Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnetic iron oxide nanogels for combined hyperthermia and drug delivery for cancer treatment
Sofia
Patri
 a,
Nguyen Thi Kim
Thanh
a,
Nguyen Thi Kim
Thanh
 *bc and
Nazila
Kamaly
*bc and
Nazila
Kamaly
 *d
*d
aDepartment of Materials, Molecular Sciences Research Hub, Imperial College London, 82 Wood Ln, London W12 0BZ, UK. E-mail: sofia.patri18@imperial.ac.uk
bUCL Healthcare Biomagnetic and Nanomaterials Laboratories, 21 Albemarle Street, London W1S 4BS, UK. E-mail: ntk.thanh@ucl.ac.uk; Web: https://www.ntk-thanh.co.uk
cBiophysic Group, Department of Physics and Astronomy, University College London, London WC1E 6BT, UK
dDepartment of Chemistry, Molecular Sciences Research Hub, Imperial College London, 82 Wood Ln, London W12 0BZ, UK. E-mail: nazila.kamaly@imperial.ac.uk
First published on 26th July 2024
Abstract
Hyperthermia and chemotherapy represent potential modalities for cancer treatments. However, hyperthermia can be invasive, while chemotherapy drugs often have severe side effects. Recent clinical investigations have underscored the potential synergistic efficacy of combining hyperthermia with chemotherapy, leading to enhanced cancer cell killing. In this context, magnetic iron oxide nanogels have emerged as promising candidates as they can integrate superparamagnetic iron oxide nanoparticles (IONPs), providing the requisite magnetism for magnetic hyperthermia, with the nanogel scaffold facilitating smart drug delivery. This review provides an overview of the synthetic methodologies employed in fabricating magnetic nanogels. Key properties and designs of these nanogels are discussed and challenges for their translation to the clinic and the market are summarised.
1. Introduction
Conventional treatments for cancer, such as chemotherapy and radiotherapy,1 while essential, are often accompanied by profound and debilitating side effects. These unwanted reactions, alongside the emergence of drug resistance, highlight the need for innovative therapeutic approaches.2In response, combinatorial approaches have emerged for cancer treatment over the years.3 Therapies such as photodynamic or ultraviolet light therapy have shown the potential to weaken cancer before chemotherapy.3 Notably, the rise of combinatorial therapy can be traced to the convergence of nanotechnology and cancer therapeutics. This has enabled the development of precision-engineered therapies with superior targeting capabilities, reduced systemic toxicity, and promising clinical outcomes.4
This review focuses on the combination of magnetic hyperthermia and chemotherapy with the use of magnetic iron oxide nanogels.
Hyperthermia and chemotherapy are established modalities in cancer treatment, each with distinct drawbacks.5 While hyperthermia poses risks of invasiveness and adverse effects such as heat-related complications including heat strokes, heat cramps, and dehydration,6 chemotherapy often inflicts severe side effects on patients due to medication toxicity. Combining the two therapies offers the advantage of localised heat and lower drug doses, as the treatment can leverage both the cytotoxic effects of the drugs and the hyperthermic action, thus enhancing therapeutic efficacy while potentially mitigating the individual drawbacks associated with each modality.
2. Magnetic hyperthermia
Hyperthermia therapy relies on the thermal effects on cancer cells.7 The susceptibility of cancer cells to heat-induced damage was demonstrated in pioneering research done by Woodhall et al. in the 1960s.8 At temperatures around 43 °C and in the absence of hyperthermia-enhancing agents, the vasculature of tumour tissues, characterised by abnormalities, experiences a notable reduction in blood flow. This reduction impairs the delivery of oxygen and nutrient supplies to the tumour cells, leading to cell death.7 At the same temperature, healthy cells exhibit resilience to thermal stress.Diverse approaches to hyperthermia treatment are employed based on tumour characteristics such as location and size. For instance, whole-body hyperthermia techniques such as hot baths or saunas are utilised for extensive skin tumours. These techniques elevate the patient's temperature up to the tolerance limit, typically around 41 °C.9 For deep-seated tumours, localised heat application is achieved through the use of metal probes.10 In recent years, the field has witnessed a shift towards less invasive yet highly efficient methods such as magnetic hyperthermia (MH).
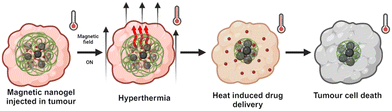
Magnetic hyperthermia harnesses the heat-generating potential of magnetic nanoparticles (NPs) when exposed to alternating magnetic fields (AMFs).11 This technique offers significant advantages, notably the ability of AMFs to penetrate tissues deeply and the option to directly administer nanoparticles into the tumour site, rendering the therapy minimally invasive,11Fig. 1.
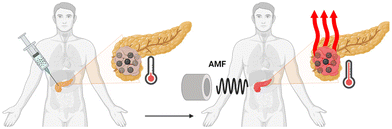 | ||
| Fig. 1 Magnetic hyperthermia due to injected nanoparticles interacting with AMF (created with BioRender.com). | ||
2.1 Iron oxide nanoparticles
Iron oxide nanoparticles (IONPs) are one of the few magnetic nanoparticles approved by the FDA for biomedical applications.10 They are made of magnetite (Fe3O4) or maghemite (γ-Fe2O3) with an inverse spinel structure.12 IONPs are non-toxic and undergo metabolisation primarily by the spleen and liver;13 the iron component holds potential for utilisation in haemoglobin production.14Both the biocompatibility and performance of IONPs are strictly related to the magnetic properties of the NP. The properties, in turn, depend on the size, crystallinity, and purity of the material.12 The ideal properties for MH are obtained with mono-dispersed and colloidally stable IONPs. This can be achieved with several synthetic strategies, such as alkaline precipitation of iron salts in water,15,16 thermal decomposition,17 polyol mediated synthesis18 and lithography.19
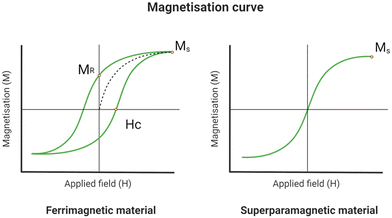
When IONPs are subjected to an external magnetic field or AMF, the electron spins orient themselves along the direction of the applied field;20 once all the spins are aligned, the material reaches magnetisation saturation (MS), represented as a plateau in magnetisation curves,21Fig. 2. These curves are obtained by measuring the magnetisations of the sample under different magnetic field strengths.12
As the AMF is removed, the material will retain some residual magnetisation (MR), resulting in a hysteresis loop.12 The remanent magnetisation is one of the causes of particles’ aggregation, which can lead to clot formation in the arteries.12 The magnetisation curves and MS values are significantly influenced by the size and morphology of the particles.22 Consequently, IONPs may display varying MS values depending on the synthetic method, batch, and degree of polydispersity. Only recently, LaGrow et al. provided a solution for the reproducible synthesis of IONP. By investigating the growth mechanism of the co-precipitation synthesis method with the aid of synchrotron X-ray diffraction in solution,23 they were able to provide a comprehensive understanding of the mechanism. This is essential not only for robust syntheses, but also for greater control over the size of the iron oxide nanoparticles, and hence the tuning of their magnetic properties.
At diameters below 25 nm, the energy needed for spin reorientation falls below the thermal energy barrier.12 Consequently, IONPs of this size range do not retain residual magnetisation upon removal of the AMF. Instead, excess magnetic energy dissipates into the surroundings as heat through the physical motion of particles (Brownian relaxation) or the rotational movement of magnetic moments (Neél relaxation).12 Materials with this characteristic are called superparamagnetic;12 this property is essential in biological applications as it prevents the aggregation of the particles.12
The magnetic field frequencies employed in MH therapy are considered safe for human health. The bottleneck when using the IONPs is that there is not enough heat generated and a high dose of IONPs is required.24 Therefore, researchers are focusing on enhancing the heating efficiency of IONPs to minimise the required quantity for treatment.
The heating efficiency of IONPs is quantified in terms of specific power absorption (SAR), representing the energy released per unit mass.17 A SAR of 300 W g−1 with a magnetic field of frequency and amplitude of 100 kHz and 10 kA m−1 respectively is appropriate for hyperthermia applications in humans.25,26
This could be achieved with 4 mL of magnetic fluid with a concentration of 120 mg mL−1, assuming a homogeneous distribution of the NPs inside a tumour with volume of 20 mL and no losses outside the tumour site.26 It is important to note that the SAR diminishes with increased tumour volumes; this highlights the importance of a precisely targeted delivery of the NPs inside the tumour.27 Commercially available IONPs can only reach 100 W g−1.28 It should be noted that the SAR depends on the IONP properties and the AMF amplitude and frequency.29
In 2013, Meffre et al. synthesised a series of core–shell iron/iron carbide nanoparticles with a SAR of 415 W g−1 in a magnetic field with a frequency of 54 kHz and amplitude of 24 kA m−1.30
In 2014, Di Corato et al. tested the SAR of several magnetic nanomaterials in solution and the cellular environment.31 Having tested single round particles, agglomerations and cubes, they reported heating up to 42 °C in a field with a frequency of up to 700 kHz and amplitude of up to 24 kA m−1. Notably, all tested nanomaterials exhibited a decrease in SAR upon cellular internalisation,31 demonstrating the influence of environmental factors on SAR.17 To achieve a SAR of approximately 300 W g−1 (ref. 27) in the cellular environment, the IONPs should have exceptionally high SAR in solution. For more information on magnetic hyperthermia, and its relation with IONP properties and the environment, we refer the reader to publications elsewhere.31–39
To summarise, the ideal IONPs for hyperthermic applications should possess the following characteristics:
• Composition: they are composed of magnetite (Fe3O4) or maghemite (γ-Fe2O3) with an inverse spinel structure.12
• Size: Their diameter should be less than 25 nm to exhibit superparamagnetic properties.40
• Specific absorption rate (SAR): they should ideally exhibit a SAR value of approximately 300 W g−1 (ref. 27) under a magnetic field frequency of 100 kHz and an amplitude of 10 kA m−1.30
The tendency of IONPs to aggregate, driven by factors such as van der Waal forces, poses potential risks of pulmonary embolism or coagulation.51 This can be counteracted by covering the IONPs in an organic coating. The coating provides steric and electrostatic repulsion while enhancing the colloidal stability and the biocompatibility of the metal oxide particles.51 In 2015, Walter et al. functionalised the surface of IONPs with PEGylated mono-phosphonated dendrons.52 Their investigation revealed that the conical architecture of the dendron contributes to the enhanced colloidal stability of the IONPs, attributed to the introduction of steric hindrance.52 Additionally, polymeric coatings like chitosan offer a means to increase the repulsive forces between the IONPs.53
Furthermore, employing a soft coating material enables IONPs to serve as a drug delivery system (DDS), facilitating combination therapy with other treatments.54–57 While hyperthermia alone may not suffice for complete cancer eradication, it can synergise with drug interventions by weakening cancer cells.7,11
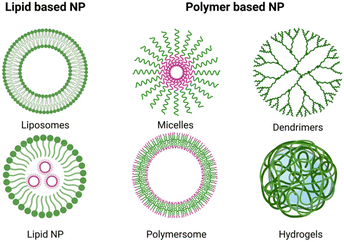
| Platform | Size | Shape and structure | MNP hybridisation method | Applications | Ref. |
|---|---|---|---|---|---|
| Dendrimers | 7–11 nm | MNPs covered in dendrimers | In situ MNP synthesis | MRI imaging | 59 and 65 |
| Polymers | 10 to 100 nm | Single MNP with a polymer shell | In situ MNP synthesis grafting | Drug delivery, MRI imaging, hyperthermia | 56, 57, 63, 64 and 66 |
| Liposomes | >100 nm | MNPs embedded in lipid bilayer | Formation of liposomes in in presence of pre-formed MNP | MRI imaging, drug release, hyperthermia | 67–69 |
| Micelles | 40 to 135 nm | IONPs encapsulated in micelles | Grafting in presence of water to induce micelles | MRI imaging | 61 |
| Microbubbles | 1–4 μm | Gas core, shell of polymer and MNP | Pre-formed MNP distributed in polymer layer during synthesis | MRI imaging, drug delivery | 55 and 62 |
| Hydrogel | 300 nm–150 μm | Hydrogel shell with IONPs core | In situ, blending, grafting | Drug delivery, hyperthermia | 70–73 |
Dendrimers are 3D macromolecules, comprising an inner core and branching outer layers.58 Such dendrimer-covered IONPs are often used in magnetic resonance imaging (MRI) as dendrimers can facilitate the penetration of IONPs inside cells.59
Micelles, colloidal dispersions of amphiphilic molecules arranged in a particle-like shape,60 also aid in cellular uptake of IONPs for imaging purposes.61
Microbubbles are spherical polymeric structures containing a gas core.62 By encapsulating IONPs in microbubbles, the IONPs can be delivered inside the cells by sonoporation.62
Polymers, such as polyethylene glycol (PEG), can be grafted on the surface of IONPs to prolong their circulation time.63 Additionally, polymers can also impart controlled drug delivery properties to IONPs: for example, Zhang et al. coated their IONPs with poly(N-isopropylacrylamide) (PNIPAAM) to facilitate the development of a magnetic DDS with thermoresponsive characteristics.64
Liposomes, hollow spherical particles composed of a lipid bilayer,12 exhibit less stability compared to other platforms, leading to potential leakage of contents over time.1
Hydrogels are formed by polymer chains crosslinked to give a 3D network with a highly porous nature that encapsulates water.12 Similar to liposomes, hydrogels are highly biocompatible but they excel in shielding the payload from degradation.12
From Table 1, it can be noted that polymers, liposomes and hydrogels are the most extensively studied for drug delivery.
3. Localised delivery of chemotherapy drugs with nanogels
Chemotherapy relies on small-molecule antiplastic drugs to either kill cancer cells or inhibit their growth.74 These drugs suffer from short circulation half-lives and poor water solubility,1 hindering their ability to reach the tumour site. Higher doses are typically administered to patients to ensure therapeutic efficacy, albeit at the cost of impacting healthy cells in the body.In recent years, these shortcomings have been addressed with the development of biomaterial carriers, DDS.4 The DDS platforms shield the drugs from the biological environment, extending their circulation time, and offering precise control over drug release kinetics and localisation through external manipulation.1 The main aim of a DDS is to enhance the therapeutic window of the drug. As Fig. 4 shows, nano-sized DDS facilitate a more uniform release of the drug over time, achieving the minimum effective level earlier than macro-sized DDS.75
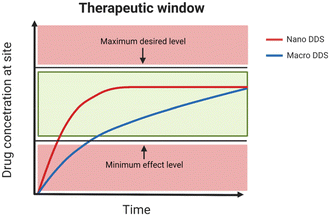 | ||
| Fig. 4 The therapeutic window for an ideal nano-sized DDS and a macro DDS (created with BioRender.com). | ||
The design of an efficient nano-carrier should address the following points:75
• Encapsulation: The nanocarrier must exhibit high encapsulation efficiency to minimise the dosage required for therapy. Additionally, the carrier's shell should effectively prevent cargo leakage before the intended release.75
• Stimuli-response: the release of the cargo should be controlled by a stimulus to prevent premature release.76
• Passive targeting: the size and morphology should be tailored to target the tumour thanks to the enhanced permeability and retention effect (EPR). EPR is caused by the abnormal growth of the tumour's vasculature and the poorly formed lymphatic systems.76
• Active targeting: Incorporating ligands capable of targeting specific receptors expressed by tumour cells enhances the accumulation of DDS within the tumour site.75
• Ease of synthesis: the synthesis of the nanocarrier should be scalable for commercialisation and the post-processing should be straightforward and time-effective.
Hydrogels and liposomes can both be engineered to meet the criteria for an ideal drug delivery system. However, hydrogels are more suitable for combined hyperthermia and chemotherapy applications due to their three-dimensional polymer network, which effectively prevents the leakage of IONPs over time.1 For other systems capable of simultaneous hyperthermia and drug delivery, the reader is invited to read the review by Hervault et al.21
3.1 Nanogels
As shown in Table 1, hydrogels incorporating IONPs may possess larger diameters that could impede biomedical applications.4 To optimise functionality, hydrogels must be sized appropriately to evade antibody interference 4 and traverse biological barriers for intracellular drug delivery.76 The nano-range size can be achieved by constraining the diameter of the hydrogels to the nano-scale (<500 nm), rendering them as nanogels. The size constraint can be achieved via traditional microemulsion polymerisations75 or more recent strategies such as micro-moulding and micro-fluidic methods.77 A diverse array of natural and synthetic polymers can be used to form nanogels78 with good biocompatibility; for biomedical applications, natural or FDA-approved polymers, such as PEG, are preferred.79Nanogels can undergo crosslinking through either physical interactions or covalent chemical bonds.79 Physically crosslinked nanogels are synthesised in mild conditions and are less likely to carry toxic contaminants, such as unreacted monomers or catalysts.79 However, these nanogels are also less stable in physiological conditions and it is more difficult to control the cargo release.
Chemically crosslinked nanogels are given by a bonding reaction between the chains of the polymers. These nanogels exhibit enhanced stability and are better suited for biomedical applications.79 Many cross-linking reactions can be used to form chemically crosslinked nanogels, such as radical polymerisation,80 click chemistry and copper-free click chemistry,81,82 amide crosslinking83 or photocrosslinking.84
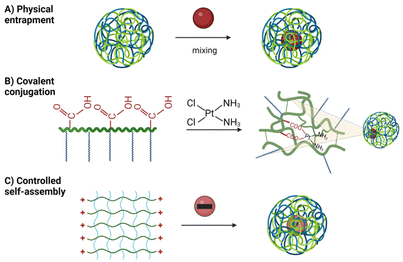
• Physical entrapment: drugs are entrapped by mixing them with pre-formed nanogel;85
• Covalent conjugation: drugs form covalent bonds with nanogels via pendant functional groups on polymer chains or the backbone.86 The disruption of these covalent bonds is necessary for drug release into the environment;87
• Self-assembly: electrostatic interactions or hydrophobic interactions facilitate drug coordination with polymer chains.88
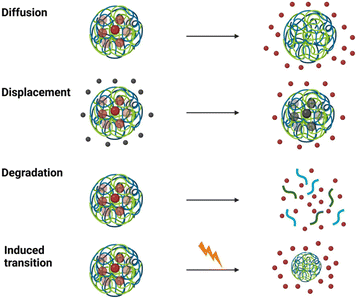
Upon reaching the target site, drug release from the nanogel is regulated by the mesh size of the network. The mesh size is influenced by crosslinking points and polymer nature.89 If the drug cannot freely travel outside of the network by diffusion, several strategies allow for a more controlled release, such as degradation, which permanently disrupts the 3D network of the gel.90
Alternatively, the nanogel can be forced to undergo an induced transition. For example, the network can be shrunk, leading to water and cargo expulsion.76 Nanogels capable of such trigger-induced changes are termed stimuli-responsive, exhibiting reversible alterations in conformation or solubility,76Fig. 6.
For combined magnetic hyperthermia and chemotherapy, the conformational change of the nanogel should be triggered by a rise in temperature, induced by the IONPs.11 The ability of the nanogel to swell or shrink is governed by a volume phase transition known as coil-to-globule transition,91Fig. 7.
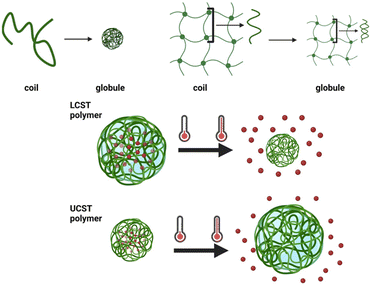 | ||
| Fig. 7 Coil to globule transition in polymer chains and nanogels; LCST and UCST polymers (created with BioRender.com). | ||
In a polymer solution, above a certain temperature known as the lower critical solution temperature (LCST), the entropy penalty becomes prohibitive to maintain the coil configuration. The penalty prompts the polymer chain to collapse onto itself to shield the hydrophobic sections from solvent molecules, forming a globule.92 The transition is governed by the ratio between the hydrophobic and hydrophilic units in the polymer.92
In a nanogel, the coil-to-globule transition results in the shrinking of the network with the expulsion of the solvent from the mesh;91 some nanogels can undergo the opposite transition, from a collapsed to a swollen state, upon reaching an upper critical solution temperature (UCST).93
Nanogels undergo this structural change faster than hydrogels as the time to achieve this transition is proportional to the square of their length.91
Thermoresponsiveness is given by a thermosensitive unit in the nanogel formulation, such as PNIPAAM, LCST = 32 °C (ref. 79) or poly(N-vinylcaprolactam) (PVCL, LCST = 32 °C).95 As the ideal temperature for hyperthermia is above 40 °C,57 as demonstrated by Wang et al.: at 40 °C, around 50% of the cancer cells died under exposure to AMF. Hence, the nanogel should be engineered to exhibit a sharp transition at this temperature.
One strategy to raise or lower the LCST of the polymer is through copolymerisation of more hydrophilic or hydrophobic monomers. By adjusting the ratio of these monomers, the desired temperature range can be achieved.96,97 However, the addition of monomers to lower the LCST often results in a broadening of the transition temperature range, complicating control.
Alternatively, the addition of branching units or the variation of the chain length has also demonstrated efficacy in altering the LCST,93,98Fig. 8.
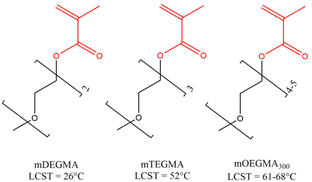 | ||
| Fig. 8 Non-linear PEG derivates and their LCST.94 mDEGMA – di(ethylene glycol) methyl ether methacrylate; mTEGMA – tri(ethylene glycol) methacrylate; mOEGMA – oligo(ethylene glycol) methyl ether methacrylate. | ||
Overall, the ideal nanogel formulation for combined hyperthermia and drug delivery should possess the following properties:
• Composition: it should be composed of FDA-approved or biocompatible polymers, such as PEG.
• Drug release: the nanogel should release its cargo in response to an external stimulus, specifically heat.
• Thermo-responsivity: it should demonstrate a distinct phase transition temperature in the range of 40–45 °C.57
4. Combinatorial therapies
While DDSs have demonstrated efficacy in mitigating chemotherapy toxicity, addressing the adaptability of tumours and achieving complete eradication of malignant growths necessitates the development of multifunctional therapies.74Petryk et al. showed that, following heat treatment, cancer cells exhibit reduced drug resistance, rendering synergistic therapy 1.5 times more effective than chemotherapy alone.99 Similar results have been reported by Wang et al.57 A Phase III clinical trial report highlighted that the combination of hyperthermia and chemotherapy doubled the life expectancy of patients with soft tissue sarcoma.5Fig. 9 shows a graphical representation of the simultaneous action of hyperthermia and chemotherapy.
To effectively combine these two different therapies, it is necessary to understand the properties of each material, how to enhance them in the therapy, and how they are affected by the biological environment.1
In the sections below, the synthesis, properties and applications of iron oxide magnetic nanogels will be covered.
5. Synthesis of magnetic nanogels
The magnetic nanogels reviewed in this study feature IONPs encapsulated within a nanogel shell. Different structures have been reported in the literature, all of which depend on the synthesis method of the magnetic nanogels.5.1 Synthesis by physical entrapment
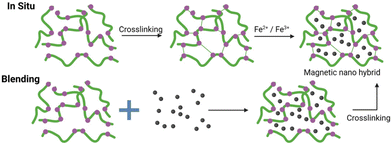
The blending and in situ are two prominent techniques relying on the physical entrapment of IONPs within the nanogel structure. Both methods have been extensively used since the early 2000s for magnetic hydrogel/nanogel synthesis,100–105Fig. 10.In the blending method, non-covalent interactions are commonly used to form stable intermediates; a subsequent crosslinking reaction forms the magnetic nanogels. Vijayan et al. achieved pre-organisation of the magnetic nanogel structure through electrostatic interactions between negatively charged citric acid-coated IONPs and the positively charged 2-(dimethylamino)ethyl methacrylate.102 Following the crosslinking reaction, a negatively charged, 80 nm spherical magnetic nanogel was formed, even if only 2.5 wt% of IONPs were incorporated in the nanogel.
In 2013, Chiu and co-workers synthesised hollow hybrid nanogels by photoinitiated polymerisation of a nanogel in the presence of citric acid-coated IONPs.106 They exploited the temporary hydrogen bonding (H-bond) of the citric acid and the PNIPAAM grafts of the nanogel precursor to form an assembly at pH 3 with a diameter of 198 nm. Covalent crosslinking of polymer chains was subsequently executed to secure a stable system with a diameter of 208 nm even at higher pH. Remarkably, approximately 44 wt% of IONPs were incorporated into the nanogels, rivalling previous literature records.106 However, the blending method offers limited control over IONP incorporation into the matrix, potentially altering predicted properties and leading to unwanted aggregate formation.103 In the study by Chiu and co-workers, the MS of the IONPs synthesised decreased from 77 emu g−1 to 36 emu g−1 post-polymerisation.106
The in situ method provides a simpler synthetic approach by utilising the micro/nanogel as a microreactor. But, as with the blending method, controlling the morphology and consequently the properties of magnetic nanogels remains challenging.103
The absence of a strong bond between the IONPs and the nanogel chains can lead to a leakage of the IONPs over time. Such leakage not only undermines the functionality of the composite, as the IONPs can no longer facilitate drug delivery, but it also poses potential toxicity concerns.107,108 By introducing a covalent bond between the IONPs and the nanogel, the composites can be constructed in a “bottom-up” fashion with higher control over the structure.109
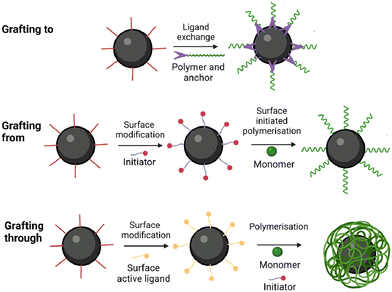
5.2 Synthesis by covalent grafting
The grafting approaches rely on the use of ligands featuring functional groups capable of binding with the surface, Fig. 11. These ligands commonly include carboxylic acids,51,110 trimethoxysilanes,17 and phosphonic acids.111,112 A key attribute of these bonds is their capacity to withstand high temperatures, a crucial requirement for hyperthermia applications.109Cazares-Cortes et al. synthesised MagNanogels by incorporating the IONPs at pH 3 utilising their positive surface charge for easy diffusion through the neutral polymer matrix.113 By increasing the pH to 7, the IONPs are neutralised and carboxylic functionalities of the nanogel can bind to the surface of the IONPs. This approach, previously used by Boularas et al.,114 resulted in a homogeneous distribution of IONPs throughout the polymer matrix without aggregation, Fig. 12. However, the MagNanogels were found to be stable only up to a wt of 37.5% iron content. Those with wt percentages of 50 and 66.7% exhibited instability due to the presence of aggregates on the surface.113
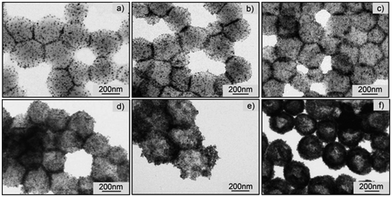 | ||
| Fig. 12 Transmission electron microscopy (TEM) images of the MagNanogels synthesised by Cazares-Cortes et al. with different IONPs content (from (a) to (f): 9.0, 16.7, 28.6, 37.5, 50.0, and 66.7 wt%).113 Reprinted with permission from E. Cazares-Cortes et al., ACS Appl. Mater. Interfaces, 2017, 9(31), 25775–25788. Copyright 2017 American Chemical Society. | ||
The modified IONPs can serve as either crosslinkers or templates around which the nanogel can be synthesised.
Gao et al. utilised the hydroxyl groups on the surface of bare IONPs to modify the surface with 2-isocyanatoethyl methacrylate (IEM).108 The resulting IEM-IONPs were employed as templates for synthesising a PVCL nanogel with miniemulsion polymerisation. However, the hydrophobic nature of the IEM-modified IONPs caused aggregation in solution (with an average particle size of 215 nm in dimethylsulfoxide, while transmission electron microscopy (TEM) analysis indicated a diameter of 18 nm); the aggregation led to the presence of multiple particles within the core of the nanogel.
Similarly, Chen et al. employed 3-(trimethoxysilyl)propyl methacrylate (TMSPMA) as a ligand, which promoted IONPs aggregation.117 These aggregated IONPs were used as crosslinking nodes in a PNIPAAM nanogel, but the resulting magnetic nanogel exhibited non-homogeneous crosslinking density. This unevenness can be attributed partially to the challenge of uniformly modifying IONPs with crosslinkers, resulting in different numbers of crosslinking sites per particle. Furthermore, the hydrophobic nature of TMSPMA contributed to the formation of clusters with varying dimensions, leading to differences in the number of crosslinking sites between the cores.
In 2021, Chou et al. obtained well-dispersed IONPs by imparting a negative charge on the surface. This was accomplished by using acrylic acid (AA) monomers as the surface active ligands.118 The study emphasised the critical nature of anchoring AA, highlighting its importance in synthesising magnetic hydrogels with small encapsulated cores of IONPs. When no anchoring step was performed and all the components were blended, minimal incorporation of IONPs in the hydrogel matrix was observed.118
In a study by Fernandez et al. in 2021, the efficacy of the grafting through method was compared with that of the blending method. Using 3-butenoic acid as the surface-active monomer, the blending method resulted in IONPs predominantly located towards the surface of a continuous macrogel matrix.119 In contrast, the grafting through method led to uniform localisation of IONPs at the centre of individual nanogels ranging between 100 and 200 nm in size.
The works by Fernandez et al. and Chou et al. demonstrate the superiority of the grafting methods over the methods utilising physical entrapment. Grafting methods offer superior control over both size and morphology, facilitating the core placement of the majority IONPs within the nanogel structure. However, the grafting to method can lead to a less dense coating due to the steric effects of bulky polymer chains.109 Grafting a monomer onto IONP surfaces ensures uniform templating of the nanogel, although monomer solubility affects morphology, with poorly dispersed monomer-IONPs resulting in undesired clustering.108,109,117 Nonetheless, the grafting through maintains composite size within the nanometer scale.119 In some instances, it is possible to achieve a single core–shell configuration below 50 nm.120 Thus, employing surface-active IONPs as templates in the grafting through method is preferred.
6. Properties
In the design of magnetic nanogels, a key concern lies in the impact of hybridisation on the properties of the individual components.91 Coating or trapping the IONPs in the matrix will reduce their rotation, which influences the SAR. On the other hand, the volume phase transition of the nanogel can be impacted as a portion of the internal volume of the nanogel is occupied by the IONPs.91 This interplay between the nanogel matrix and the encapsulated IONPs underscores the importance of understanding and optimising the hybridisation process to achieve desired properties in magnetic nanogels.6.1 Magnetic properties
As mentioned, achieving effective hyperthermia treatment requires magnetic nanogels with high heating ability. However, the encapsulation of IONPs hinders their rotational movement, thereby reducing their Brownian relaxation and leading to a decrease in MS and SAR, Table 2.Medeiros et al. reported that magnetic hydrogels with the lowest percentage of iron content (0.08 wt%) exhibited the lowest MS per mass unit of sample.122 However, having a high concentration of IONPs in the core can adversely affect the thermo-responsiveness of the polymer.
Zhang and co-workers synthesised magnetic nanogels by polymerising PNIPAAM and chitosan in the presence of oleic acid-coated IONPs with the blending method.123 The magnetic nanogel exhibited a MS value of 9.3 emu g−1 sample, significantly lower than the 64.3 emu g−1 of the bare IONPs. As the oleic acid-coated IONPs also showed a decrease in MS (35.8 emu g−1 sample), they concluded that:
• The oleic acid shell around the nanoparticles reduced the magnetic properties of the sample;
• The presence of a thick carbon shell in the magnetic nanogels reduced the magnetism of the particles by disrupting the surface anisotropy and the alignment of the surface spins.123
However, when subjected to an AMF, an increase in solution temperature was observed, dependent on the concentration of magnetic nanogels (10 g mL−1 15–35 °C and 20 mg mL−1 15–45 °C). Zhang and co-workers measure a SAR value between 10 and 15 W g−1 when exposing the magnetic nanogels to an AMF with an amplitude of 20 kA m−1 and a frequency of 360 kHz. This demonstrates that magnetic nanogels do not necessarily require high MS to release heat to the environment; however, the SAR value is too low for hyperthermic applications.123 Similarly, in the study by Chiu and co-workers, the MS of the sample containing the IONPs synthesised with blending method decreased from 77 emu g−1 to 36 emu g−1 post-polymerisation.106
In the study by Gao et al., minimal differences were observed in the MS of the bare IONPs, the ligand-modified IONPs and the magnetic nanogel synthesised with the grafting through method (68 emu g−1, 64 emu g−1 and 60 emu g−1 respectively).108 However, no experiments to record the SAR value were performed. Similarly, the coating did not significantly impact the MS values of the magnetic nanogels synthesised by Lin and co-worker.124
Recently, Cazares-Cortes et al. reported that the incorporation of 37.5 wt% IONPs in their nanogel through grafting to method did not affect the MS (98 and 96 emu g−1). Under an AMF with a frequency of 335 kHz and an amplitude of 7 kA m−1, they recorded a SAR value of 47 W g−1, comparable to that of the IONPs alone (SAR of 55 W g−1). Their findings indicate that the right polymer formulation will not affect the magnetic properties of the IONPs,113 but the SAR is lower than the ideal one for biomedical applications.27 Other studies in the literature have also reported no reduction in the MS of the magnetic core.102,125 To illustrate the potential of their MagNanogel for hyperthermia therapy, Cazares-Cortes et al. demonstrated an increase of the water temperature from 20 °C to 40 °C in a solution of MagNanogels exposed to AMF.113 Under AMF for 30 min, they observed a size change from 462 nm to 430 nm. This study showcased how IONPs can induce thermo-responsive behaviour in the surrounding polymer matrix.113
6.2 Thermo-responsiveness
When IONPs are encapsulated in the nanogel matrix, they could hinder the coil-to-globule transition and impact the LCST value of the composite.For instance, Fernandez et al. utilised MEO2MA as the thermosensitive polymer (LCST = 26 °C), but observed a transition temperature of 21.9 °C in the magnetic microgels.
The change in LCST is dependent on the synthesis of the magnetic nanogel and the chosen polymer. Chen et al. found that PNIPAAM magnetic microgels exhibited a transition temperature of around 33 °C, similar to that of PNIPAAM microgels (32 °C).117
Additionally, the percentage of iron content can affect the size change at the LCST.122 Chou et al. recorded the transition temperature for magnetic nanogels with a P(PNIPAAM-co-AA) polymer blend synthesised with the grafting through method. The magnetic nanogels with a higher percentage of IONPs in the core exhibited less shrinkage.118
In another study by Gao et al., magnetic nanogels synthesised with grafting through method with PVCL showed a clear size reduction (440 nm to 380 nm) in the temperature range between 28 °C to 42 °C, with an LCST of 32.5 °C.108 No change was observed before or after this range. However, for a more precise therapy, the temperature range should be narrow to allow for a sharp decrease in size. Liu et al. achieved a narrow temperature range for their PVCL-based magnetic nanogel with the grafting through method, which shrank from 530 to 300 nm within approximately a 4 °C range, with an LCST of 40 °C.125
The environment can also influence the LCST of the polymer. Jiang et al. calculated an LCST of 40 °C for their magnetic nanogel with PNIPAAM.126 However, by adjusting the pH of the medium, they were able to modify the LCST: at pH 6.8, the LCST was 37 °C due to the reduced hydrophilicity of the less ionised carboxyl groups of the PNIPAAM.
For hyperthermia applications, the nanogel should respond to temperatures above the body's and be stable in an acidic environment to prevent premature drug release.125
6.3 Drug release
Chemotherapy drugs can be loaded into magnetic nanogels and held in the matrix through various non-covalent interactions, such as hydrogen bonding108 and electrostatic interactions,127 or covalent conjugation.126Liu et al. applied an external AMF to induce the release in their magnetic nanogels. The release of cargo only slightly increased with AMF.125 Investigations revealed that the drug release was primarily driven by the dissociation of the nanogel shell in the presence of glucose. Consequently, the group concluded that AMF-triggered release made only a small contribution to the overall drug release mechanism.
Similarly, Gao et al. observed an increase in the percentage of drugs released by their magnetic nanogels when the solution temperature was increased to 37 °C. However, no tests were performed in the presence of an AMF. This suggests that drug release from this composite could occur simply by injecting it into the body due to the wide temperature range triggering release.108
Cazares-Cortes et al. were able to load up to 63% of doxorubicin (DOX) in their MagNanogel and tested the release under heat, pH and magnetic trigger. By raising the temperature of a water bath to 50 °C and 70 °C, they could achieve up to 32 and 36% of release after 4 h.113 Under AMF with amplitude 12 kA m−1 and frequency 335 Hz, the group achieved up to 47% DOX release. It is worth noting that close to 100% release was obtained when the pH was lowered from 7.5 to 5 as the MagNanogels are sensitive to the acidic environment.
The characteristics of magnetic nanogels are dependent upon both the size of IONPs and the polymer utilised. However, magnetic nanogels synthesised via the grafting through technique preserve the inherent properties of their constituent elements. This phenomenon stems from the enhanced control achieved through covalent synthetic methods for incorporating IONPs, contrasting with the limitations of physical entrapment approaches. However, very few studies in the literature present a complete set of data, in particular regarding the response to the AMF and measurement of the SAR value. Among the reported magnetic nanogels, the work by Cazares-Cortes et al. appears to be the most promising for drug release and thermo-responsiveness. Despite preserving the magnetic properties of the IONPs post-nanogel synthesis and promising experiments in solution, the relatively low SAR value (47 W g−1) might hinder biomedical applications in vivo.
7 Applicability of magnetic nanogels for cancer treatment
7.1 Biocompatibility of magnetic nanogels
Several studies have reported the good biocompatibility of magnetic nanogels,95 which seem to be less cytotoxic to healthy cells than certain chemotherapy drugs, such as DOX,113,124 while exhibiting higher efficiency against cancer cells.113,127The magnetic nanogel developed by Cazares-Cortes et al. showed viability close to 100% for cells treated with 2 mM of the composites, while cells treated with DOX alone showed viability of only 60%.113
Magnetic polymers containing poly(acrylic acid), such as those synthesised by Sundaresan et al., also exhibited good biocompatibility (80% viability with 0.5 mg mL−1), challenging the toxicity associated with the acrylate units.128 The magnetic nanogel developed by Mdlovu et al. contains pluronic F127 (PF) and polyethylenimine (PEI). PEI is known for its cell toxicity.124 No cell death was observed in HepG2 cells after 24 h and 48 h with concentrations of up to 75 μL mL−1 of the magnetic nanogel.
Once DOX was loaded in the magnetic nanogel and tested against cancer cells, the complex was found to be less cytotoxic than the drug alone.124 However, when the cells were exposed to an external magnetic field, the viability was lower than that observed with DOX alone. The team was able to verify an increased presence of DOX inside the cells when delivered with the magnetic nanogel. This suggests that the PF/PEI nanogel can destabilise the endosomal barriers and facilitate penetration of DOX through the cellular membrane.
Jiang et al. found no iron accumulation in the animals’ organs after 21 d from the treatment with the magnetic nanogel. Histopathological exams on the liver, spleen, brain, and other organs did not show any abnormal results.126
7.2 Efficiency of the combined hyperthermia/drug delivery system
Despite the abundance of literature on the synthesis and properties of magnetic nanogels, few studies have extended their investigations to include in vitro experiments. To the authors’ knowledge, no paper has explored the in vivo efficacy of magnetic nanogels for combined hyperthermia and drug delivery. Therefore, in the in vivo section, the two cited works should be considered exemplary studies outlining the necessary experiments for a comprehensive investigation.Cazares-Cortes et al. compared the viability of cancer cells with and without the contribution of the AMF. Empty magnetic nanogels under AMF did not affect the cells’ viability. After loading DOX in the magnetic nanogel, 75% of the cells died after exposure to AMF (with a local temperature of 65 °C). As 50% died without AMF, the paper concluded that hyperthermia enhances the cytotoxicity of DOX but is not efficient on its own.113
Contrary to Cazares-Cortes et al.,113 Indulekha et al. reported that 2 mg mL−1 of the empty magnetic nanogels under AMF caused 51% of cell death.95 After DOX loading, the magnetic nanogels were found to be toxic to 50% of the cells without AMF and to 80% with AMF. It should be noted that the DOX-loaded magnetic nanogels in a water bath at 43 °C released 60% of DOX under AMF and 40% without. Hence, the highest cytotoxicity of the DOX-loaded magnetic nanogels under AMF could be attributed to a higher amount of DOX released in the cells rather than due to the hyperthermic action.95
A study conducted by Hayashi et al. demonstrates the synergistic effect of combined therapy in a mouse model with xenografted tumours. In their experiment, IONPs coated with polymer chains were injected into the mice, followed by the application of an AMF.130
Thermal imaging of the animals revealed the localised “hot-spot” effect generated by the IONPs when exposed to the AMF, Fig. 13. Subsequent monitoring of tumour reduction over several days post-treatment showed a significant decrease in tumour size.
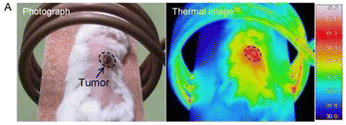 | ||
| Fig. 13 Thermal image of a mouse injected with magnetic polymer grafts.130 Adapted with permission from M. Hayashi et al., Theranostics, 2014, 4, 834–84. Copyright 2014 Ivyspring International Publisher. | ||
Fig. 14 depicts the mice 45 d after the injection, illustrating an 80% reduction in tumour size in mice treated with either hyperthermia or chemotherapy alone. Notably, in mice treated with the magnetic composite under AMF, the tumour completely disappeared after 12 d. Furthermore, the survival rate of the mice treated with the combined chemotherapy and hyperthermia was 100% after 200 d from the treatment. Mice treated with the individual treatments died after 100 d from the procedure.130
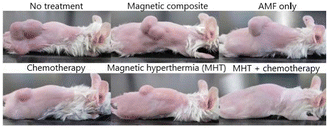 | ||
| Fig. 14 Pictures of tumour size after 45 d of treatment.130 Adapted with permission from M. Hayashi et al., Theranostics, 2014, 4, 834–84. Copyright 2014 Ivyspring International Publisher. | ||
This data suggests a synergistic mechanism wherein magnetic hyperthermia treatment reduces the volume of tumour cells, facilitating the penetration and efficacy of chemotherapy drugs throughout the tumour tissue.130
To conclude, several formulations in the literature have demonstrated the non-toxicity of magnetic nanogels. However, there is a lack of in vitro and in vivo studies showing the efficacy of magnetic nanogels for combined hyperthermia and chemotherapy. The in vitro works done by Indulekha et al.131 and by Cazares-Cortes et al.113 have both shown that around 75 to 80% of cancer cells can be killed with DOX-loaded magnetic nanogels under AMF. As the SAR of the MagNanogels by Cazares-Cortes et al. might not be ideal for human applications, there is an urgent need for in vivo studies.
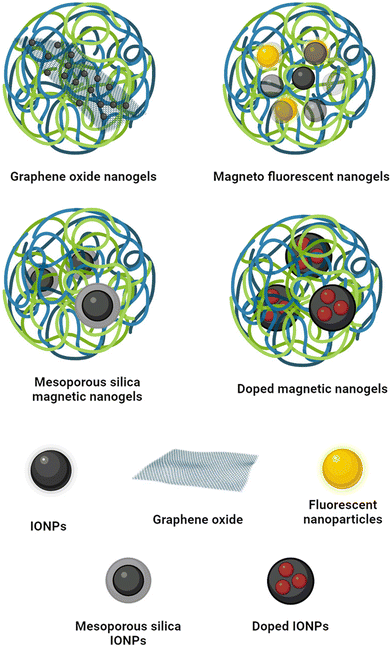
8. Beyond iron oxide magnetic nanogels
In recent years, the field of magnetic nanogels has witnessed significant advancements, with the integration of various materials to enhance their properties and broaden their applications, Fig. 15.8.1 Magnetic graphene oxide nanogels
The incorporation of graphene oxide (GO) into magnetic nanogels offers several advantages,132 including increased drug-loading capacity and the ability for magnetic tracking and smart drug delivery.133Bardajee et al. demonstrated a method for modifying GO with magnetic nanoparticles to create magnetic GO (MGO). While the MGO nanogels showed high drug loading efficiency (more than 80% for DOX), the relatively low MS of 0.08 emu g−1 limited their suitability for applications such as hyperthermia, MRI, or magnetic guiding.132
In contrast, Kunene et al. achieved higher MS and superparamagnetic behaviour for both MGO and MGO nanogels (71.62 and 67.61 emu g−1, respectively).134
Despite the reduction in porosity due to the presence of nanogels, they demonstrated higher loading efficiency of MGO nanogels compared to MGO alone. Moreover, they observed accelerated drug release from MGO nanogels under the influence of an AMF, high temperatures, and acidic pH conditions.134
8.2 Magnetic-fluorescent nanogels
The integration of quantum dots (QDs) into magnetic nanogel formulations offers the potential to impart fluorescence to the structure, enabling sensing capabilities135 and cellular imaging.136Shen et al. synthesised a luminescent-magnetic nanogel by incorporating pre-formed IONPs and QD during the polymerisation of chitosan.136 The composite showed strong orange/yellow emission, making it suitable for imaging purposes. However, the presence of cadmium in the QDs had a slight impact on cell viability due to its toxicity.136
In a different approach, Wang et al. developed a core–shell nanogel containing iron oxide crystals and non-toxic137 fluorescent carbon dots (CDs).138 Similar to magnetic nanogels, these composite nanogels were capable of releasing heat induced by the IONPs under an AMF. Additionally, the CDs enabled the nanogels to absorb near-infrared (NIR) wavelengths, allowing them to heat the environment upon NIR irradiation.138 Consequently, these nanogels could be utilised for both hyperthermia and photothermal therapy, with triggering mechanisms involving either an external AMF or NIR irradiation. Furthermore, the magnetic-fluorescent nanogels could be employed for fluorescent tracking, as the luminescence intensity was dependent on the size, with emission intensity increasing as the matrix shrank.138
8.3 Mesoporous silica magnetic nanogels
Coating the IONPs with a mesoporous silica shell (MSS) is one of the most widely used strategies to improve the biocompatibility of the IONPs and increase the particles’ surface area for improved drug loading abilities.139 While silica itself does not release drugs on cue, incorporating a nanogel coating can enable smart release mechanisms.140Mdlovu et al. demonstrated that incorporating stimuli-responsive nanogels enhances the loading efficiency of IONPs coated in mesoporous silica.124 Their magnetic nanogel showed a sustained release for over 70 h, with the highest release (94.41%) reached at an acidic pH of 5.4 and 42 °C due to the thermoresponsivity of the nanogel. Although these conditions mimic the altered physiology of the tumour environment, release experiments in the presence of an AMF were not conducted. In vitro and in vivo experiments showed no toxicity during histopathological analyses and enhanced tumour reduction in mice.124
The presence of silica on the surface of IONPs can significantly reduce their magnetism.140,141 Despite this, the MSS-IONPs nanogel reported by Keshavarz et al. exhibited a SAR value of 108.6 W g−1 and a temperature of 58 °C within 5 min of exposure to an AMF.140 The frequency and intensity of the magnetic field were 315 kHz and 14.3 kA m−1 respectively. The application of an AMF increased cargo release from 15% to 70%, demonstrating potential applications in hyperthermia and triggered drug release.
8.4 Doped mangnetic nanogels
Doping IONPs with other elements, such as manganese or zinc, can enhance their magnetic properties, stability, and SAR, making them more suitable for biomedical applications.142Monfared et al. utilised manganese zinc ferrite nanoparticles as the magnetic component in their PNIPAAM nanogel formulation.143 The resulting nanogel exhibited a SAR of 62.4 W g−1 in an AMF with 10 kA m−1 amplitude and 400 kHz frequency. The nanogel showed the highest sustained release of the drug when incubated at 44 °C for 24 h. However, it's important to note that no experiments were conducted to investigate drug release induced by an AMF. This aspect is crucial for evaluating the feasibility of using such nanogels in magnetic field-triggered drug delivery systems. Further studies are needed to explore the responsiveness of these doped IONP-based nanogels to external magnetic stimuli and their potential for controlled drug release in hyperthermia applications.
9. Challenges for clinical applications
The translation of research findings from the laboratory to clinical trials faces numerous challenges, resulting in a limited number of studies advancing to clinical stages.4 Several reasons contribute to this gap between research and clinical application, as outlined in various reviews144–146 and have been summarised in this section. The lack of international standards for assessing the safety of nanomedicines poses a significant obstacle to their commercialisation.146 Existing toxicological guidelines within both the European and American Pharmacopeias address general safety assessments, but they are not specific to nanomaterials. Nanoparticles exhibit unique behaviours due to their size, necessitating specialised regulations for their production and physicochemical testing.144,145 Furthermore, guidelines for studying the pharmacokinetics profiles of nanomaterials are lacking in regulatory agencies such as the Food and Drug Administration (FDA), the Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMA). Nanomaterials’ pharmacokinetics and pharmacodynamics profiles are more complex than traditional drugs, involving factors like protein corona formation, intricate metabolic pathways, and potential body accumulation.147Understanding how nanomaterials behave in the biological environment is crucial before their commercialisation.146 While in vitro and small animal studies provide valuable insights, the human biological environment differs significantly, making it challenging to predict nanomedicine behaviour post-administration.4 Despite the extensive research on nanomaterials, there is a significant gap in toxicological studies specific to nanomaterials.147 Developing new nanotoxicological assays, such as microfluidics or organ bioprinting, is essential for translating nanomedicines into clinical applications.147
Furthermore, the absence of specific regulations on good manufacturing practices, laboratory practices, and clinical practices for nanomaterials poses a challenge.147 International regulations could help prevent instances such as the removal of approved nanotechnological formulations from the market due to unforeseen negative side effects.144
The transition from laboratory-scale development to industrial-scale production presents a significant obstacle in the commercialisation of nanomaterials and nanomedicine. Firstly, nanoparticle production is exceptionally sensitive to minute variations.148 Factors like stirring speed and agitation, crucial in maintaining formulation integrity, can exert substantial influence.149 Scaling up to larger batches highlights the issue and ensuring reproducibility becomes increasingly difficult. Any inconsistency in batch-to-batch uniformity could result in altered properties and physicochemical profiles of the drug.148 However, the multitude of parameters necessary to ensure product uniformity renders purity validation challenging. Moreover, the synthetic pathways of nanomedicines often involve multi-step, complex processes that are low-yielding and high-cost, dissuading pharmaceutical manufacturing efforts.148 The presence of solvents and potentially toxic components such as unreacted monomers or ligands complicates purification procedures, without guaranteeing product purity and safety.148 Finally, verifying the stability of nanomedicine throughout production, storage, and administration is paramount, as even slight alterations could impact drug toxicity.148 These various challenges collectively contribute to the complexity of establishing the requisite quality control assessments necessary for the commercialisation of nanomedicines.149
Despite FDA approval or clinical trials for both IONPs145 and nanogels,144 no magnetic nanogel has entered human trials. While NanoTherm® (IONPs coated in aminosilane) has been successfully commercialised,10 ThermoDox® (a liposome formulation) reached phase III clinical trial before being halted due to insufficient evidence of efficacy.150 Despite this setback, the success of NanoTherm® paves the way for magnetic nanogel formulations as efficient synergistic therapies against cancer.
Finally, the intrinsic limitations of the magnetic field applicable in hyperthermia therapy pose a challenge to the development of magnetic nanogels.151 The heat generated during hyperthermia therapy is partly due to eddy currents, which are induced by metallic materials in magnetic fields and are quantified by the following equation:
| P = σt(πμ0)2(H0f)2r2 |
• The possibility of establishing a correlation with the local SAR limits for MRI.
• SARMAX can be easily estimated from calculations, simulations, or direct measurements.
• This parameter is reliable and adaptable, accounting for hot spots and non-axial field geometries.
In the study by Kwok et al., the patients did not experience any discomfort during the hyperthermia experiments.160
Overcoming these challenges requires interdisciplinary collaborations, innovative approaches, and sustained investment in translational research. Despite hurdles, ongoing efforts aim to advance the clinical translation of nanomaterial-based therapies, ultimately improving patient outcomes and advancing healthcare.
10. Conclusions
Combining hyperthermia and chemotherapy through magnetic nanogels presents a promising approach in cancer treatment, offering synergistic therapeutic effects and addressing the limitations of individual treatments.3,4While various synthesis strategies exist for magnetic nanogels, optimising their magnetic properties, thermo-responsiveness, drug-loading capacity, and biocompatibility are crucial for their effectiveness.76 Optimisation of the magnetic properties and SAR for hyperthermic properties is still needed to achieve the optimal value for biomedical applications. Numerous studies in this review have thoroughly investigated the thermo-responsive characteristics of magnetic nanogels, additional research focusing on drug release upon exposure to AMF is needed.
Although there is ongoing debate regarding the cytotoxicity of hyperthermia alone, studies have demonstrated the efficacy of magnetic nanogels in combined hyperthermia and chemotherapy drug delivery in vitro. However, challenges such as the complexity of magnetic nanogels, limited understanding of their behaviour in vivo, and their multi-component nature hinder their translation into clinical applications.146 Conducting in vivo comparisons to assess the potential increased toxicity of magnetic nanogels compared to individual hyperthermia and chemotherapy treatments would advance the field towards commercialisation. Data on the tumour regression, toxicity and survival rate of mice treated with magnetic nanogels could bridge the gap between the bench and the market. Similarly, a comparative study against other formulations for combined hyperthermia and chemotherapy, such as magnetic liposomes, would demonstrate the advantages of nanogels over other combined therapy approaches.
Most studies in the literature utilise spherical IONPs in magnetic nanogels, but exploring alternative morphologies such as cubes and nanoflowers could offer enhanced heating abilities and therapeutic efficacy. Further research into the properties and behaviour of magnetic nanogels, along with the development of standardised protocols, will be crucial for advancing their clinical translation and realising their potential in cancer therapy.107
Author contributions
Sofia Patri: Conceptualisation (supporting); writing – original draft (lead); writing – review and editing (equal). Nguyen T. K. Thanh: Conceptualisation (lead); writing – review and editing (equal). Nazila Kamaly: Conceptualisation (lead); writing – review and editing (equal).Data availability
As this is a review article, the Data availability statement does not apply.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank for the financial support the EPSRC and SFI Centre for Doctoral Training in Advanced Characterisation of Materials (Grant Ref EP/S023259/1).References
- Y. Sun and E. Davis, Nanomaterials, 2021, 11, 746 CrossRef CAS PubMed.
- D. Longley and P. Johnston, J. Pathol., 2005, 205, 275–292 CrossRef CAS PubMed.
- K. Bhatia, Bhumika and A. Das, Life Sci., 2020, 258, 118134 CrossRef CAS PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- R. D. Issels, L. H. Lindner, J. Verweij, R. Wessalowski, P. Reichardt, P. Wust, P. Ghadjar, P. Hohenberger, M. Angele, C. Salat, Z. Vujaskovic, S. Daugaard, O. Mella, U. Mansmann, H. R. D. T. Knösel, S. Abdel-Rahman, M. Schmidt, W. Hiddemann, K.-W. Jauch, C. Belka and A. Gronchi, JAMA Oncol., 2018, 4, 483–492 CrossRef PubMed.
- Z. Behrouzkia, Z. Joveini, B. Keshavarzi, N. Eyvazzadeh and R. Aghdam, Oman Med. J., 2016, 31, 89–90 CrossRef CAS PubMed.
- B. Hildebrandt, P. Wust, O. Ahlers, A. Dieing, G. Sreenivasa, T. Kerner, R. Felix and H. Riess, Crit. Rev. Oncol., 2002, 43, 33–56 CrossRef PubMed.
- B. Woodhall, K. Pickrell, N. Georgiade, M. Mahaley and H. Dukes, Ann. Surg., 1960, 151, 750–758 CrossRef CAS PubMed.
- P. Wust, B. Hildebrandt, G. Sreenivasa, B. Rau, J. Gellermann, H. Riess, R. Felix and P. Schlag, Lancet Oncol., 2002, 3, 487–497 CrossRef CAS PubMed.
- F. Soetaert, P. Korangath, D. Serantes, S. Fiering and R. Ivkov, Recent Adv. Drug Delivery Res., 2020, 163, 65–83 CrossRef PubMed.
- E. Guisasola, A. Baeza, L. Asín, J. M. dela Fuente and M. Vallet-Regí, Small Methods, 2018, 2, 1800007–1800018 CrossRef.
- D. Ortega, in Magnetic Nanoparticles: From Fabrication to Clinical Applications: Structure and Magnetism in Magnetic Nanoparticles, ed. N. T. K. Thanh, CRC Press, Taylor & Francis group, 1st edn, 2012 Search PubMed.
- N. Hoshyar, S. Gray, H. Han and G. Bao, Nanomedicine, 2016, 11, 673–692 CrossRef CAS PubMed.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- N. Feltin and M. P. Pileni, Langmuir, 1997, 13, 3927–3933 CrossRef CAS.
- P. Majewski and B. Thierry, Crit. Rev. Solid State Mater. Sci., 2007, 32, 203–215 CrossRef CAS.
- L. Asín, G. Stepien, M. Moros, R. M. Fratila and J. M. de la Fuente, in Clinical Applications of magnetic nanoparticles, Magnetic Nanoparticles for Cancer Treatment Using Magnetic Hyperthermia, ed. N. T. K. Thanh, CRC Press, Taylor & Francis group, 1st edn, 2019 Search PubMed.
- L. Lartigue, P. Hugounenq, D. Alloyeau, S. P. Clarke, M. Lévy, J.-C. Bacri, R. Bazzi, D. F. Brougham, C. Wilhelm and F. Gazeau, ACS Nano, 2012, 6, 10935–10949 CrossRef CAS PubMed.
- B. S. Kwon, W. Zhang, Z. Li and K. M. Krishnan, Adv. Mater. Interfaces, 2015, 2, 1400511 CrossRef.
- P. Atkins, J. D. Paula and J. Keeler, in Atkins’ Physical chemistry, Oxford University Press, Oxford, 11th edn, 2018, ch. 15, p. 675 Search PubMed.
- A. Hervault and N. T. K. Thanh, Nanoscale, 2014, 6, 11553–11573 RSC.
- Q. Li, C. W. Kartikowati, S. Horie, T. Ogi, T. Iwaki and K. Okuyama, Sci. Rep., 2017, 7, 9894 CrossRef PubMed.
- A. P. LaGrow, M. O. Besenhard, A. Hodzic, A. Sergides, L. K. Bogart, A. Gavriilidis and N. T. K. Thanh, Nanoscale, 2019, 11, 6620–6628 RSC.
- N. A. Stocke, P. Sethi, A. Jyoti, R. Chan, S. M. Arnold, J. Z. Hilt and M. Upreti, Biomaterials, 2017, 120, 115–125 CrossRef CAS PubMed.
- U. Gneveckow, A. Jordan, R. Scholz, V. Brüβ, N. Waldöfner, J. Ricke, A. Feussner, B. Hildebrandt, B. Rau and P. Wust, Med. Phys., 2004, 31, 1444–1451 CrossRef PubMed.
- M. Johannsen, U. Gneveckow, L. Eckelt, A. Feussner, N. Waldöfner, R. Scholz, S. Deger, P. Wust, S. A. Loening and A. Jordan, Int. J. Hyperthermia, 2005, 21, 637–647 CrossRef CAS PubMed.
- P. Wust, U. Gneveckow, M. Johannsen, D. Böhmer, T. Henkel, F. Kahmann, J. Sehouli, R. Felix, J. Ricke and A. Jordan, Int. J. Hyperthermia, 2006, 22, 673–685 CrossRef CAS PubMed.
- M. Kallumadil, M. Tada, T. Nakagawa, M. Abe, P. Southern and Q. A. Pankhurst, J. Magn. Magn. Mater., 2009, 321, 1509–1513 CrossRef CAS.
- C. Martinez-Boubeta, K. Simeonidis, A. Makridis, M. Angelakeris, O. Iglesias, P. Guardia, A. Cabot, L. Yedra, S. E. F. Peiro, Z. Saghi, P. A. Midgley, I. C.-L. D. Serantes and D. Baldomir, Sci. Rep., 2013, 3, 1652–1659 CrossRef PubMed.
- A. Meffre, B. Mehdaoui, V. Kelsen, P. F. Fazzini, J. Carrey, S. Lachaize, M. Respaud and B. Chaudret, Nano Lett., 2012, 12, 4722–4728 CrossRef CAS PubMed.
- R. D. Corato, A. Espinosa, L. Lartigue, M. Tharaud, S. Chat, T. Pellegrino, C. Ménager, F. Gazeau and C. Wilhelm, Biomaterials, 2014, 35, 6400–6411 CrossRef PubMed.
- E. Y. K. N. Suriyanto and S. D. Kumar, Biomed. Eng. OnLine, 2017, 16, 1–22 CrossRef PubMed.
- M. Suto, Y. Hirota, H. Mamiya, A. Fujita, R. Kasuya, K. Tohji and B. Jeyadevan, J. Magn. Magn. Mater., 2009, 321, 1493–1496 CrossRef CAS.
- X. Peng, B. Wang, Y. Yang, Y. Zhang, Y. Liu, Y. He, C. Zhang and H. Fan, ACS Biomater. Sci. Eng., 2019, 5, 1635–1644 CrossRef CAS PubMed.
- S. Mahjoob and K. Vafai, Int. J. Heat Mass Transfer, 2009, 52, 1608–1618 CrossRef CAS.
- E. C. Abenojar, S. Wickramasinghe, J. Bas-Concepcion and A. C. S. Samia, Prog. Nat. Sci.: Mater. Int., 2016, 26, 440–448 CrossRef CAS.
- S. Laurent, S. Dutz, U. O. Häfeli and M. Mahmoudi, Adv. Colloid Interface Sci., 2011, 166, 8–23 CrossRef CAS PubMed.
- D. Chang, M. Lim, J. A. C. M. Goos, R. Qiao, Y. Y. N. Y. Ng, F. M. Mansfeld, M. Jackson, T. P. Davis and M. Kavallaris, Front. Pharmacol., 2018, 9, 1–20 CrossRef PubMed.
- S. Dutz and R. Hergt, J. Am. Chem. Soc., 2014, 25, 452001–452029 Search PubMed.
- K. M. Krishnan, IEEE Trans. Magn., 2010, 46, 2523–2558 CAS.
- H. Arami, A. Khandhar, D. Liggitt and K. M. Krishnan, Chem. Soc. Rev., 2015, 44, 8576–8607 RSC.
- U. Patil, S. Adireddy, A. Jaiswal, S. Mandava, B. Lee and D. Chrisey, Int. J. Mol. Sci., 2015, 16, 24417–24450 CrossRef CAS PubMed.
- Q. Feng, Y. Liu, J. Huang, K. Chen, J. Huang and K. Xiao, Sci. Rep., 2018, 2, 2082–2095 CrossRef PubMed.
- T. K. Jain, M. K. Reddy, M. A. Morales, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharmaceutics, 2008, 5, 316–327 CrossRef CAS PubMed.
- G. Liu, J. Gao, H. Ai and X. Chen, Small, 2013, 9, 1533–1545 CrossRef CAS PubMed.
- M. Mahmoudi, H. Hofmann, B. Rothen-Rutishauser and A. Petri-Fink, Chem. Rev., 2012, 112, 2323–2338 CrossRef CAS PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS PubMed.
- D. D. Stueber, J. Villanova, I. Aponte, Z. Xiao and V. L. Colvin, Pharmaceutics, 2021, 13, 943–969 CrossRef CAS PubMed.
- L. Yildirimer, N. T. Thanh, M. Loizidoua and A. M. Seifalianh, Nano Today, 2011, 6, 585–607 CrossRef CAS PubMed.
- A. Walter, A. Garofalo, A. Parat, H. Martinez, D. Felder-Flesch and S. Begin-Colin, Nanotechnol. Rev., 2015, 4, 581–593 CAS.
- A. Walter, A. Garofalo, A. Parat, J. Jouhannaud, G. Pourroy, E. Voirin, S. Laurent, P. Bonazza, J. Taleb, C. Billotey, L. V. Elst, R. N. Muller, S. Begin-Colin and D. Felder-Flesch, J. Mater. Chem. B, 2015, 3, 1484–1494 RSC.
- Z.-T. Tsai, J.-F. Wang, H.-Y. Kuo, C.-R. Shen, J.-J. Wang and T.-C. Yen, J. Magn. Magn. Mater., 2010, 322, 208–213 CrossRef CAS.
- P. T. Yin, S. Shah, N. Pasquale, O. Garbuzenko, T. Minko and K. Lee, Biomaterials, 2016, 81, 46–57 CrossRef CAS PubMed.
- C.-H. Fan, C.-Y. Ting, H.-J. Lin, C.-H. Wang, H.-L. Liu, T.-C. Yen and C.-K. Yeh, Biomaterials, 2013, 34, 3706–3715 CrossRef CAS PubMed.
- A. Hervault, M. Lim, C. Boyer, A. Dunn, D. Mott, S. Maenosono and N. Thanh, Nanoscale, 2016, 8, 12152–12161 RSC.
- L. Wang, A. Hervault, P. Southern, O. Sandre, F. Couillaud and N. Thanh, J. Mater. Chem. B, 2020, 8, 10527–10539 RSC.
- K. Madaan, S. Kumar, N. Pooni, V. Lather and D. Pandita, J. Pharm. BioAllied Sci., 2014, 6, 139–150 CrossRef PubMed.
- J. W. Bulte, T. Douglas, B. Witwer, S. C. Zhang, E. Strable, B. K. Lewis, H. Zywickea, B. Miller, P. van Gelderen, B. M. Moskowitz, I. D. Duncan and J. A. Frank, Nat. Biotechnol., 2001, 19, 1141–1147 CrossRef CAS PubMed.
- N. Hanafy, M. El-Kemary and S. Leporatti, Cancers, 2018, 10, 238 CrossRef PubMed.
- B.-S. Kim, J.-M. Qiu, J.-P. Wang and T. A. Taton, Nano Lett., 2005, 5, 1987–1991 CrossRef CAS PubMed.
- F. Yang, Y. Li, Z. Chen, Y. Zhang, J. Wu and N. Gu, Biomaterials, 2009, 30, 3882–3890 CrossRef CAS PubMed.
- J. Xie, C. Xu, N. Kohler and S. S. Y. Hou, Adv. Mater., 2007, 19, 3163–3166 CrossRef CAS.
- J. L. Zhang, R. S. Srivastava and R. D. K. Misra, Langmuir, 2007, 23, 6342–6351 CrossRef CAS PubMed.
- E. Strable, J. W. M. Bulte, B. Moskowitz, K. Vivekanandan, M. Allen and T. Douglas, Chem. Mater., 2001, 13, 2201–2209 CrossRef CAS.
- T. K. Jain, M. A. Morales, S. K. Sahoo, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharm., 2005, 2, 194–205 CrossRef CAS PubMed.
- Y. Chen, A. Bose and G. D. Bothun, ACS Nano, 2010, 4, 3215–3221 CrossRef CAS PubMed.
- A. Hardiansyah, L.-Y. Huang, M.-C. Yang, T.-Y. Liu, S.-C. Tsai, C.-Y. Yang, C.-Y. Kuo, T.-Y. Chan, H.-M. Zou, W.-N. Lian and C.-H. Lin, Nanoscale Res. Lett., 2014, 9, 497 CrossRef PubMed.
- Z. Al-Ahmady, N. Lozano, K.-C. Mei, W. T. Al-Jamal and K. Kostarelos, Int. J. Pharm., 2016, 154, 133–142 CrossRef PubMed.
- H. Liu, C. Wang, Q. Gao, X. Liu and Z. Tong, Acta Biomater., 2010, 6, 275–281 CrossRef CAS PubMed.
- R. Narain, M. Gonzales, A. S. Hoffman, P. S. Stayton and K. M. Krishnan, Langmuir, 2007, 23, 6299–6304 CrossRef CAS PubMed.
- S. A. Meenach, C. G. Otu, K. W. Anderson and J. Z. Hilt, Int. J. Pharm., 2012, 427, 177–184 CrossRef CAS PubMed.
- S. Dagdelen, M. Mackiewicz, M. Osial, E. Waleka-Bargiel, J. Romanski, P. Krysinski and M. Karbarz, J. Mater. Sci., 2023, 58, 4094–4114 CrossRef CAS.
- H. Ma, G. Yu, J. Cheng, L. Song, Z. Zhou, Y. Zhao, Q. Zhao, L. Liu, X. Wei and M. Yang, Biomacromolecules, 2023, 24, 868–885 CrossRef CAS PubMed.
- R. T. Chacko, J. Ventura, J. Zhuang and S. Thayumanavan, Adv. Drug Delivery Rev., 2012, 64, 836–851 CrossRef CAS PubMed.
- A. A. Ali, A. Al-Othman and M. H. Al-Sayah, J. Controlled. Release, 2022, 351, 476–503 CrossRef CAS PubMed.
- J. K. Oh, R. Drumright, D. J. Siegwart and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 448–477 CrossRef CAS.
- E. Mauri, G. Perale and F. Rossi, ACS Appl. Nano Mater., 2018, 1, 6525–6541 CrossRef CAS.
- H. Wang, L. Gao, T. Fan, C. Zhang, B. Zhang, O. A. Al-Hartomy, A. Al-Ghamdi, S. Wageh, M. Qiu and H. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 54621–54647 CrossRef CAS PubMed.
- J. K. Oh, D. J. Siegwart, H. Lee, G. Sherwood, L. Peteanu, J. O. Hollinger, K. Kataoka and K. Matyjaszewski, J. Am. Chem. Soc., 2007, 129, 5939–5945 CrossRef CAS PubMed.
- D. Steinhilber, M. Witting, X. Zhang, M. Staegemann, F. Paulus, W. Friess, S. Küchler and R. Haag, J. Controlled. Release, 2019, 169, 289–295 CrossRef PubMed.
- Y.-Y. Li, J. Yang, W.-B. Wu, X.-Z. Zhang and R.-X. Zhuo, Langmuir, 2009, 25, 1923–1926 CrossRef CAS PubMed.
- J. Zhuang, S. Jiwpanich, V. D. Deepak and S. Thayumanavan, ACS Macro Lett., 2012, 1, 175–179 CrossRef CAS PubMed.
- Y.-C. Wang, J. Wu, Y. Li, J.-Z. Du, Y.-Y. Yuan and J. Wang, Chem. Commun., 2010, 46, 3520–3522 RSC.
- K. Akiyoshi, S. Kobayashi, S. Shichibe, D. Mix, M. Baudys, S. W. Kim and J. Sunamoto, J. Controlled. Release, 1998, 5, 313–320 CrossRef PubMed.
- W. Jin, P. Xu, Y. Zhan, Y. Shen, E. A. V. Kirk, B. Alexander, W. J. Murdoch, L. Liu and D. D. Isaak, Drug Delivery, 2007, 14, 279–286 CrossRef CAS PubMed.
- G. W. Ashley, J. Henise, R. Reid and D. V. Santi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 2318–2323 CrossRef CAS PubMed.
- T. K. Bronich, S. V. Vinogradov and A. V. Kabanov, Nano Lett., 2001, 1, 535–540 CrossRef CAS.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- T. O′shea, E. K. A. A. Aimetti, V. Yesilyurt and R. Langer, Adv. Mater., 2015, 27, 65–72 CrossRef PubMed.
- B. Sung, M.-H. Kim and L. Abelmann, Bioeng. Transl. Med., 2021, 6, e10190 CrossRef CAS PubMed.
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Mater. Sci. Eng., R, 2015, 95, 1–49 CrossRef PubMed.
- M. Karimi, P. S. Zangabad, A. Ghasemi, M. Amiri, M. Bahrami, H. Malekzad, H. G. Asl, Z. Mahdieh, M. Bozorgomid, A. Ghasemi, M. R. R. T. Boyuk and M. R. Hamblin, ACS Appl. Mater. Interfaces, 2016, 8, 21107–21133 CrossRef CAS PubMed.
- N. Badi, Prog. Polym. Sci., 2017, 66, 54–79 CrossRef CAS.
- S. Indulekha, P. Arunkumar, D. Bahadur and R. Srivastava, Colloids Surf., B, 2017, 155, 304–313 CrossRef CAS PubMed.
- S. J. Lue, C.-H. Chen and C.-M. Shih, J. Macromol. Sci., Part B: Phys., 2011, 50, 563–579 CrossRef CAS.
- L. Yu, T. Ci, S. Zhou, W. Zeng and J. Ding, Biomater. Sci., 2013, 1, 411–420 RSC.
- S. Han, M. Hagiwara and T. Ishizone, Macromolecules, 2003, 36, 8312–8319 CrossRef CAS.
- A. A. Petryk, A. J. Giustini, R. E. Gottesman, P. A. Kaufman and P. J. Hoopess, Int. J. Hyperthermia, 2013, 29, 845–851 CrossRef CAS PubMed.
- Q. Cui, S. Zhu, Y. Yan, Q. Ye, U. Ziener and Z. Cao, J. Nanosci. Nanotechnol., 2015, 15, 1533–4880 CrossRef PubMed.
- D. Müller-Schulte and T. Schmitz-Rode, J. Magn. Magn. Mater., 2006, 301, 267–271 CrossRef.
- V. M. Vijayan, A. E. Beeran, S. J. Shenoy, J. Muthu and V. Thomas, ACS Appl. Bio Mater., 2019, 2, 757–768 CrossRef CAS PubMed.
- J. Zhang, S. Xu and E. Kumacheva, J. Am. Chem. Soc., 2004, 126, 7908–7914 CrossRef CAS PubMed.
- B. Brugger and W. Richtering, Adv. Mater., 2007, 19, 2973–2978 CrossRef CAS.
- M. Hayati, G. R. Bardajee, M. Ramezani, S. S. Hosseini and F. Mizani, Polym. Int., 2020, 69, 156–164 CrossRef CAS.
- W.-H. Chiang, V. T. Ho, H.-H. Chen, W.-C. Huang, Y.-F. Huang, S.-C. Lin, C.-S. Chern and H.-C. Chiu, Langmuir, 2013, 29, 6434–6443 CrossRef CAS PubMed.
- A. Pikabea and J. Forcada, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3573–3586 CrossRef CAS.
- F. Gao, X. Wu, D. Wu, J. Yu, J. Yao, Q. Qi, Z. Cao, Q. Cui and Y. Mi, Colloids Surf., A, 2020, 587, 124363 CrossRef CAS.
- S. Kurzhals, R. Zirbs and E. Reimhult, ACS Appl. Mater. Interfaces, 2015, 7, 19342–19352 CrossRef CAS PubMed.
- J. Xie, C. Xu, N. Kohler, Y. Hou and S. Sun, Adv. Mater., 2007, 19, 3163–3166 CrossRef CAS.
- C. Lu, L. R. Bhatt, H. Y. Jun, S. H. Parkc and K. Y. Chai, J. Mater. Chem., 2012, 22, 19806–19811 RSC.
- M. A. White, J. A. Johnson, J. T. Koberstein and N. J. Turro, J. Am. Chem. Soc., 2006, 128, 11356–11357 CrossRef CAS PubMed.
- E. Cazares-Cortes, A. Espinosa, J.-M. Guigner, A. Michel, N. Griffete, C. Wilhelm and C. Ménager, ACS Appl. Mater. Interfaces, 2017, 9, 25775–25788 CrossRef CAS PubMed.
- M. Boularas, E. Gombart, J.-F. Tranchant, L. Billon and M. Save, Rapid Commun., 2015, 36, 79–83 CrossRef CAS PubMed.
- S. F. Medeiros, J. O. Filizzola, V. F. Fonseca, P. F. Oliveira, T. M. Silva, A. Elaissari and A. M. Santos, Mater. Lett., 2015, 160, 522–525 CrossRef CAS.
- R. D. Piazza, E. da Silva Nunes, W. R. Viali, S. W. da Silva, F. H. Aragón, J. A. H. Coaquira, P. C. de Morais, R. F. C. Marques and M. J. Júnior, Carbohydr. Polym., 2017, 178, 378–385 CrossRef CAS PubMed.
- T. Chen, Z. Cao, X. Guo, J. Nie, J. Xu, Z. Fan and B. Du, Polymer, 2011, 52, 172–179 CrossRef CAS.
- F.-Y. Chou, C.-M. Shih, M.-C. Tsai, W.-Y. Chiu and S. J. Lue, Polymer, 2012, 53, 2839–2846 CrossRef CAS.
- A. Lapresta-Fernández, A. Salinas-Castillo and L. F. Capitán-Vallvey, Sci. Rep., 2021, 11, 3947 CrossRef PubMed.
- M. Rahimi, M. Yousef, Y. Cheng, E. I. Meletis, R. C. Eberhart and K. Nguyen, J. Nanosci. Nanotechnol., 2009, 9, 4128–4134 CrossRef CAS PubMed.
- S. Sayadnia, E. Arkan, R. Jahanban-Esfahlan, S. Sayadnia and M. Jaymand, Polym. Adv. Technol., 2021, 32, 262–271 CrossRef CAS.
- S. F. Medeiros, J. O. Filizzola, P. F. Oliveira, T. M. Silva, B. R. Lara, M. V. Lopes, B. Rossi-Bergmann, A. Elaissari and A. M. Santos, Mater. Lett., 2016, 175, 296–299 CrossRef CAS.
- P. Li, A. M. Zhu, Q. L. Liu and Q. G. Zhang, Ind. Eng. Chem. Res., 2008, 47, 7700–7706 CrossRef CAS.
- N. V. Mdlovu, R.-S. Juang, M.-T. Weng, Y.-S. Lin and K.-S. Lin, ACS Appl. Nano Mater., 2023, 6, 8416–8433 CrossRef CAS.
- J. Liu, C. Detrembleur, A. Debuigne, M.-C. D. Pauw-Gillet, S. Mornet, L. V. Elst, S. Laurent, E. Duguet and C. Jérôme, J. Mater. Chem. B, 2014, 2, 1009–1023 RSC.
- L. Jiang, Q. Zhou, K. Mu, H. Xie, Y. Zhu, W. Zhu, Y. Zhao, H. Xu and X. Yang, Biomaterials, 2013, 30, 7418–7428 CrossRef PubMed.
- C. Yao, Y. Yuan and D. Yang, ACS Appl. Bio Mater., 2018, 1, 2012–2020 CrossRef CAS PubMed.
- V. Sundaresan, J. U. Menon, M. Rahimi, K. T. Nguyen and A. S. Wadajkar, Int. J. Pharm., 2014, 446, 1–7 CrossRef PubMed.
- X. Zhang, P. Wei, Z. Wang, Y. Zhao, W. Xiao, Y. Bian, D. Liang, Q. Lin, W. Song, W. Jiang and H. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 15956–15969 CrossRef CAS PubMed.
- K. Hayashi, M. Nakamura, H. Miki, S. Ozaki, M. Abe, T. Matsumoto, W. Sakamoto, T. Yogo and K. Ishimura, Theranostics, 2014, 4, 834–844 CrossRef PubMed.
- S. Indulekha, P. Arunkumar, D. Bahadur and R. Srivastava, Colloids Surf., B, 2017, 155, 304–313 CrossRef CAS PubMed.
- G. Bardajee and Z. Hooshyar, Polym. Bull., 2018, 75, 5403–5419 CrossRef CAS.
- R. Gonzalez-Rodriguez, E. Campbell and A. Naumov, PLoS One, 2019, 14, e0217072 CrossRef CAS PubMed.
- S. C. Kunene, K.-S. Lin, M.-T. Weng, M. J. C. Espinoza and C.-M. Wu, J. Ind. Eng. Chem., 2021, 104, 93–105 CrossRef CAS.
- A. Riedinger, M. P. Leal, S. R. Deka, C. George, I. R. Franchini, A. Falqui, R. Cingolani and T. Pellegrino, Nano Lett., 2011, 11, 3136–3141 CrossRef CAS PubMed.
- J.-M. Shen, X.-M. Guan, X.-Y. Liu, J.-F. Lan, T. Cheng and H.-X. Zhang, Bioconjugate Chem., 2012, 23, 1010–1021 CrossRef CAS PubMed.
- Y.-Y. Liu, N.-Y. Yu, W.-D. Fang, Q.-G. Tan, R. Ji, L.-Y. Yang, S. Wei, X.-W. Zhang and A.-J. Miao, Nat. Commun., 2021, 12, 812 CrossRef CAS PubMed.
- H. Wang, J. Yi, S. Mukherjee, P. Banerjeea and S. Zhou, Nanoscale, 2014, 6, 13001–13011 RSC.
- Z. Shuting, W. Jing, A. Xianquan and Z. Yan, Front. Bioeng. Biotechnol., 2022, 9, 39008–39016 Search PubMed.
- H. Keshavarz, A. Khavandi, S. Alamolhoda and M. R. Naimi-Jamal, RSC Adv., 2020, 10, 39008–39016 RSC.
- W. Pon-On, T. Tithito, W. Maneeprakorn, T. Phenrat and I.-M. Tang, Mater. Sci. Eng., C, 2019, 97, 23–30 CrossRef CAS PubMed.
- L. Wang, S.-M. Lai, C.-Z. Li, H.-P. Yu, P. Venkatesan and P.-S. Lai, Pharmaceutics, 2022, 14, 1000 CrossRef CAS PubMed.
- A. Monfared, A. Zamanian, I. Sharifi and M. Mozafari, Mater. Chem. Phys., 2019, 26, 44–50 CrossRef.
- F. Ali, K. Neha and S. Parveen, J. Drug Delivery Sci. Technol., 2023, 80, 104118 CrossRef CAS.
- A. Narang, R.-K. Chang and M. A. Hussain, J. Pharm. Sci., 2013, 102, 3867–3882 CrossRef CAS PubMed.
- M. Fonseca, I. Jarak, F. Victor, C. Domingues, F. Veiga and A. Figueiras, Materials, 2024, 17, 319 CrossRef CAS PubMed.
- C. Dominguez, A. Santos, C. Alvarez-Lorenzo, A. Concheiro, I. Jarak, F. Veiga, I. Barbosa, M. Dourado and A. Figueiras, ACS Nano, 2022, 16, 9994–10041 CrossRef PubMed.
- S. Hua, M. B. C. de Matos, J. M. Metselaar and G. Storm, Front. Pharmacol., 2018, 9, 1–14 CrossRef PubMed.
- R. Paliwal, R. J. Babu and S. Palakurthi, AAPS PharmSciTech, 2014, 15, 1527–1534 CrossRef CAS PubMed.
- M. Regenold, P. Bannigan, J. C. Evans, A. Waspe, M. J. Temple and C. Allen, Nanomedicine, 2022, 50, 102484 CrossRef PubMed.
- R. Hergt and S. Dutz, J. Magn. Magn. Mater., 2007, 311, 187–192 CrossRef CAS.
- W. J. Atkinson, I. A. Brezovich and D. P. Chakraborty, IEEE Trans. Biomed. Eng., 1984, 31, 70–75 CAS.
- U. Gneveckow, A. Jordan, R. Scholz, V. Brüβ, N. Waldöfner, J. Ricke, A. Feussner, B. Hildebrandt and B. R. P. Wust, Med. Phys., 2004, 31, 1444–1451 CrossRef PubMed.
- A. Jordan, R. Scholz, K. Maier-Hauff, F. van Landeghem, N. Waldoefner, U. Teichgraeber, J. Pinkernelle, H. Bruhn, F. Neumann, B. Thiesen, A. von Deimling and R. Felix, J. Neurooncol., 2006, 78, 7–14 CrossRef CAS PubMed.
- J.-H. Lee, J. T. Jang, J. S. Choi, S. H. Moon, S. H. Noh, J. W. Kim, J.-G. Kim, I.-S. Kim, K. I. Park and J. Cheon, Nat. Nanotechnol., 2011, 6, 418–422 CrossRef CAS PubMed.
- S. Kossatz, R. Ludwig, H. Dharing, V. Ettelt, G. Rimkus, M. Marciello, G. Salas, V. Patel, F. J. Teran and I. Hilger, Pharm. Res., 2014, 31, 3274–3288 CrossRef CAS PubMed.
- J. Pan, Y. Xu, Q. Wu, P. Hu and J. Shi, J. Am. Chem. Soc., 2021, 143, 8116–8128 CrossRef CAS PubMed.
- C. Caizer, Nanomaterials, 2021, 11, 40–60 CrossRef CAS PubMed.
- B. H. de la Parte, I. Rodrigo, J. Gutiérrez-Basoa, S. I. Correcher, C. M. Medina, J. J. Echevarría-Uraga, J. A. Garcia, F. Plazaola and I. García-Alonso, Cancers, 2022, 14, 3084–3098 CrossRef PubMed.
- M. K. Y. Kwok, C. C. J. Maley, A. Dworkin, S. Hattersley, P. Southern and Q. A. Pankhurst, Appl. Phys. Lett., 2023, 122, 240502–240511 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |