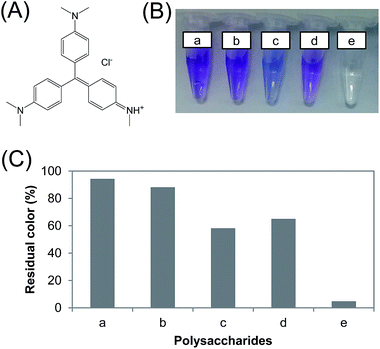 |
| | Fig. 4 (A) The chemical structure of methyl violet 2B. (B) Color changes and (C) degree of residual color after reaching adsorption equilibrium. Polysaccharide adsorbents: (a) no addition; (b) succinoglycan; (c) N-benzyltriazole derivatized succinoglycan; (d) dextran; (e) N-benzyltriazole derivatized dextran (10 g L−1 adsorbent). | |
As another key, the surface morphology of N-benzyltriazole derivatized dextran, with its roughness, supports the effective adsorption of dye (Fig. 2A). The craterous surface structure of N-benzyltriazole derivatized dextran may allow methyl violet 2B to be trapped and adsorbed into its holes.36 Conversely, N-benzyltriazole derivatized succinoglycan shows a laminar sheet-like surface,23 and parent dextran has a spherical structure with the average diameter of 20.03 μm (Fig. S2†). In reality, the uneven surface of the N-benzyltriazole derivatized dextran was filled after the adsorption of dye, as shown in Fig. 2B. Morphological changes were also observed for the adsorption by other adsorbents.4,37 From the plot profiles of the selected 50 μm line in the SEM data (according to Fiji image processing package), the 8 potholes of 2–5 μm width and the flattened surface are described in blue and red line, respectively (Fig. 2C). The gray scale can be considered as an underestimation due to the image treatment software effect, however the degree of surface roughness is clearly differentiated. These results indicate that various non-covalent bonds including π–π interaction and its uneven surface work at the present dye adsorption.
3.5. FT-IR spectra of N-benzyltriazole derivatized dextran/methyl violet 2B composite
After adsorption methyl violet 2B onto N-benzyltriazole derivatized dextran, the precipitated composite was analyzed with FT-IR spectroscopy. Fig. 5 shows shifts or changes of IR absorption peaks, indicating interactions of the adsorbent and methyl violet. All the observed changes are summarized in Table S3,† and the characteristic change is present at C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and aromatic C
C and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. A disappearance in the C
C stretching vibration. A disappearance in the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretch may be attributed to the electrostatic interaction of tertiary ammonium ion of methyl violet with the terminal alkyne group of N-benzyltriazole derivatized dextran (Fig. 1). A novel peak (1587 cm −1) belong to methyl violet 2B was formed, and band shifts took place from 1643 to 1637 cm −1. This result demonstrates the additional function of residual alkyne group in N-benzyltriazole derivatized dextran for electrostatic interaction with methyl violet. Similar observations have also been reported in methylene blue/exfoliated graphene oxide composite.38
C stretch may be attributed to the electrostatic interaction of tertiary ammonium ion of methyl violet with the terminal alkyne group of N-benzyltriazole derivatized dextran (Fig. 1). A novel peak (1587 cm −1) belong to methyl violet 2B was formed, and band shifts took place from 1643 to 1637 cm −1. This result demonstrates the additional function of residual alkyne group in N-benzyltriazole derivatized dextran for electrostatic interaction with methyl violet. Similar observations have also been reported in methylene blue/exfoliated graphene oxide composite.38
 |
| | Fig. 5 FT-IR spectra of N-benzyltriazole derivatized dextran before (in red) and after adsorption (in blue). | |
3.6. Effect of pH on methyl violet 2B removal
Adsorption processes are affected by solution pH, as it influences not only the surface charge of the adsorbent but also the chemistry of dye in solution.39 We depict the effect of pH on dye removal by N-benzyltriazole derivatized dextran (Fig. 6); we found that the dye adsorption decreased as the pH increased. Under alkaline condition, the hydrogen bonds stabilizing the adsorbate–adsorbent interaction can be destroyed, and the N-benzyltriazole derivatized dextran may adopt another conformation because of charge repulsion. Whereas the opposite trend was observed in the adsorption of basic dye onto sunflower seed hull and bagasse fly ash.3,4
 |
| | Fig. 6 Effect of pH on dye removal by N-benzyltriazole derivatized dextran (100 ppm dye, 10 g L−1 adsorbent; buffers used: pH 2, HCl–KCl buffer; pH 4, citrate buffer; pH 7, phosphate buffer; pH 9, borate buffer; pH 10, NaHCO3·NaOH buffer; pH 11, NaHCO3·NaOH buffer). | |
3.7. Effect of temperature, activation energy, and a thermodynamic study
The adsorption studies were carried out at different temperatures, ranging from 15 to 75 °C (Table 2 and Fig. S5†). The adsorption capacity and the rate constant were found to increase with increasing temperature, indicating that the adsorption is an endothermic process. This may be due to an increase in the mobility of the dye or a change of active sites within the internal structure of the N-benzyltriazole derivatized dextran as the temperature increases. The present dye adsorption follows the pseudo-second order model with the best correlation coefficient (r2 > 0.99); using the rate constant for pseudo-second order adsorption (k2) at several temperatures, the energy of activation (Ea) was calculated to be 33.96 kJ mol−1 from an Arrhenius plot (Fig. S6A† and Table 2). In general, activation energies less than 40 kJ mol−1 indicate a physisorption process, while those higher than 40 kJ mol−1 signify chemisorption.40 Thus, the present adsorption of methyl violet 2B by N-benzyltriazole derivatized dextran can be regarded as predominantly physisorption.
Table 2 Activation parameters for dye adsorption onto N-benzyltriazole derivatized dextran (25 ppm dye; 1 g L−1 adsorbent)
| Temperature (°C) |
k2 (mg g−1 min−1) |
Ea (kJ mol−1) |
r2 |
ΔH# (kJ mol−1) |
ΔS# (J mol−1 K−1) |
ΔG# (kJ mol−1) |
r2 |
| 15 |
0.0031 |
33.96 |
0.9602 |
31.33 |
−183.548 |
84.19 |
0.9531 |
| 35 |
0.0068 |
87.86 |
| 45 |
0.0148 |
89.70 |
| 55 |
0.0221 |
91.53 |
| 75 |
0.0310 |
95.20 |
Table 3 shows thermodynamic parameters as obtained from the intercept and slope of a plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc vs. 1/T (Fig. S7†). The positive ΔH0 value, 16.47 kJ mol−1, also indicates that the adsorption is an endothermic process; furthermore, the magnitude of this value provides information about the type of sorption. In principle, the heat evolved during physical adsorption is of the same order of magnitude as the heat of condensation, i.e., 2.1–20.9 kJ mol−1, while the heat of chemisorption generally falls in the range of 80–200 kJ mol−1.41 Although the low value of ΔS0 implies that no remarkable change in entropy occurred during adsorption, the positive value may be attributed to an increased randomness at the solid–solution interface during adsorption. The negative values of ΔG0 (−3.90, −5.61, and −6.71 kJ mol−1) at the given temperatures (288, 308, and 328 K) indicate that the adsorption processes at all three recorded temperatures are spontaneous.
Kc vs. 1/T (Fig. S7†). The positive ΔH0 value, 16.47 kJ mol−1, also indicates that the adsorption is an endothermic process; furthermore, the magnitude of this value provides information about the type of sorption. In principle, the heat evolved during physical adsorption is of the same order of magnitude as the heat of condensation, i.e., 2.1–20.9 kJ mol−1, while the heat of chemisorption generally falls in the range of 80–200 kJ mol−1.41 Although the low value of ΔS0 implies that no remarkable change in entropy occurred during adsorption, the positive value may be attributed to an increased randomness at the solid–solution interface during adsorption. The negative values of ΔG0 (−3.90, −5.61, and −6.71 kJ mol−1) at the given temperatures (288, 308, and 328 K) indicate that the adsorption processes at all three recorded temperatures are spontaneous.
Table 3 Thermodynamic parameters of dye adsorption onto N-benzyltriazole derivatized dextran (25 ppm dye; 1 g L−1 adsorbent)
| Temperature (K) |
Kc |
ΔG0 (kJ mol−1) |
ΔH0 (kJ mol−1) |
ΔS0 (J mol−1 K−1) |
r2 |
| 288 |
1.6293 |
−3.8993 |
16.4677 |
71.0308 |
0.9746 |
| 308 |
2.1905 |
−5.6067 |
| 328 |
2.4629 |
−6.7130 |
4. Conclusion
In the present study, N-benzyltriazole derivatized dextran was successfully synthesized by a click reaction. It was then evaluated for its effectiveness as a polysaccharide adsorbent for the removal of methyl violet dye from water. Equilibrium and kinetic studies were carried out for the adsorption of methyl violet onto N-benzyltriazole derivatized dextran. The monolayer adsorption capacity was determined to be 95.24 mg of dye per gram of N-benzyltriazole derivatized dextran. The adsorption efficiency was found to be dependent on the initial dye concentration, solution pH, adsorbate contact time, and temperature. The adsorption kinetics followed a pseudo-second order model. A thermodynamic study supported the theory that the adsorption of methyl violet 2B onto N-benzyltriazole derivatized dextran is a spontaneous and endothermic process at the studied temperatures. We suggest that methyl violet removal by N-benzyltriazole derivatized dextran is caused by physical adsorption based on hydrogen bonding, van der Waals interactions, and π-stacking interactions, and electrostatic interactions. The present study indicates great potential for the removal of cationic dyes from aqueous solutions using natural biomaterials. Furthermore, the practical use of tailor made polysaccharide-derivative adsorbents to remove various dye and organic pollutants as target chemicals could be expected in the environmental field.
Acknowledgements
This paper was supported by the KU Research Professor Program of Konkuk University. This research was also supported by the National Research Foundation of Korea Grant, funded by the Korean Government (NRF-2013R1A1A2012568 and NRF-2011-619-E0002), and by the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012-0006686). SDG.
References
- Y. Anjaneyulu, N. S. Chary and D. S. S. Raj, Rev. Environ. Sci. Bio/Technol., 2005, 4, 245–273 CrossRef CAS.
- N. Koprivanac and H. Kusic, Hazardous organic pollutants in colored wastewaters, Nova Science Publishers, Hauppauge, 2009 Search PubMed.
- I. D. Mall, V. C. Srivastava and N. K. Agarwal, Dyes Pigm., 2006, 69, 210–223 CrossRef CAS PubMed.
- B. Hameed, J. Hazard. Mater., 2008, 154, 204–212 CrossRef CAS PubMed.
- M. S. Lucas and J. A. Peres, Dyes Pigm., 2006, 71, 236–244 CrossRef CAS PubMed.
- H. S. Rai, M. S. Bhattacharyya, J. Singh, T. Bansal, P. Vats and U. Banerjee, Crit. Rev. Environ. Sci. Technol., 2005, 35, 219–238 CrossRef CAS.
- B. Shi, G. Li, D. Wang, C. Feng and H. Tang, J. Hazard. Mater., 2007, 143, 567–574 CrossRef CAS PubMed.
- Y. S. Al-Degs, M. I. El-Barghouthi, A. H. El-Sheikh and G. M. Walker, Dyes Pigm., 2008, 77, 16–23 CrossRef CAS PubMed.
- G. Walker and L. Weatherley, Water Res., 1997, 31, 2093–2101 CrossRef CAS.
- S. Lambert, N. Graham, C. Sollars and G. Fowler, Water Sci. Technol., 1997, 36, 173–180 CrossRef CAS.
- V. Dhanapal and K. Subramanian, Carbohydr. Polym., 2014, 108, 65–74 CrossRef CAS.
- G. Crini and P.-M. Badot, Prog. Polym. Sci., 2008, 33, 399–447 CrossRef CAS PubMed.
- A. Jeanes, C. A. Wilham and J. C. Miers, J. Biol. Chem., 1948, 176, 603–615 CAS.
- A. Misaki, M. Torii, T. Sawai and I. J. Goldstein, Carbohydr. Res., 1980, 84, 273–285 CrossRef CAS.
- M. Naessens, A. Cerdobbel, W. Soetaert and E. J. Vandamme, J. Chem. Technol. Biotechnol., 2005, 80, 845–860 CrossRef CAS PubMed.
- R. Mehvar, J. Controlled Release, 2000, 69, 1–25 CrossRef CAS.
- B. Porsch and L.-O. Sundelöf, J. Chromatogr. A, 1994, 669, 21–30 CrossRef CAS.
- K. Nwe and M. W. Brechbiel, Cancer Biother.Radiopharm., 2009, 24, 289–302 CrossRef CAS PubMed.
- S.-I. Hakomori, J. Biochem., 1964, 55, 205–208 CAS.
- M. N. Tahir, C. Bork, A. Risberg, J. C. Horst, C. Komoß, A. Vollmer and P. Mischnick, Macromol. Chem. Phys., 2010, 211, 1648–1662 CrossRef CAS PubMed.
- P. F. Tankam, P. Mischnick, H. Hopf and P. G. Jones, Carbohydr. Res., 2007, 342, 2031–2048 CrossRef CAS PubMed.
- L.-X. Wang, Y. Wang, B. Pellock and G. C. Walker, J. Bacteriol., 1999, 181, 6788–6796 CAS.
- E. Cho, K. Kim, M. N. Tahir, J. Y. Lee and S. Jung, Notes, 2014, 35, 2589 CAS.
- W. Weber and J. Morris, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–60 Search PubMed.
- J. W. Grissom, T. L. Calkins, H. A. McMillen and Y. Jiang, J. Org. Chem., 1994, 59, 5833–5835 CrossRef CAS.
- D. de Belder, Polysaccharides in Medicinal Applications, Marcel Dekker, New York, NY, 1996, pp. 505–524 Search PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- Y.-S. Ho, W.-T. Chiu and C.-C. Wang, Bioresour. Technol., 2005, 96, 1285–1291 CrossRef CAS PubMed.
- A. Akgerman and M. Zardkoohi, J. Chem. Eng. Data, 1996, 41, 185–187 CrossRef CAS.
- S. V. Mohan and J. Karthikeyan, Environ. Pollut., 1997, 97, 183–187 CrossRef CAS.
- R. D. Johnson and F. H. Arnold, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1995, 1247, 293–297 CrossRef.
- P. Atkins, Physical Chemistry, Oxford University Press, 1998 Search PubMed.
- L. Ai, M. Li and L. Li, J. Chem. Eng. Data, 2011, 56, 3475–3483 CrossRef CAS.
- S. Ghorai, A. Sarkar, M. Raoufi, A. B. Panda, H. Schönherr and S. Pal, ACS Appl. Mater. Interfaces, 2014, 6, 4766–4777 CAS.
- Y. Li, B. Chen and L. Zhu, Bioresour. Technol., 2010, 101, 7307–7313 CrossRef CAS PubMed.
- H. L. Parker, A. J. Hunt, V. L. Budarin, P. S. Shuttleworth, K. L. Miller and J. H. Clark, RSC Adv., 2012, 2, 8992–8997 RSC.
- S. Dawood and T. K. Sen, Water Res., 2012, 46, 1933–1946 CrossRef CAS PubMed.
- G. Ramesha, A. V. Kumara, H. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270–277 CrossRef CAS PubMed.
- N. M. Mahmoodi, B. Hayati, M. Arami and C. Lan, Desalination, 2011, 268, 117–125 CrossRef CAS PubMed.
- T. Anirudhan and P. Radhakrishnan, J. Chem. Thermodyn., 2008, 40, 702–709 CrossRef CAS PubMed.
- Y. Liu and Y.-J. Liu, Sep. Purif. Technol., 2008, 61, 229–242 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03317a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), synthesized from sucrose by Leuconostoc mesenteroides.13 The general structure of dextrans consists of a linear backbone of repeating α-1,6-linked D-glucopyranosyl units branched with α-1,2, α-1,3, and α-1,4 linkages, and is viscoelastic in water.14 Since dextrans are non-toxic, biocompatible, and inert in physiological conditions, they have been targeted for use in some industrial fields, such as food, cosmetics, and pharmaceuticals.15,16 To further the potential applications of dextrans, it is possible to modify their hydroxyl groups with specific substituents; for instance, cross-linked and carboxymethyl- and diethylaminoethyl-derivatized dextrans have been utilized as chromatographic separation resins.17
000), synthesized from sucrose by Leuconostoc mesenteroides.13 The general structure of dextrans consists of a linear backbone of repeating α-1,6-linked D-glucopyranosyl units branched with α-1,2, α-1,3, and α-1,4 linkages, and is viscoelastic in water.14 Since dextrans are non-toxic, biocompatible, and inert in physiological conditions, they have been targeted for use in some industrial fields, such as food, cosmetics, and pharmaceuticals.15,16 To further the potential applications of dextrans, it is possible to modify their hydroxyl groups with specific substituents; for instance, cross-linked and carboxymethyl- and diethylaminoethyl-derivatized dextrans have been utilized as chromatographic separation resins.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, from Leuconostoc sp.) were purchased from Fluka analytical and Sigma-Aldrich Co, respectively. Diethyl ether (99.5%) from J.T.Baker and dimethyl sulfoxide (DMSO, 99.9%) from Sigma-Aldrich Co. were used as organic solvents. All other chemicals used were of analytical reagent grade.
000, from Leuconostoc sp.) were purchased from Fluka analytical and Sigma-Aldrich Co, respectively. Diethyl ether (99.5%) from J.T.Baker and dimethyl sulfoxide (DMSO, 99.9%) from Sigma-Aldrich Co. were used as organic solvents. All other chemicals used were of analytical reagent grade.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 150 mL). After dissolution, benzyl azide (2 equiv. per alkynyl group) was added, followed by a freshly prepared 1 M aqueous solution of sodium L-ascorbate (20 mol% per alkynyl group) and CuSO4·5H2O (5 mol% per alkynyl group). The reaction mixture was stirred at room temperature for 96 h.21 The product was dialyzed (molecular weight cutoff (MWCO) > 10
1 v/v, 150 mL). After dissolution, benzyl azide (2 equiv. per alkynyl group) was added, followed by a freshly prepared 1 M aqueous solution of sodium L-ascorbate (20 mol% per alkynyl group) and CuSO4·5H2O (5 mol% per alkynyl group). The reaction mixture was stirred at room temperature for 96 h.21 The product was dialyzed (molecular weight cutoff (MWCO) > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against deionized water and the final solution was lyophilized.
000) against deionized water and the final solution was lyophilized.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2 = ln
k2 = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT
A − Ea/RT
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k2/T = ln(kB/h) + (ΔS#/R) − (ΔH#/RT)
k2/T = ln(kB/h) + (ΔS#/R) − (ΔH#/RT)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc vs. 1/T yields a straight line with −ΔH0/R and ΔS0/R as its slope and intercept, respectively.
Kc vs. 1/T yields a straight line with −ΔH0/R and ΔS0/R as its slope and intercept, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc = ΔS0/R − ΔH0/RT
Kc = ΔS0/R − ΔH0/RT

![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and aromatic C
C and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. A disappearance in the C
C stretching vibration. A disappearance in the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretch may be attributed to the electrostatic interaction of tertiary ammonium ion of methyl violet with the terminal alkyne group of N-benzyltriazole derivatized dextran (Fig. 1). A novel peak (1587 cm −1) belong to methyl violet 2B was formed, and band shifts took place from 1643 to 1637 cm −1. This result demonstrates the additional function of residual alkyne group in N-benzyltriazole derivatized dextran for electrostatic interaction with methyl violet. Similar observations have also been reported in methylene blue/exfoliated graphene oxide composite.38
C stretch may be attributed to the electrostatic interaction of tertiary ammonium ion of methyl violet with the terminal alkyne group of N-benzyltriazole derivatized dextran (Fig. 1). A novel peak (1587 cm −1) belong to methyl violet 2B was formed, and band shifts took place from 1643 to 1637 cm −1. This result demonstrates the additional function of residual alkyne group in N-benzyltriazole derivatized dextran for electrostatic interaction with methyl violet. Similar observations have also been reported in methylene blue/exfoliated graphene oxide composite.38

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc vs. 1/T (Fig. S7†). The positive ΔH0 value, 16.47 kJ mol−1, also indicates that the adsorption is an endothermic process; furthermore, the magnitude of this value provides information about the type of sorption. In principle, the heat evolved during physical adsorption is of the same order of magnitude as the heat of condensation, i.e., 2.1–20.9 kJ mol−1, while the heat of chemisorption generally falls in the range of 80–200 kJ mol−1.41 Although the low value of ΔS0 implies that no remarkable change in entropy occurred during adsorption, the positive value may be attributed to an increased randomness at the solid–solution interface during adsorption. The negative values of ΔG0 (−3.90, −5.61, and −6.71 kJ mol−1) at the given temperatures (288, 308, and 328 K) indicate that the adsorption processes at all three recorded temperatures are spontaneous.
Kc vs. 1/T (Fig. S7†). The positive ΔH0 value, 16.47 kJ mol−1, also indicates that the adsorption is an endothermic process; furthermore, the magnitude of this value provides information about the type of sorption. In principle, the heat evolved during physical adsorption is of the same order of magnitude as the heat of condensation, i.e., 2.1–20.9 kJ mol−1, while the heat of chemisorption generally falls in the range of 80–200 kJ mol−1.41 Although the low value of ΔS0 implies that no remarkable change in entropy occurred during adsorption, the positive value may be attributed to an increased randomness at the solid–solution interface during adsorption. The negative values of ΔG0 (−3.90, −5.61, and −6.71 kJ mol−1) at the given temperatures (288, 308, and 328 K) indicate that the adsorption processes at all three recorded temperatures are spontaneous.



