Synthesis, crystal structure, DFT, and photovoltaic studies of BaCeCuS3†
Abstract
We report the synthesis of single crystals of the quaternary sulfide, BaCeCuS3, for the first time by heating a polycrystalline sample of BaCeCuS3 with an excess of KCl flux inside a vacuum-sealed fused silica tube. The crystal structure of the compound is determined by a single-crystal X-ray diffraction study which shows that it crystallizes in the primitive orthorhombic crystal system (space group: Pnma) with cell constants of a = 10.6740(13) Å, b = 4.1200(6) Å, and c = 13.409(2) Å. The structure is best described as a pseudo-two-dimensional structure where tetrahedral CuS4 and octahedral CeS6 units serve as the main building blocks. The polyanionic 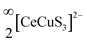 layers in the structure are separated by the presence of electropositive Ba2+ cations. The optical absorption study on the polycrystalline sample of BaCeCuS3 shows a semiconducting nature with a direct bandgap of 1.8(2) eV consistent with the green color of the sample. The temperature-dependent thermal conductivity (ktot) measurements on the polycrystalline BaCeCuS3 sample reveal an extremely low value of ktot (0.32 W m−1 K−1) at 773 K. DFT calculations were carried out to obtain the electronic structure of the title compound. Our theoretical studies predict a high value of the thermoelectric figure of merit (>1) for BaCeCuS3 by optimization of the charge carriers. To also explore a practical application of BaCeCuS3, a liquid junction solar cell was fabricated: TiO2/CdS/BaCeCuS3/Sn2−/S2−/MWCNTs@Ni, and the cell delivered an efficiency enhanced by ∼45% compared to the one without the sulfide. The performance improvement is affected by the hole conducting properties of BaCeCuS3, which allows facile hole transfer from CdS to the polysulfide, increases the charge separation, and hence increases the efficiency.
layers in the structure are separated by the presence of electropositive Ba2+ cations. The optical absorption study on the polycrystalline sample of BaCeCuS3 shows a semiconducting nature with a direct bandgap of 1.8(2) eV consistent with the green color of the sample. The temperature-dependent thermal conductivity (ktot) measurements on the polycrystalline BaCeCuS3 sample reveal an extremely low value of ktot (0.32 W m−1 K−1) at 773 K. DFT calculations were carried out to obtain the electronic structure of the title compound. Our theoretical studies predict a high value of the thermoelectric figure of merit (>1) for BaCeCuS3 by optimization of the charge carriers. To also explore a practical application of BaCeCuS3, a liquid junction solar cell was fabricated: TiO2/CdS/BaCeCuS3/Sn2−/S2−/MWCNTs@Ni, and the cell delivered an efficiency enhanced by ∼45% compared to the one without the sulfide. The performance improvement is affected by the hole conducting properties of BaCeCuS3, which allows facile hole transfer from CdS to the polysulfide, increases the charge separation, and hence increases the efficiency.

- This article is part of the themed collection: New Journal of Chemistry Selected Articles in Physical and Materials Chemistry from India


 Please wait while we load your content...
Please wait while we load your content...