Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coordination polymer glass from a protic ionic liquid: proton conductivity and mechanical properties as an electrolyte†
Tomohiro
Ogawa
 ab,
Kazuki
Takahashi
c,
Sanjog S.
Nagarkar
a,
Koji
Ohara
d,
You-lee
Hong
e,
Yusuke
Nishiyama
ab,
Kazuki
Takahashi
c,
Sanjog S.
Nagarkar
a,
Koji
Ohara
d,
You-lee
Hong
e,
Yusuke
Nishiyama
 ef and
Satoshi
Horike
ef and
Satoshi
Horike
 *abgh
*abgh
aAIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Yoshida-Honmachi, Sakyo-ku, Kyoto 606-8501, Japan. E-mail: horike@icems.kyoto-u.ac.jp
bInstitute for Integrated Cell-Material Sciences, Institute for Advanced Study, Kyoto University, Yoshida-Honmachi, Sakyo-ku, Kyoto 606-8501, Japan
cAdvanced Research and Innovation Center, DENSO Corporation, 500-1 Minamiyama, Komenoki-cho, Nisshin, Aichi 470-0111, Japan
dDiffraction and Scattering Division, Center for Synchrotron Radiation Research, Japan Synchrotron Radiation Research Institute (JASRI), Kouto, Sayo, Hyogo 679-5198, Japan
eRIKEN-JEOL Collaboration Center, Yokohama, Kanagawa 230-0045, Japan
fJEOL RESONANCE Inc., Akishima, Tokyo 196-8558, Japan
gDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan
hDepartment of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong, 21210, Thailand
First published on 17th April 2020
Abstract
High proton conducting electrolytes with mechanical moldability are a key material for energy devices. We propose an approach for creating a coordination polymer (CP) glass from a protic ionic liquid for a solid-state anhydrous proton conductor. A protic ionic liquid (dema)(H2PO4), with components which also act as bridging ligands, was applied to construct a CP glass (dema)0.35[Zn(H2PO4)2.35(H3PO4)0.65]. The structural analysis revealed that large Zn–H2PO4−/H3PO4 coordination networks formed in the CP glass. The network formation results in enhancement of the properties of proton conductivity and viscoelasticity. High anhydrous proton conductivity (σ = 13.3 mS cm−1 at 120 °C) and a high transport number of the proton (0.94) were achieved by the coordination networks. A fuel cell with this CP glass membrane exhibits a high open-circuit voltage and power density (0.15 W cm−2) under dry conditions at 120 °C due to the conducting properties and mechanical properties of the CP glass.
Introduction
Proton conductive materials are important components of various electrochemical devices.1–3 One significant device is the fuel cell, and anhydrous proton conductors as electrolytes working at 120–200 °C have long been in high demand.4 In the past decade, a number of studies on the proton conductivity of coordination polymers (CPs) and metal–organic frameworks (MOFs) have been reported.5,6 Crystalline CP/MOFs have advantages for high proton conductivity because of their tailorable pores and ability to accommodate guest molecules. However, they are intrinsically non-moldable because of their crystalline nature, and the grain boundary causes gas leaking and additional resistance in the electrolyte layers. The preparation of mechanically stable membranes is one of the bottlenecks in electrolyte developments.7 More recently, CP/MOFs in liquid and glassy states have received attention as moldable amorphous materials.8–13 The phase transitions of CP/MOFs from crystals to liquids and glasses provide the option of use as a grain boundary-free monolith, which is a promising property for solid electrolytes.8 In spite of their potential, there are still a limited number of reports on proton-conductive CP/MOFs glass, and there are no reports of CP/MOFs to satisfy the criteria for sufficient proton conductivity (above 10 mS cm−1) under anhydrous conditions.14To develop a CP glass with high intrinsic proton conductivity, we focused on protic ionic liquids which are known as representative anhydrous proton conducting liquids.15,16 They are a subset of ionic liquids synthesized from Brønsted acids and bases. A number of combinations of acids and bases to create protic ionic liquids have been reported, and the optimal compositions achieved high proton conductivity (reaching 50 mS cm−1 at 120 °C) and high chemical and thermal stabilities.17 Protic ionic liquids exhibiting high proton conductivity typically exhibit low viscosity.18,19 However, low viscosity liquids are difficult to use in membranes, which results in a lower performance.20–23 Furthermore, high mobility of both cations and anions results in a low transport number of protons. This low transport number leads to a decrease in the open-circuit voltage (OCV) in fuel cells.21,24 There is also scientific interest in the methodology to improve transport numbers while keeping the high inherent conductivity of protic ionic liquids.
In this study, we propose a new approach for the design of a proton conductor based on a CP glass synthesized from a protic ionic liquid and metal ions. Appropriately selected metal ions can interact with the anions to form a CP with the desirable characteristics of moldability, proton conductivity, as well as a high transport number (Fig. 1A left). To date, there have been no reports on metal ion incorporation into protic ionic liquids for the modulation of proton conducting dynamics.16 Here, a CP glass consisting of a protic ionic liquid (dema)(H2PO4−) (dema = diethylmethylammonium cation) and Zn2+ was synthesized as a glassy coordination polymer. The structural analysis of the coordination network in the CP glass elucidates the enhanced proton conductivity, transport number, and mechanical properties, as well as performance in a H2/O2 fuel cell under anhydrous conditions.
Results and discussion
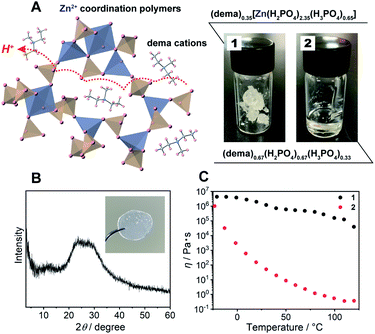
As a protic ionic liquid, (dema)(H2PO4) (proton conductivity of 6.5 mS cm−1 at 120 °C) is a candidate for further evaluation.17 The monoanionic H2PO4− can work both as a proton carrier and a bridging ligand (Fig. 1A left). Redox inactive Zn2+ is used as a metal ion, which is often used for CP/MOF glasses.8,9 The amorphous compound containing Zn2+, dema, and H2PO4− was synthesized by neutralization of zinc oxide (ZnO), diethylmethylamine and phosphoric acid (H3PO4) (Scheme S1† top). Grinding these materials afforded 1 as a viscoelastic solid (Fig. 1A). As a reference for 1, we also prepared (dema)(H2PO4) (2) according to the method available in the literature (Scheme S1,† bottom).17The chemical compositions of 1 and 2 were characterized by solid-state magic angle spinning nuclear magnetic resonance (SSNMR) (Fig. S1†) and elemental analysis. The proton peaks were assigned to either dema or H2PO4−/H3PO4, and both 1 and 2 contained no detectable water molecules. The observed integration value of the 1H signals of dema (dema![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2PO4− = 1
H2PO4− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.6) was different from the theoretical chemical composition of the starting materials (dema
7.6) was different from the theoretical chemical composition of the starting materials (dema![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2PO4− = 1
H2PO4− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Previous reports identified that highly stable protic ionic liquids have a pKa difference between the cation and the anion which is larger than 10.18,25 The difference in the pKa value between H3PO4 (2.1) and the diethylmethylammonium cation (10.5), ΔpKa, was 8.4, and the neutral amine was partially evaporated during the drying process (Scheme S1†). Both SSNMR and the elemental analysis indicated an identical composition of 1, i.e., (dema)0.35[Zn(H2PO4)2.35(H3PO4)0.65]. Partial evaporation of the amine was also observed for 2, even though we followed the reported synthesis method.171H SSNMR gave the composition of 2 as (dema)0.67(H2PO4)0.67(H3PO4)0.33. Furthermore, the 31P SSNMR spectrum of 1 exhibits a single peak at 0.2 ppm (20 °C, Fig. S2†), which suggests that the phosphoric acid species remains in the form of orthophosphoric acids after drying.26,27 The powder X-ray (Cu Kα) diffraction (PXRD) pattern of 1 at room temperature exhibited no Bragg peaks, and 1 was assignable to an amorphous state (Fig. 1B). The IR spectrum was measured to obtain information about the proton conductive groups (Fig. S3†). However, the spectrum shape was very broad and difficult to use for assignments. This is probably due to its amorphous nature as it contains many different configurations of cations and coordination structures. The thermal properties of 1 were subsequently determined by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC, Fig. S4†). There was no weight loss up to 200 °C. In addition, the DSC curves of 1 showed a glass transition at −22 °C. It has no first-order phase transition corresponding to crystallization under the measurement conditions. Based on its amorphous diffraction pattern and glass transition behaviour, the synthesized 1 can be categorized as a CP glass.
3). Previous reports identified that highly stable protic ionic liquids have a pKa difference between the cation and the anion which is larger than 10.18,25 The difference in the pKa value between H3PO4 (2.1) and the diethylmethylammonium cation (10.5), ΔpKa, was 8.4, and the neutral amine was partially evaporated during the drying process (Scheme S1†). Both SSNMR and the elemental analysis indicated an identical composition of 1, i.e., (dema)0.35[Zn(H2PO4)2.35(H3PO4)0.65]. Partial evaporation of the amine was also observed for 2, even though we followed the reported synthesis method.171H SSNMR gave the composition of 2 as (dema)0.67(H2PO4)0.67(H3PO4)0.33. Furthermore, the 31P SSNMR spectrum of 1 exhibits a single peak at 0.2 ppm (20 °C, Fig. S2†), which suggests that the phosphoric acid species remains in the form of orthophosphoric acids after drying.26,27 The powder X-ray (Cu Kα) diffraction (PXRD) pattern of 1 at room temperature exhibited no Bragg peaks, and 1 was assignable to an amorphous state (Fig. 1B). The IR spectrum was measured to obtain information about the proton conductive groups (Fig. S3†). However, the spectrum shape was very broad and difficult to use for assignments. This is probably due to its amorphous nature as it contains many different configurations of cations and coordination structures. The thermal properties of 1 were subsequently determined by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC, Fig. S4†). There was no weight loss up to 200 °C. In addition, the DSC curves of 1 showed a glass transition at −22 °C. It has no first-order phase transition corresponding to crystallization under the measurement conditions. Based on its amorphous diffraction pattern and glass transition behaviour, the synthesized 1 can be categorized as a CP glass.
The mechanical properties of 1 were evaluated from viscosity and dynamic mechanical analyses (DMA). The viscosity of 1 showed a higher value than that of 2 in the measurement temperature range (−20 to 120 °C) (Fig. 1C). The viscosity values of 1 are lower than the viscosity at the Littleton softening point (106.6) and higher than that at the working point (104).28 Soda lime glass exhibits these viscosities around 700–800 °C. The viscosity of 1 at room temperature to 120 °C is suitable for making a large transparent monolith by an annealing process (Fig. 1B; inset). Furthermore, DMA of 1 also demonstrated a higher shear modulus than that of 2 (Fig. S5†). The G′′ (the loss modulus) of 1 is lower than G′ (the storage modulus) at each temperature (−20 to 120 °C). This suggests that 1 has no fluidity even above the glass transition temperature.
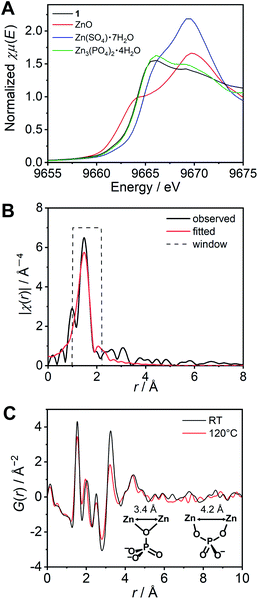
We subsequently characterized the amorphous structure of 1. X-ray absorption fine structure (XAFS) analysis is often used for consideration of the coordination geometry and average coordination number of Zn2+.29–31 The XAFS spectra of the K-edge region of Zn2+ for 1 and the reference samples were measured and analysed (Fig. S6†). As references, three Zn2+ salts adopting different coordination geometries, i.e., ZnO (tetrahedral), Zn(SO4)·7H2O (octahedral), and Zn3(PO4)2·4H2O (tetrahedral![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octahedral = 2
octahedral = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)32 were also measured. The edges of the spectra of the reference samples showed a relationship between the energy and the coordination number. The X-ray absorption near edge structure (XANES) region of 1 (Fig. 2A) exhibited a similar energy and shape to that of the model crystal Zn3(PO4)2·4H2O, which indicates that the averaged coordination number of 1 is in the range of 4–6, and close to 4.67 (tetrahedral
1)32 were also measured. The edges of the spectra of the reference samples showed a relationship between the energy and the coordination number. The X-ray absorption near edge structure (XANES) region of 1 (Fig. 2A) exhibited a similar energy and shape to that of the model crystal Zn3(PO4)2·4H2O, which indicates that the averaged coordination number of 1 is in the range of 4–6, and close to 4.67 (tetrahedral![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octahedral = 2
octahedral = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The resulting k3 weighted radial distribution function of 1 was fitted using the FEFF calculation (Fig. 2B). The obtained converged parameter gave a reasonable coordination number (4.6 ± 0.4), which is close to the proposed model Zn3(PO4)2·4H2O. The fitted coordination number of 4.6 can only be explained by some H2PO4−/H3PO4 coordinating more than two Zn2+ ions. These results suggest that H2PO4− acts as a bridging ligand and that a Zn2+–H2PO4−/H3PO4 coordination network structure is formed in 1.
1). The resulting k3 weighted radial distribution function of 1 was fitted using the FEFF calculation (Fig. 2B). The obtained converged parameter gave a reasonable coordination number (4.6 ± 0.4), which is close to the proposed model Zn3(PO4)2·4H2O. The fitted coordination number of 4.6 can only be explained by some H2PO4−/H3PO4 coordinating more than two Zn2+ ions. These results suggest that H2PO4− acts as a bridging ligand and that a Zn2+–H2PO4−/H3PO4 coordination network structure is formed in 1.
The reduced pair distribution function (PDF), G(r), was conducted using a wide Q range diffraction measured at the synchrotron (incident energy = 61.377 keV). The G(r) of 1 exhibited five distinct peaks (at 1.54, 2.03, 2.51 3.25, and 4.42 Å) observed below 5 Å (Fig. 2C). A related crystal structure of zinc phosphate (Zn3(PO4)2·4H2O)32 was used to consider the possible Zn2+–Zn2+ distances in the Zn2+–H2PO4−/H3PO4 network structure. In the model crystal structure, two types of Zn2+–Zn2+ distances were found to be shorter than 5 Å. The shortest Zn2+–Zn2+ distance (3.38 Å) was formed via one μ-oxygen atom of the PO43− anion. Another Zn2+–Zn2+ distance (4.22 Å) bridged by two oxygen atoms of a PO43− was also found (Fig. 2C inset). The peaks obtained in the PDF are in good agreement with the two aforementioned Zn2+–Zn2+ distances. In addition to the comparison with the model crystal, the atomic scattering factor of Zn2+ is much higher than that of the other atoms. Therefore, the two peaks (3.25 and 4.42 Å) can be assigned to Zn2+–Zn2+. The PDF measured at 120 °C also showed similar peaks. The Zn2+–Zn2+ correlations are retained at high temperatures, even the intensity of the peak at 3.3 Å is slightly lower than that obtained at room temperature. The XAFS and PDF analyses of 1 suggest the formation of coordination networks.
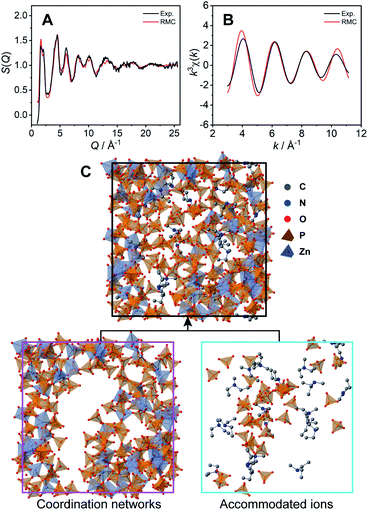
The coordination network structure was demonstrated by reverse Monte Carlo (RMC) structural modelling using the X-ray experimental structural factor S(Q) and EXAFS k3χ(k) data at room temperature with several coordination number constraints. In this simulation, the atoms (Zn: 60, P: 180, O: 720, N: 21, C: 105, and H: 693) were placed in a cubic box. The size of the cubic box was determined by the experimental density (1.87 g cm−3) at room temperature. The calculated S(Q) (Fig. 3A) and k3χ(k) (Fig. 3B) of the RMC structural modelling indicated good agreement with the experimental values. The resulting RMC modelling structure of a cubic unit cell is presented in Fig. 3C (Table S2†). The averaged coordination number of Zn2+ is 4.4, which is close to that obtained by the XAFS measurements (4.6).
The obtained CP structure contains all 60 Zn2+ atoms and 147 H2PO4−/H3PO4 molecules (Fig. 3C bottom left). In the structure, dema and free H2PO4−/H3PO4 are found in the voids of the three-dimensional coordination network (Fig. 3C bottom right). The RMC modelling structure suggests that bulky dema and free H2PO4−/H3PO4 are surrounded by coordination networks. The structure can play the role of exclusive proton conductivity with a low energy barrier.
The ion conductivity of 1 was determined by variable temperature AC impedance spectroscopy under a dry inert atmosphere (Fig. 4A and S7†). The Nyquist plots at 120 °C are shown with fitted curves from an equivalent circuit model (Fig. S8†). The equivalent circuit contains R1, R2, and Q1, where R1 is the bulk resistance of 1 or 2, and the parallel connection of R2 and Q1 represents combined resistance and constant phase elements from the electric double layer, respectively (Fig. S9 and S10†).33 The conductivity reached σ = 13.3 mS cm−1 at 120 °C, which is approximately twice as high as that of 2 (σ = 6.5 mS cm−1).17 In protic ionic liquids, the lower viscosity results in higher conductivity due to the contribution of the vehicle mechanism.18 The conductivities of 1 are higher than that of 2 at each temperature, whereas the viscosity of 1 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η−1 = −5.6 P−1 at 120 °C) is significantly higher than that of 2 (log
η−1 = −5.6 P−1 at 120 °C) is significantly higher than that of 2 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η−1 = −0.57 P−1 at 120 °C). This suggests that in comparison to 2, the proton-conducting mechanism of 1 is dominated more by the Grotthuss mechanism. The temperature dependence of both 1 and 2 showed non-Arrhenius type plots which are frequently seen in ionic liquids and their polymer composites.25,34 These temperature profiles were analysed by curve fitting using the Vogel–Fulcher–Tammann (VFT) equation (eqn (1), see ESI† for details) to obtain the parameters for proton conductivity.35,36
η−1 = −0.57 P−1 at 120 °C). This suggests that in comparison to 2, the proton-conducting mechanism of 1 is dominated more by the Grotthuss mechanism. The temperature dependence of both 1 and 2 showed non-Arrhenius type plots which are frequently seen in ionic liquids and their polymer composites.25,34 These temperature profiles were analysed by curve fitting using the Vogel–Fulcher–Tammann (VFT) equation (eqn (1), see ESI† for details) to obtain the parameters for proton conductivity.35,36
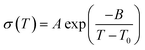 | (1) |
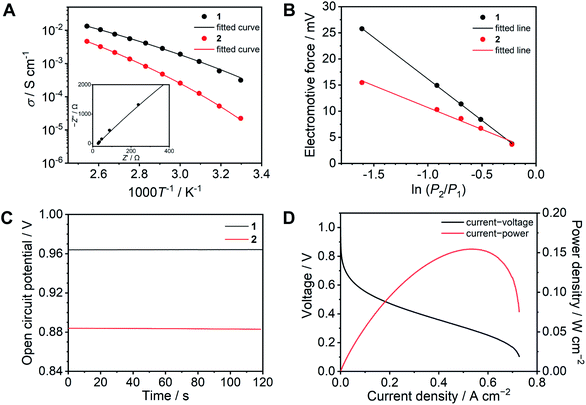 | ||
| Fig. 4 Proton conducting properties of 1 (black) and 2 (red). (A) Ion conductivity as a function of temperature under anhydrous conditions. The solid lines are fitting curves determined using the VFT equation (eqn (1)). The inset exhibits the Nyquist plots of 1 at 120 °C. (B) Electromotive force measured under different relative hydrogen gas pressure. (C) OCV of a H2/O2 fuel cell at 120 °C without humidification. (D) I–V (black) and I–W (red) curves of a H2/O2 fuel cell with 1 as an electrolyte at 120 °C without humidification. | ||
The resulting fitting parameters are in good agreement with the experimental results (Fig. 4A, solid lines) and are summarized in Table S1.† The smaller A value of 1 than that of 2 suggests that the concentration of conducting ions does not contribute to the higher conductivity. The higher conductivity of 1 is attributed to the lower pseudo activation energy (B). This suggests that the coordination network provides a better proton-conducting path than fluidic liquid states.
To obtain further insight, electrochemical measurements were subsequently carried out in a H2/O2 fuel cell. The transport number is defined as the ratio of the electric current derived from an ion of interest or target ion to the total electric current.37 In the case of protic ionic liquids, a transport number is usually in the range of 0.5–0.6 which means that the counter anions contribute to the ion conduction.17,38–40 The transport numbers of 1 and 2 were determined by electromotive force (EMF) measurements between different hydrogen partial pressure values (−ln(P2/P1) = 0.22, 0.51, 0.69, 0.92, and 1.61) at 120 °C (Fig. 4B) using eqn (2) (see ESI†).41 The resulting transport number of 1 is 0.94, which indicates that the ion conductivity of 1 is predominantly attributed to the protons. On the other hand, the obtained transport number for 2 is 0.49. The high transport number of 1 suggests that the movement of anions is significantly suppressed by coordination bond formation with Zn2+. To confirm the impact of the high transport number of 1, we measured the OCV in a H2/O2 fuel cell at 120 °C (Fig. 4C). The OCV of 1 is 0.96 V and it is intact over 10 minutes, it is also higher than that of 2 (0.88 V), while the observed OCV of 1 is lower than the theoretical value of 1.16 V at 120 °C,42 it is comparative to the highest OCV values of CP/MOFs and protic ionic liquids at 120 °C under anhydrous conditions reported so far.23,43,44
The I–V curve of the H2/O2 fuel cell with 1 was determined at 120 °C under anhydrous conditions (Fig. 4D). The electrolyte membrane was prepared by impregnation of 1 into a polytetrafluoroethylene (PTFE) membrane. The I–V curve was measured using 100% H2 and O2 gases. We also attempted to evaluate the I–V curve for 2, however, this was unsuccessful under the same conditions. This is because of high fluidity and because it is hard to suppress the gas permeation using 2 as the electrolyte. The observed maximum power density was 150 mW cm−2. Among the fuel cell performance in related conditions at 120 °C, this value is higher than that of the reported CP/MOFs,44,45 xerogels,46 covalent organic frameworks (COFs),7,47 and membrane composite of protic ionic liquids21,40 and as high as the composite of functionalized graphene oxide and Nafion (150 mW cm−2) measured under humidified conditions (RH = 25%).48 Furthermore, neat 1 was tested as an electrolyte layer without the PTFE membrane (Fig. S11†). The OCV value of the fuel cell with neat 1 exhibited almost the same value (0.96 V). This suggests that the CP glass can be used directly as an electrolyte, although the OCV stability during measurement (10 min) is slightly lower than that measured with the PTFE membrane.
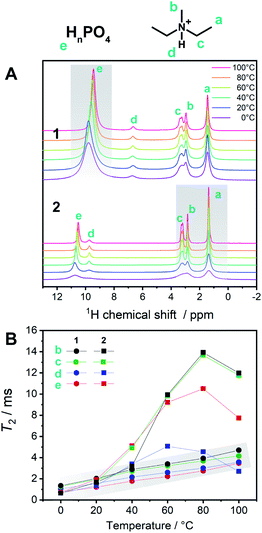
To describe the dynamics of the coordination network and protons in 1, we carried out 1H SSNMR from 0 to 100 °C for 1 and 2 (Fig. 5A). The chemical shifts of the protons on the aliphatic carbons of dema (4–1 ppm) were independent of the temperature and were identical for 1 and 2. On the other hand, the proton signals of H2PO4−/H3PO4 were observed at 10–9.5 (1) and 11–10.4 (2) ppm, respectively. Hydrogen bond formation has been reported in protic ionic liquids containing proton-donating and accepting anions such as HSO4− and H2PO4−.19,23 When the hydrogen bonds are formed in liquid states or solutions, downfield shifts are often observed.16 A dimer formation via two hydrogen bonds in H2PO4− is formed and these protons trapped by the dimer structure are not favourable for proton conduction. The presence of Zn2+ decreases the hydrogen bonds between H2PO4−/H3PO4 by forming a coordination network. Considering the temperature dependence of 1, only the acidic proton exhibited a relatively high dependence of the chemical shifts and peak broadening on temperature, whereas both the cations and anions of 2 showed temperature-dependent broadening. This difference suggests that the conductivity of 1 is mainly affected by the proton of H2PO4−/H3PO4, which is in good agreement with other measurements. It was reported that the ion conductivities of protic ionic liquids are related not only to the protons but also to the cations and anions.23 The observed spin–spin relaxation time (T2) of 2 is much longer than that of 1 in the temperature region of 0–100 °C (Fig. 5B). The shorter T2 for 1 means that it exhibits more solid-like behaviour, and 2 is in a more isotropic fluid state.
These SSNMR analyses support the observation that the coordination networks in 1 enhance the conductivity and increase the proton transport number.
Conclusions
In conclusion, we propose an approach for creating a CP glass from a protic ionic liquid for solid-state anhydrous proton conductors. The reaction of Zn2+ and protic ionic liquid (dema)(H2PO4−) formed a Zn2+–H2PO4−/H3PO4 network structure, and dema cations were surrounded by this CP network. The synthesized compound 1 is a glassy solid exhibiting sufficient mechanical strength to form a membrane. The amorphous structure was characterized by XAFS and PDF, which indicates the formation of a coordination network. The three-dimensional coordination polymer structure was proposed by RMC modeling, which was in good agreement with the experimental data. The formation of the extended networks prohibits the movement of both cations (dema) and anions (H2PO4−). This coordination driven structure prevents formation of hydrogen bonds, and the glassy solid 1 shows higher proton conductivity (13.3 mS cm−1) than liquid 2 at 120 °C. Consequently, we observed exclusive proton conduction in the structure. This is confirmed by the high transport number (0.94) and OCV (0.96 V). These proton-conducting and viscoelastic properties of 1 demonstrated the highest performance in a H2/O2 fuel cell (0.15 W cm−2) among the reported CP/MOF conductors. The characteristic proton-conducting properties and structural analyses indicate that the coordination network formation from protic ionic liquids is promising for the modification of mechanical properties and proton conductivity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society of the Promotion of Science (JSPS) for a Grant-in-Aid for Scientific Research (B) (JP18H02032) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and Strategic International Collaborative Research Program (SICORP) and Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) from the Japan Science and Technology, Japan. The synchrotron radiation experiments were performed using the BL14B2, BL02B2, and BL04B2 beamlines at SPring-8 with the approval of JASRI (Proposal No. 2018A1753, 2018B1311, and 2019A1292). We acknowledge Dr Naoki Ogiwara for the XAFS measurements.Notes and references
- Q. Li, R. He, J. O. Jensen and N. J. Bjerrum, Chem. Mater., 2003, 15, 4896–4915 CrossRef CAS.
- H. Zhang and P. K. Shen, Chem. Rev., 2012, 112, 2780–2832 CrossRef CAS PubMed.
- A. Chandan, M. Hattenberger, A. El-Kharouf, S. F. Du, A. Dhir, V. Self, B. G. Pollet, A. Ingram and W. Bujalski, J. Power Sources, 2013, 231, 264–278 CrossRef CAS.
- C. Wieser, Fuel Cells, 2004, 4, 245–250 CrossRef CAS.
- J. Escorihuela, R. Narducci, V. Compañ and F. Costantino, Adv. Mater. Interfaces, 2019, 6, 1801146 Search PubMed.
- S. S. Bao, G. K. H. Shimizu and L. M. Zheng, Coord. Chem. Rev., 2019, 378, 577–594 CrossRef CAS.
- H. S. Sasmal, H. B. Aiyappa, S. N. Bhange, S. Karak, A. Halder, S. Kurungot and R. Banerjee, Angew. Chem., Int. Ed., 2018, 57, 10894–10898 CrossRef CAS PubMed.
- D. Umeyama, S. Horike, M. Inukai, T. Itakura and S. Kitagawa, J. Am. Chem. Soc., 2015, 137, 864–870 CrossRef CAS PubMed.
- T. D. Bennett, Y. Yue, P. Li, A. Qiao, H. Tao, N. G. Greaves, T. Richards, G. I. Lampronti, S. A. Redfern, F. Blanc, O. K. Farha, J. T. Hupp, A. K. Cheetham and D. A. Keen, J. Am. Chem. Soc., 2016, 138, 3484–3492 CrossRef CAS PubMed.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- A. Qiao, T. D. Bennett, H. Tao, A. Krajnc, G. Mali, C. M. Doherty, A. W. Thornton, J. C. Mauro, G. N. Greaves and Y. Yue, Sci. Adv., 2018, 4, eaao6827 CrossRef PubMed.
- S. Horike, S. S. Nagarkar, T. Ogawa and S. Kitagawa, Angew. Chem., Int. Ed., 2020, 59, 6652–6664 CrossRef CAS.
- L. Frentzel-Beyme, M. Kloss, P. Kolodzeiski, R. Pallach and S. Henke, J. Am. Chem. Soc., 2019, 141, 12362–12371 CrossRef CAS PubMed.
- P. Ramaswamy, N. E. Wong and G. K. Shimizu, Chem. Soc. Rev., 2014, 43, 5913–5932 RSC.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2015, 115, 11379–11448 CrossRef CAS PubMed.
- H. Nakamoto and M. Watanabe, Chem. Commun., 2007, 2539–2541, 10.1039/b618953a.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- J. P. Belieres and C. A. Angell, J. Phys. Chem. B, 2007, 111, 4926–4937 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764–9773 CrossRef CAS.
- T. Yasuda and M. Watanabe, MRS Bull., 2013, 38, 560–566 CrossRef CAS.
- M. Watanabe, M. L. Thomas, S. Zhang, K. Ueno, T. Yasuda and K. Dokko, Chem. Rev., 2017, 117, 7190–7239 CrossRef CAS PubMed.
- R. E. Rosli, A. B. Sulong, W. R. W. Daud, M. A. Zullzifley, T. Husaini, M. I. Rosli, E. H. Majlan and M. A. Haque, Int. J. Hydrogen Energy, 2017, 42, 9293–9314 CrossRef CAS.
- M. S. Miran, H. Kinoshita, T. Yasuda, M. A. Susan and M. Watanabe, Phys. Chem. Chem. Phys., 2012, 14, 5178–5186 RSC.
- J. W. Wiench, M. Pruski, B. Tischendorf, J. U. Otaigbe and B. C. Sales, J. Non-Cryst. Solids, 2000, 263, 101–110 CrossRef.
- G. Walter, U. Hoppe, J. Vogel, G. Carl and P. Hartmann, J. Non-Cryst. Solids, 2004, 333, 252–262 CrossRef CAS.
- J. E. Shelby, Introduction to Glass Science and Technology, The Royal Society of Chemistry, Cambridge UK, 2nd edn, 2005 Search PubMed.
- Y. Zou, H. Xu, G. Wu, Z. Jiang, S. Chen, Y. Huang, W. Huang and X. Wei, J. Phys. Chem. B, 2009, 113, 2066–2070 CrossRef CAS PubMed.
- P. D'Angelo, A. Zitolo, F. Ceccacci, R. Caminiti and G. Aquilanti, J. Chem. Phys., 2011, 135, 154509 CrossRef.
- F. Sessa, V. Migliorati, A. Serva, A. Lapi, G. Aquilanti, G. Mancini and P. D'Angelo, Phys. Chem. Chem. Phys., 2018, 20, 2662–2675 RSC.
- L. Herschke, V. Enkelmann, I. Lieberwirth and G. Wegner, Chem. Eur. J., 2004, 10, 2795–2803 CrossRef CAS PubMed.
- J. R. M. Evgenij Barsoukov, Impedance Spectroscopy Theory, Experiment, and Applications, John Wiley & Sons, Inc., New Jersey, US, 2nd edn, 2005 Search PubMed.
- C. Brigouleix, M. Anouti, J. Jacquemin, M. Caillon-Caravanier, H. Galiano and D. Lemordant, J. Phys. Chem. B, 2010, 114, 1757–1766 CrossRef CAS.
- G. S. Fulcher, J. Am. Ceram. Soc., 1925, 8, 339–355 CrossRef CAS.
- M. A. Susan, T. Kaneko, A. Noda and M. Watanabe, J. Am. Chem. Soc., 2005, 127, 4976–4983 CrossRef CAS PubMed.
- M. Galiński, A. Lewandowski and I. Stępniak, Electrochim. Acta, 2006, 51, 5567–5580 CrossRef.
- A. Noda, M. A. B. H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- H. Nakamoto, A. Noda, K. Hayamizu, S. Hayashi, H.-o. Hamaguchi and M. Watanabe, J. Phys. Chem. C, 2007, 111, 1541–1548 CrossRef CAS.
- S. Y. Lee, T. Yasuda and M. Watanabe, J. Power Sources, 2010, 195, 5909–5914 CrossRef CAS.
- T. Norby, Solid State Ionics, 1988, 28, 1586–1591 CrossRef.
- U. S. Department of Energy, Fuel Cell Handbook, EG&G Technical Services, Inc., Maryland, U.S., 7th edn, 2016 Search PubMed.
- M. A. Haque, A. B. Sulong, K. S. Loh, E. H. Majlan, T. Husaini and R. E. Rosli, Int. J. Hydrogen Energy, 2017, 42, 9156–9179 CrossRef CAS.
- M. Inukai, S. Horike, T. Itakura, R. Shinozaki, N. Ogiwara, D. Umeyama, S. S. Nagarkar, Y. Nishiyama, M. Malon, A. Hayashi, T. Ohhara, R. Kiyanagi and S. Kitagawa, J. Am. Chem. Soc., 2016, 138, 8505–8511 CrossRef CAS.
- D. Gui, X. Dai, Z. Tao, T. Zheng, X. Wang, M. A. Silver, J. Shu, L. Chen, Y. Wang, T. Zhang, J. Xie, L. Zou, Y. Xia, J. Zhang, J. Zhang, L. Zhao, J. Diwu, R. Zhou, Z. Chai and S. Wang, J. Am. Chem. Soc., 2018, 140, 6146–6155 CrossRef CAS.
- H. B. Aiyappa, S. Saha, P. Wadge, R. Banerjee and S. Kurungot, Chem. Sci., 2015, 6, 603–607 RSC.
- D. B. Shinde, H. B. Aiyappa, M. Bhadra, B. P. Biswal, P. Wadge, S. Kandambeth, B. Garai, T. Kundu, S. Kurungot and R. Banerjee, J. Mater. Chem. A, 2016, 4, 2682–2690 RSC.
- H. Zarrin, D. Higgins, Y. Jun, Z. Chen and M. Fowler, J. Phys. Chem. C, 2011, 115, 20774–20781 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TG, DTA, Nyquist plots, fitting parameters, experimental details. See DOI: 10.1039/d0sc01737j |
| This journal is © The Royal Society of Chemistry 2020 |