C-2 auxiliaries for stereoselective glycosylation based on common additive functional groups†
Received
19th December 2019
, Accepted 16th January 2020
First published on 16th January 2020
Abstract
The stereoselective introduction of the glycosidic bond is one of the main challenges in chemical oligosaccharide synthesis. Stereoselective glycosylation can be achieved using neighbouring group participation of a C-2 auxiliary or using additives, for example. Both methods aim to generate a defined reactive intermediate that reacts in a stereoselective manner with alcohol nucleophiles. This inspired us to develop new C-2 auxiliaries based on commonly used additive functionalities such as ethers, phosphine oxides and tertiary amides. Good 1,2-trans-selectivity was observed for the phosphine oxide and amide-based auxiliaries expanding the toolbox with new auxiliaries for stereoselective glycosylation reactions.
Introduction
Carbohydrates play an essential role in biological systems. A major challenge in relating carbohydrate structure to function is the limited availability of structurally well-defined oligosaccharides and glycoconjugates. Such well-defined oligosaccharides can be obtained by chemical synthesis. The major challenge in oligosaccharide synthesis is the stereoselective introduction of the glycosidic bonds.1,2 Stereoselective glycosylation can be achieved using neighbouring group participation (NGP) of a C-2 auxiliary or using additives, for example. Both methods aim to generate a defined reactive intermediate that reacts in a stereoselective manner with alcohol nucleophiles (Fig. 1A). For example, NGP of C-2 auxiliaries such as esters, picolyl ethers,3 aryl nitriles4 and ethers5 mainly leads to 1,2-trans-glycosides, whilst other C-2 auxiliaries based on esters, thioethers6–11 and selenoethers12 mainly lead to 1,2-cis-glycosides. Alternatively, the use of additives for stereoselective glycosylations requires a non-assisting functionality at C-2.13 A Lewis base additive, such as nitriles,14 ethers,15,16 sulfides,17 phosphine oxides,18–20 iodide based reagents21,22 and (form)amides,19,20,23 is added to stabilize the glycosyl cation and introduce facial selectivity in the subsequent nucleophilic displacement by the glycosyl acceptor. Inspired by both these approaches we set out to develop new C-2 auxiliaries based on recently reported additives. To this end, new C-2 auxiliaries based on linear and cyclic ethers, phosphine oxides, and amide functionalities were prepared (Fig. 1B). Their glycosylation properties were established with a number of glycosyl acceptors. The ether-based auxiliaries showed very modest stereoselectivity whilst the phosphine oxide and amide based auxiliaries lead to the stereoselective formation of 1,2-trans glycosides.
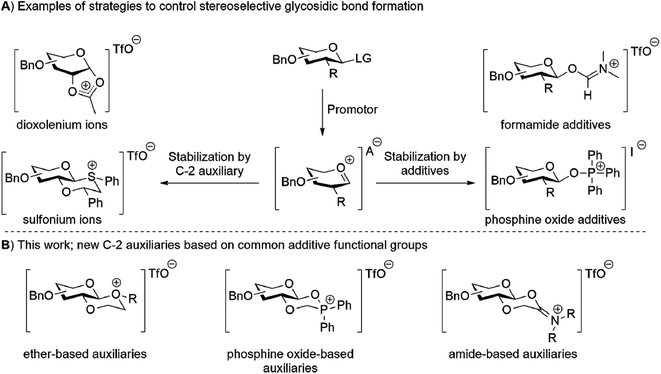 |
| | Fig. 1 (A) The two main strategies for stereoselecitve glycosylation. The oxocarbenium ion is trapped either by a C-2 auxiliary or additive to control the structure of the reactive intermediate. (B) Newly developed C-2 auxiliaries based on common additive functional groups. | |
Results and discussion
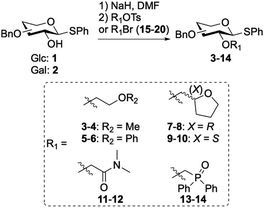
Six auxiliaries based on ethers, phosphine oxide and amides were designed and installed on the C-2 position of glucose and galactose leading to a set of 12 glycosyl donors (3–14, Table 1). All glycosyl donors were prepared starting from benzyl protected D-glucal and D-galactal.11 Oxidation of the glycal led to the corresponding α-1,2-anhydro sugar24 which was reacted with sodium thiophenolate to yield corresponding β-thioglycosides 1 and 2 in moderate yields (55–65%).25 The C-2 auxiliary was introduced using sodium hydride and subsequent addition of the appropriate bromide or tosylate (15–20) resulted in moderate to good yields of coupled products 3–14 (54–86%, Table 1).
Table 1 Synthesis of glycosyl donors equipped with ether, phosphine oxide and N,N-dimethylamide auxiliaries (3–14)
|
|
| Entry |
Product |
Type |
Yield [%] |
| Reagents and conditions: 1-Bromo-2-methoxyethane (15), (2-bromo-ethoxy)benzene (16), 2-bromo-N,N-dimethylacetamide (17), (bromomethyl)diphenylphosphine oxide (18) or (R)- or (S)-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate (19–20) (4 eq.), NaH (2–4 eq.), DMF, 0 °C to rt, 16 h. |
| 1 |
3
|
Glc |
59 |
| 2 |
4
|
Gal |
73 |
| 3 |
5
|
Glc |
86 |
| 4 |
6
|
Gal |
69 |
| 5 |
7
|
Glc |
54 |
| 6 |
8
|
Gal |
83 |
| 7 |
9
|
Glc |
72 |
| 8 |
10
|
Gal |
66 |
| 9 |
11
|
Glc |
58 |
| 10 |
12
|
Gal |
65 |
| 11 |
13
|
Glc |
45 |
| 12 |
14
|
Gal |
41 |
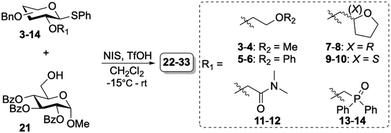
Donors 3–14 were glycosylated with glycosyl acceptor (21) under premix condition using the commonly employed N-iodosuccinimide (NIS) triflic acid (TfOH) promotor system.26 The glycosylation reactions were carried out in CH2Cl2 at −15 °C and the stereoselectivity was determined using NMR analysis of the crude reaction mixtures after work-up. The corresponding purified disaccharides (22–33) were obtained in moderate to excellent yields with α/β-selectivities ranging from non-selective to high β-selectivity (Table 2). Donors containing ether-based auxiliaries (3–6) were unselective and no clear change in selectivity over the different donors was observed (entries 1–4 and 7–10). Chiral auxiliaries have shown to induce more stereoselectivity in glycosylation reactions.8,27 Therefore, better stereoselectivities were expected for chiral THF-based auxiliaries (7–10). Nevertheless, donors 7–10 did not show any stereoselectivity (entries 3–4 and 9–10). In contrast, the N,N-dimethylamide auxiliary proved the be β-selective for glucose and moderately β-selective (α/β = 20/80) for galactose (entries 5 and 11, respectively). This β-selectivity was surprising compared to findings by Mong and coworkers, where DMF as additive induced α-selectivity.23 Similarly, phosphine oxide auxiliaries 13–14 induced high β-selectivity (entries 6 and 12), in contrast to the α-selectivity observed when an additive combination of triphenylphosphine oxide/TMSI was used.19 Generally, the galacto-series were more α-selective compared to the gluco-series counterparts consistent with earlier observed trends.6,11,28
Table 2 Glycosylation results of glycosyl donors 3–14 with glycosyl acceptor 21
|
|
| Entry |
Donor |
Type |
α/βa |
Yieldb [%] |
Product |
|
α/β ratios were determined by NMR spectroscopy29,30 of the crude reaction mixture.
Isolated yields.
|
| 1 |
3
|
Glc |
50/50 |
96 |
22
|
| 2 |
5
|
Glc |
50/50 |
54 |
24
|
| 3 |
7
|
Glc |
37/63 |
85 |
26
|
| 4 |
9
|
Glc |
37/63 |
55 |
28
|
| 5 |
11
|
Glc |
0/100 |
57 |
30
|
| 6 |
13
|
Glc |
11/89 |
67 |
32
|
| 7 |
4
|
Gal |
60/40 |
95 |
23
|
| 8 |
6
|
Gal |
60/40 |
77 |
25
|
| 9 |
8
|
Gal |
45/55 |
81 |
27
|
| 10 |
10
|
Gal |
55/45 |
73 |
29
|
| 11 |
12
|
Gal |
20/80 |
43 |
31
|
| 12 |
14
|
Gal |
17/83 |
68 |
33
|
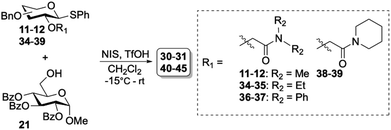
The high β-selectivity observed for glycosyl donor 11, led us to further investigate the amide containing auxiliaries. The amide functionality is more amendable for easy alteration in substitution pattern and holds better prospects for removal compared to the phosphine oxide auxiliaries which also demonstrated good selectivity. Hence, we set out to further optimize the amide auxiliaries starting with exploring the influence of the amide substitution pattern on the stereoselectivity. To this end, we prepared diethyl- (34–35), diphenyl- (36–37) and cyclic amide derivatives (38–39) according to the protocol described in Table 1. These syntheses resulted in moderate to good yields (32–77%) of glycosyl donors 34–39. Glycosylations of 34–39 also showed excellent β-selectivity for the gluco-type donors (Table 3, entries 1–4). For the galacto-type donors, β-selectivity was moderate and increased with the introduction of bulky substituents on the amide (Table 3, entries 5–7). The piperidine derived amide auxiliary gave selectivity comparable to the dimethyl auxiliary (Table 3, entries 8).
Table 3 Glycosylation results using a series of amide-based auxiliaries
|
|
| Entry |
Donor |
Type |
α/βa |
Yieldb [%] |
Product |
|
α/β ratios were determined by NMR spectroscopy29,30 of the crude reaction mixture.
Isolated yields.
|
| 1 |
11
|
Glc |
0/100 |
57 |
30
|
| 2 |
34
|
Glc |
0/100 |
42 |
40
|
| 3 |
36
|
Glc |
0/100 |
86 |
42
|
| 4 |
38
|
Glc |
3/97 |
40 |
44
|
| 5 |
12
|
Gal |
20/80 |
43 |
31
|
| 6 |
35
|
Gal |
17/83 |
44 |
41
|
| 7 |
37
|
Gal |
11/89 |
71 |
43
|
| 8 |
39
|
Gal |
20/80 |
42 |
45
|
In addition to the amide substituents we investigated the glycosyl acceptor scope using a broader range of glycosyl acceptors for both gluco- and galacto-type glycosyl donors containing the dimethylamide auxiliary. Glycosylation with primary alcohols (21, 46 and 47) resulted moderate to good yield (39% to 61%) with excellent β-selectivity for gluco-type donors (Table 4, entries 1–3). However, secondary and tertiary alcohols gave poorer to no selectivity (α/β = 33/67 and 50/50 respectively) and resulted in moderate yields (Table 4, entries 4 and 5). Glycosylations with galacto-type donors were less β-selective compared to their gluco-type counterparts (Table 4). These glycosylations resulted in low to moderate yield (22 to 56%) and moderate to non-selective for the β-anomer (α/β = 20/80 to 50/50). Again, glycosylation with secondary and tertiary alcohols gave poorer selectivity compared to primary alcohols (Table 4, entries 6–10).
Table 4 Glycosylation results for glycosyl donors 11 and 12 using a broader range of glycosyl acceptors
|
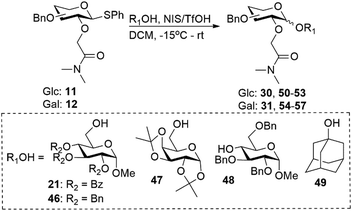
|
| Entry |
Donor type |
R1OH |
α/βa |
Yieldb [%] |
Product |
|
α/β ratios were determined by NMR spectroscopy29,30 of the crude reaction mixture.
Isolated yields.
α/β ratios were determined by NMR spectroscopy after removal of the acceptor residues by silica gel flash column chromatography (30 to 80% EtOAc in n-heptane).
|
| 1 |
Glc |
21
|
0/100 |
57 |
30
|
| 2 |
Glc |
46
|
0/100c |
39 |
50
|
| 3 |
Glc |
47
|
10/90 |
61 |
51
|
| 4 |
Glc |
48
|
33/67 |
30 |
52
|
| 5 |
Glc |
49
|
50/50 |
34 |
53
|
| 6 |
Gal |
21
|
0/100 |
43 |
31
|
| 7 |
Gal |
46
|
25/75c |
40 |
54
|
| 8 |
Gal |
47
|
20/80 |
56 |
55
|
| 9 |
Gal |
48
|
50/50 |
22 |
56
|
| 10 |
Gal |
49
|
40/60 |
28 |
57
|
To identify reaction intermediates for gluco-type donors 11 and 13, variable temperature (VT) NMR experiments were performed. Glycosyl donor 11 was reacted with Ph2SO and Tf2O in presence of TTBP at −80 °C in CD2Cl2 (Fig. 2). In a control experiment using glycosyl donor 11 and this promotor system (Tf2O, Ph2SO) no significant changes in yields or selectivity were observed indicating that the promotor system does not influence the glycosylation outcome. Directly after the addition of Tf2O the α-iminium intermediate (11α) was formed (Fig. 2b–f). In addition, a small amount of β-iminium ion (11β) was observed (α/β = 5/1). The ratio of intermediate 11α and 11β did not change even upon heating to room temperature and remained stable at this temperature (Fig. 2d).
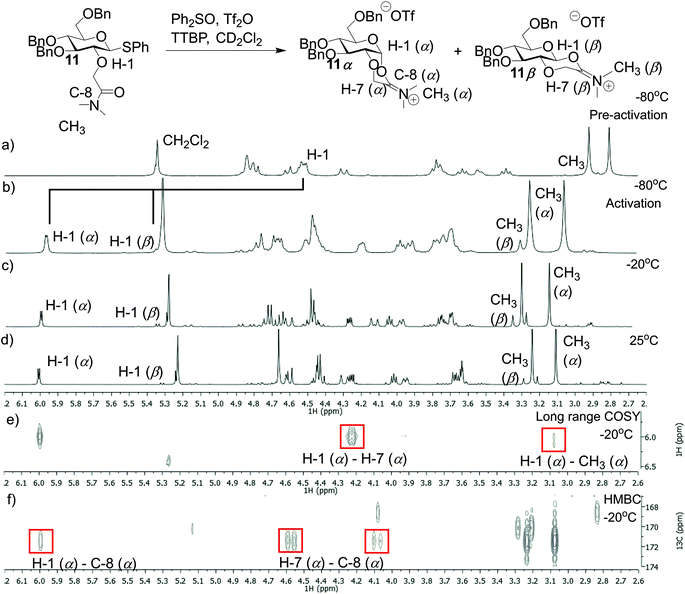 |
| | Fig. 2 VT-NMR spectra of the iminium ion derived from 11 (a) 1H NMR spectrum of 11 at −80 °C in CD2Cl2. (b) 1H NMR of 11 at −78 °C after the addition of Tf2O. (c) 1H NMR of 11 at −20 °C. (d) 1H NMR at 25 °C. (e) Long range COSY at −20 °C. (f) 1H/13C HMBC at −20 °C. | |
Additionally, we performed VT NMR experiments under the same conditions for glycosyl donor 13 starting at −20 °C (Fig. 3a and c). After addition of Tf2O, the expected α- (13α) and β-phosphonium ions (13β) were observed in a α/β-ratio of 5/2 (Fig. 3). Both reaction intermediates were stable at 10 °C and decomposed slowly at room temperature. Again, the ratio of 13α and 13β did not change upon heating. Based on these VT NMR results, the α-intermediates predominate for both the amide and phosphine oxide auxiliaries and may be reactive intermediates with strong nucleophiles. However, the erosion of stereoselectivity when glycosylating with secondary and tertiary alcohols suggests that other reactive intermediates such as the oxocarbenium ion may be responsible for disaccharide formation in these cases.
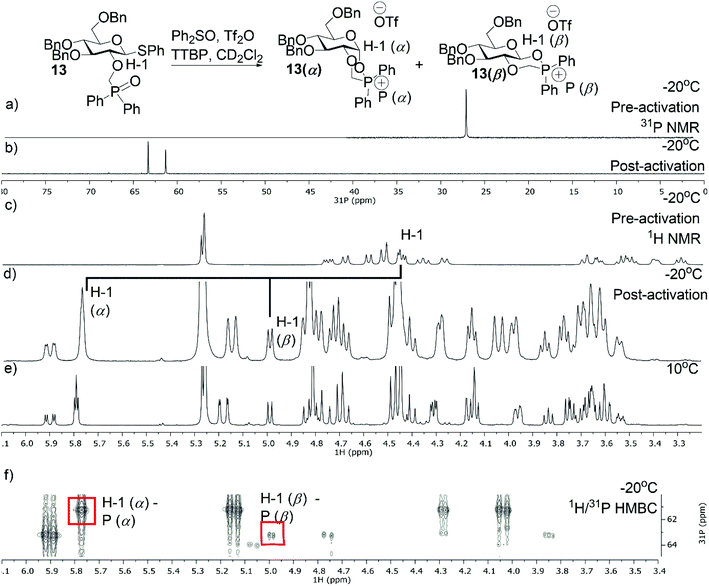 |
| | Fig. 3 VT-NMR spectra of the phosphonium ion derived from 13 (a) 31P NMR spectrum of 13 at −20 °C in CD2Cl2. (b) 31P NMR of 13 at −20 °C after the addition of Tf2O. (c) 1H NMR spectrum of 13 at −20 °C in CD2Cl2. (d) 1H NMR of 13 at −20 °C after the addition of Tf2O. (e) 1H NMR of 13 at 10 °C. (f) 1H/31P HMBC at −20 °C. | |
Finally, we explored the removal of amide auxiliaries. Using disaccharide 50 as a model substrate, the tertiary amide was hydrolysed to yield the carboxylic acid.31,32 After a simple workup the carboxylic acid was converted to the acyl azide, heated to form the isocyanate and reacted with t-BuOH at 100 °C to yield the Boc-protected amine. After a short workup the Boc group was removed and the amine was subsequently treated with 1.0 M NaOH in which it was eliminated to yield the unprotected 2-OH (58) in 50% overall yield from 50 (Scheme 1).33
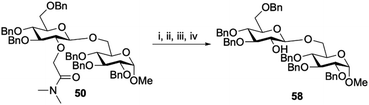 |
| | Scheme 1 Removal of the auxiliary. Reagents and conditions (i) H2O (2.2 eq.), t-BuOK (5.9 eq.), THF, rt; (ii) DIPEA (1.2 eq.), DPPA (1.1 eq.), DMF, 0 °C – rt; (iii) t-BuOH, DMF, 100 °C; (iv) NaOH, THF, EtOH, 60 °C. | |
In conclusion, we prepared a series glycosyl donor containing new C-2 auxiliaries based on commonly used additives for stereoselective glycosylation. The ether based auxiliaries gave rather unselective glycosylation reactions whilst tertiary amides and our phosphine oxide based auxiliaries showed good to absolute β-selectivity with reactive glycosyl acceptors. VT-NMR experiments confirmed the formation of reaction intermediates resulting from NGP of the C-2 auxiliaries. Potentially, chiral auxiliaries based on these functional groups can be developed to obtain even better stereoselectivity.
Experimental section
General conditions
1H and 13C NMR spectra were recorded on a Bruker 400 or 500 MHz spectrometer. Chemical shifts are reported in parts per million (ppm) relative to tetramethylsilane (TMS) or residual solvents as the internal standard. NMR data is presented as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, dd = doublet of doublets, m = multiplet and/or multiple resonances), coupling constant (J) in hertz (Hz), integration. All NMR signals were assigned on the basis of 1H NMR, 13C NMR, 31P NMR, COSY, HSQC, and TOCSY experiments. NMR data is presented for the major anomer. Mass spectra were recorded on an JEOL AccuTOF CS JMS-T100CS mass spectrometer. Automatic flash column chromatography was performed using Biotage Isolera Spektra One, using SNAP cartridges (Biotage, 30–100 μm, 60 Å), 4–50 g. TLC analysis was conducted on silica gel F254 (Merck KGaA) with detection by UV absorption (254 nm) where applicable; by spraying with 10% sulfuric acid in methanol followed by charring at ≈300 °C or by spraying with KMnO4 stain consisting of (0.06 M KMnO4, 0.5 M K2CO3 and 0.02 M NaOH in water) after gently heating of the plate. DCM, THF, and toluene were freshly distilled. Molecular sieves (4 Å) were flame-activated under a vacuum prior to use. All inert reactions were carried out under an argon atmosphere using flame-dried flasks.
General procedure A
5.0 grams (0.035 mol, 1.0 eq.) D-glucal or D-galactal was dissolved in dry DMF (170 mL). 7.0 grams (0.18 mol, 5.0 eq.) NaH (60% dispersion in paraffin oil) was added on ice. The reaction was stirred for 15 minutes and 21 mL (0.18 mol, 5.0 eq.) benzyl bromide was added. The reaction was stirred at room temperature until TLC indicated full consumption of starting material (16 hours). The reaction was quenched with methanol and the solution was concentrated in vacuo. The resulting oil was taken up in ethyl acetate and washed with water (3 × 100 mL) and brine (1 × 100 mL). The organic layer was dried over MgSO4 (anhydrous), filtrated and evaporated in vacuo to result the crude product. The benzylated products were obtained by purification of the crude product through silica gel flash column chromatography.
General procedure B
To a cooled (0 °C) solution of 4.0 grams (9.6 mmol, 1.0 eq.) benzylated glycal in DCM (40 mL) were added acetone (4 mL) and saturated aqueous NaHCO3 (68 mL). The mixture was stirred vigorously, and a solution of oxone (19.2 mmol, 2 eq.) in H2O (24 mL) was added dropwise over 15 min. The mixture was stirred vigorously at 0 °C for 30 min and then at rt until TLC indicated consumption of the starting material. The organic phase was separated, and the aqueous phase was extracted with DCM (2 × 40 mL). The combined organic phases were dried over Na2SO4 and concentrated in vacuo. The crude mixture was dissolved in dry THF (80 mL). The solution was cooled to −78 °C. MS (4 Å) and 2.2 grams (15 mmol, 1.6 eq.) sodium thiophenolate (90%) were added under an inert atmosphere. 1.0 mL (0.096 mmol, 0.1 eq.) ZnCl2 (1.0 M in Et2O) was added and the mixture was stirred for 3 days allowing it to warm up to room temperature. An aqueous solution of 1.0 M NaOH (80 mL) was added to quench the reaction. The mixture was filtrated and the organic layer was separated from the aqueous layer. The organic layer was washed with brine (80 mL), dried over Na2SO4 (anhydrous), filtrated and evaporated in vacuo. The crude product was purified with silica gel flash column chromatography to obtain the pure product. If the product was strongly coloured, the product was dissolved in DCM, norrit was added, filtrated over Celite and evaporated in vacuo to yield the product.
General procedure C
An anomeric thioether with free 2-OH (1 or 2, 1.0 eq.) was dissolved in dry DMF (0.1 M) under an inert atmosphere at 0 °C. 60% NaH dispersion in paraffin oil was added (2.0 eq. for the preparation of 11–14 and 34–39, 4.0 eq. for 3–10). After stirring for 15 minutes, ice was removed and the corresponding bromide or tosylate (4.0 eq.) was added. The reaction was stirred for overnight after which the reaction was quenched with methanol and the solvent was evaporated in vacuo. The donors were obtained by purification through silica gel flash column chromatography.
General procedure D
The corresponding glycosyl donor (1.0 eq.) and the corresponding glycosyl acceptor (2.0 eq.) were dissolved in dry DCM (0.02 M and 0.04 M respectively). MS (4 Å) were added and the mixture was cooled to −15 °C. NIS (1.1 eq.) was added followed by the addition of a catalytic amount TfOH (0.1 eq.). The reaction was stirred for 2 h allowing it to slowly reach room temperature. The reaction was quenched with TEA and taken up in EtOAc (20 mL). The solution was filtrated, washed with aqueous thiosulfate solution (10%, 20 mL) and washed with brine (20 mL). The organic layer was dried over MgSO4 (anhydrous), filtrated and the solvent was evaporated in vacuo to yield the crude product. The crude product was dissolved in CDCl3 and analysed by quantitative HSQC to determine the selectivity. After analysis the crude product was purified by flash column chromatography to obtain the product.
VT-NMR studies procedure
Glycosyl donor (15 mg, 1.0 eq.), Ph2SO (1.2 eq.) and TTBP (2.5 eq.) were dissolved in DCM-d2 (1 mL) under inert atmosphere. MS (4 Å) were added and the solution was stirred for 1.5 h. In a second vial under inert atmosphere was stirred 1.0 mL of DCM-d2 for 1.5 h over molecular sieves (4 Å). The donor solution was transferred to an NMR tube and was analysed at low temperature by NMR. To the DCM-d2 was added Tf2O (1.1 eq.) and the solution was added at −78 °C to the tube. The tube was quickly shaken and transferred to the NMR. The reaction was first analysed by NMR at low temperature and was measured at increasing temperatures.
3,4,6-Tri-O-benzyl-D-glucal.
Using general procedure A starting from D-glucal (5.14 g, 35.2 mmol), the crude product was purified by silica gel flash column chromatography (0% to 30% EtOAc in n-heptane), affording 3,4,6-tri-O-benzyl-D-glucal (14.1 g, 98%) as white solid. TLC: Rf = 0.61 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.50–7.13 (m, 15H), 6.42 (dd, J = 6.1, 1.4 Hz, 1H, H-1), 4.87 (dd, J = 6.2, 2.7 Hz, 1H, H-2), 4.83 (d, J = 11.3 Hz, 1H), 4.66–4.62 (m, 2H), 4.61–4.54 (m, 3H), 4.21 (ddd, J = 6.1, 2.7, 1.4 Hz, 1H, H-3), 4.06 (ddd, J = 8.3, 5.1, 2.8 Hz, 1H, H-5), 3.86 (dd, J = 8.7, 6.2 Hz, 1H, H-4), 3.83–3.74 (m, 2H, H-6). 13C NMR (126 MHz, CDCl3): δC 144.9 (CH, C-1), 138.5, 138.3, 138.2 (all quaternary), 128.6, 128.5, 128.5, 128.1, 127.9, 127.9, 127.8 (all aromatic), 100.1 (CH, C-2), 77.0 (CH, C-5) 75.9 (CH, C-3), 74.6 (CH, C-4), 73.9 (CH2), 73.7 (CH2), 70.6 (CH2), 68.7 (CH2, C-6). HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C27H28O4: 439.18853, found [M + Na]+: 439.18934.
3,4,6-Tri-O-benzyl-D-galactal.
Using general procedure A starting from D-galactal (5.02 g, 35.0 mmol), the crude product was purified by silica gel flash column chromatography (0% to 30% EtOAc in n-heptane), affording 3,4,6-tri-O-benzyl-D-galactal (13.6 g, 96%) as white solid. TLC:Rf = 0.57 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.36–7.23 (m, 15H), 6.36 (dd, J = 6.2, 1.5 Hz, 1H, H-1), 4.90–4.83 (m, 2H, H-2), 4.68–4.59 (m, 3H), 4.50 (d, J = 11.9 Hz, 1H), 4.42 (d, J = 11.9 Hz, 1H), 4.19 (tdd, J = 5.2, 2.6, 1.2 Hz, 2H, H-3, H-5), 3.95 (ddd, J = 4.0, 2.5, 1.3 Hz, 1H, H-4), 3.78 (dd, J = 10.2, 7.2 Hz, 1H, H-6), 3.65 (dd, J = 10.1, 5.1 Hz, 1H, H-6). 13C NMR (126 MHz, CDCl3): δC 144.3 (CH, C-1), 138.7, 138.5, 138.2 (all quaternary), 128.6, 128.5, 128.3, 128.0, 127.8, 127.7, 127.6 (all aromatic), 100.1 (CH, C-2), 75.8 (CH, C-5), 73.6 (CH2), 73.5 (CH2), 71.4 (CH, C-4), 71.0 (CH, C-3), 70.9 (CH2), 68.6 (CH2, C-6). HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C27H28O4: 439.18853, found [M + Na]+: 439.18909.
3,4,6-Tri-O-benzyl-1-thio-β-D-glucopyranoside (1).
Using general procedure B starting from 1 (4.0 gram, 9.6 mmol), the crude product was purified by silica gel flash column chromatography (0% to 15% EtOAc in n-heptane), affording 1 (2.9 g, 55%) as yellow/white solid. TLC:Rf = 0.51 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3) δH 7.63–7.54 (m, 2H), 7.38–7.14 (m, 18H), 4.90 (d, J = 11.2 Hz, 1H), 4.86–4.81 (m, 2H), 4.64–4.53 (m, 3H), 4.50 (d, J = 9.7 Hz, 1H, H-1), 3.79 (dd, J = 11.0, 1.9 Hz, 1H, H-6), 3.74 (dd, J = 11.0, 4.4 Hz, 1H, H-6), 3.63–3.57 (m, 2H, H-3, H-4), 3.56–3.46 (m, 2H, H-2, H-5), 2.39 (d, J = 2.1 Hz, 1H). 13C NMR (126 MHz, CDCl3) δC 138.6, 138.4, 138.2 (all quaternary), 133.1 (aromatic), 131.9 (quaternary), 129.1, 128.7, 128.6, 128.5, 128.2, 128.1, 128.1, 128.0, 127.8, 127.7 (all aromatic), 88.2 (CH, C-1), 86.1 (CH, C-3), 79.6 (CH, C-5), 77.5 (CH, C-4), 75.5 (CH2), 75.2 (CH2), 73.6 (CH2), 72.7 (CH, C-2), 69.2 (CH2 C-6). HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C33H34O5S: 565.20246, found [M + Na]+: 565.20159.
3,4,6-Tri-O-benzyl-1-thio-β-D-galactopyranoside (2).
Using general procedure B starting from 2 (4.0 gram, 9.6 mmol), the crude product was purified by silica gel flash column chromatography (0% to 5% Et2O in toluene), affording 2 (3.4 g, 65%) as yellow/white solid. TLC:Rf = 0.31 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.62–7.50 (m, 2H), 7.42–7.14 (m, 18H), 4.89 (d, J = 11.4 Hz, 1H), 4.76–4.65 (m, 2H), 4.57 (d, J = 11.5 Hz, 1H), 4.53 (d, J = 9.6 Hz, 1H, H-1), 4.51–4.42 (m, 2H), 4.06–3.93 (m, 2H, H-2, H-4), 3.67–3.75 (m, 3H, H-5, H-6), 3.47 (dd, J = 9.2, 2.7 Hz, 1H, H-3), 2.43 (d, J = 2.1 Hz, 1H, OH) ppm; 13C NMR (126 MHz, CDCl3): δC 138.8, 138.1, 138.0, 132.7 (all quaternary), 132.3, 129.0, 128.7, 128.6, 128.3, 128.1, 128.0, 128.0, 127.9, 127.8, 127.7, 127.6 (all aromatic), 88.7 (CH, C-1), 83.4 (CH, C-3), 77.8 (CH, C-5), 74.6, 73.7 (both CH2), 73.4 (CH, C-4), 72.6 (CH2), 69.2 (CH, C-2), 68.8 (CH2, C-6) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C33H34O5S: 565.20246, found [M + Na]+: 565.20265.
Phenyl 3,4,6-tri-O-benzyl-2-O-(2-methoxyethoxy)-1-thio-β-D-glucopyranoside (3).
Using general procedure C starting from 1 (101 mg, 0.186 mmol) and 1-bromo-2-methoxyethane (15), the crude product was purified by silica gel flash column chromatography (0% to 10% EtOAc in n-heptane), affording 3 (65 mg, 59%) as white oil. TLC:Rf = 0.54 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3) δH 7.58–7.55 (m, 2H), 7.38–7.17 (m, 18H), 4.98 (d, J = 10.7 Hz, 1H), 4.82 (d, J = 10.9 Hz, 2H), 4.63 (d, J = 9.8 Hz, 1H, H-1), 4.60–4.55 (m, 2H), 4.52 (d, J = 12.0 Hz, 1H), 4.01 (ddd, J = 10.3, 5.6, 3.4 Hz, 1H, H-6), 3.87 (ddd, J = 10.1, 6.4, 3.5 Hz, 1H, H-6), 3.76 (dd, J = 10.8, 2.0 Hz, 1H), 3.72–3.65 (m, 2H, H-3), 3.62–3.51 (m, 3H, H-5), 3.47 (ddd, J = 9.7, 4.8, 2.0 Hz, 1H, H-4), 3.36 (s, 3H), 3.34 (t, J = 9.0 Hz, 1H, H-2) ppm. 13C NMR (126 MHz, CDCl3) δC 138.7, 138.4, 138.2, 134.0 (all quaternary), 132.0, 129.0, 128.6, 128.6, 128.5, 128.1, 128.1, 127.9, 127.8, 127.8, 127.7, 127.5 (all aromatic), 87.5 (CH, C-1), 86.7 (CH, C-3), 81.9 (CH, C-2), 79.2 (CH, C-4), 77.8 (CH, C-5), 75.9 (CH2), 75.2 (CH2), 73.5 (CH2), 72.5 (CH2), 72.2 (CH2), 69.2 (CH2), 59.1 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C36H40O6S: 623.24433, found [M + Na]+: 623.24404.
Phenyl 3,4,6-tri-O-benzyl-2-O-(2-methoxyethoxy)-1-thio-β-D-galactopyranoside (4).
Using general procedure C starting from 2 (100 mg, 0.184 mmol) and 1-bromo-2-methoxyethane (15), the crude product was purified by silica gel flash column chromatography (0% to 15% EtOAc in n-heptane), affording 4 (81 mg, 73%) as white solid. TLC:Rf = 0.40 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.56–7.50 (m, 2H), 7.41–7.22 (m, 16H), 7.21–7.13 (m, 3H), 4.93 (d, J = 11.6 Hz, 1H), 4.81–4.69 (m, 2H), 4.60 (d, J = 9.6 Hz, 1H, H-1), 4.57 (d, J = 11.6 Hz, 1H), 4.48–4.36 (m, 2H), 3.94–3.85 (m, 3H, H-5, H-6), 3.75 (t, J = 9.4 Hz, 1H, H-2), 3.67–3.48 (m, 6H, H-3, H-4, H-6), 3.35 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 138.9, 138.6, 138.0, 134.4 (all quaternary), 131.6, 128.9, 128.5, 128.5, 128.3, 128.0, 128.0, 127.9, 127.8, 127.8, 127.6, 127.1 (all aromatic), 87.8 (CH, C-1), 84.0 (CH, C-3), 78.5 (CH, C-2), 77.4 (CH, C-4), 74.6 (CH, C-5), 73.9, 73.7, 73.0, 72.6 (all CH2), 72.3 (CH2, C-6), 68.9 (CH2), 59.0 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C36H40O6S: 623.24433, found [M + Na]+: 623.24509.
Phenyl 3,4,6-tri-O-benzyl-2-O-(2-phenoxyethoxy)-1-thio-β-D-glucopyranoside (5).
Using general procedure C starting from 1 (80 mg, 0.15 mmol) and (2-bromoethoxy)benzene (16), the crude product was purified by silica gel flash column chromatography (0% to 10% EtOAc in n-heptane), affording 5 (84 mg, 86%) as white solid. TLC:Rf = 0.55 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.57–7.53 (m, 2H), 6.92 (tt, J = 7.5, 1.1 Hz, 1H), 6.87–6.83 (m, 2H), 5.02 (d, J = 10.7 Hz, 1H), 4.84 (dd, J = 10.8, 3.9 Hz, 2H), 4.62 (d, J = 9.8 Hz, 1H, H-1), 4.60–4.57 (m, 2H), 4.52 (d, J = 12.0 Hz, 1H), 4.22–4.14 (m, 2H), 4.13–4.06 (m, 2H), 3.77 (dd, J = 10.9, 2.0 Hz, 1H, H-6), 3.73–3.66 (m, 2H, H-3, H-6), 3.61 (t, J = 9.4 Hz, 1H, H-4), 3.49 (ddd, J = 9.6, 4.8, 1.9 Hz, 1H, H-5), 3.39 (dd, J = 9.8, 8.6 Hz, 1H, H-2) ppm. 13C NMR (126 MHz, CDCl3): δC 158.9, 138.6, 138.4, 138.2, 134.0 (all quaternary), 132.0, 129.5, 129.0, 128.6, 128.6, 128.5, 128.2, 128.1, 127.9, 127.9, 127.8, 127.7, 127.6, 120.9, 114.7 (all aromatic), 87.6 (CH, C-1), 86.6 (CH, C-3), 81.8 (CH, C-2), 79.2 (CH, C-5), 77.8 (CH, C-4), 76.0 (CH2), 75.2 (CH2), 73.6 (CH2), 71.8 (CH2), 69.2 (CH2, C-6), 67.4 (CH2) ppm. HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C41H42O6S: 685.25998, found [M + Na]+: 685.25843.
Phenyl 3,4,6-tri-O-benzyl-2-O-(2-phenoxyethoxy)-1-thio-β-D-galactopyranoside (6).
Using general procedure C starting from 2 (80 mg, 0.15 mmol) and (2-bromoethoxy)benzene (16), the crude product was purified by silica gel flash column chromatography (0% to 10% EtOAc in n-heptane), affording 6 (84 mg, 69%) as white solid. TLC:Rf = 0.56 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.55–7.50 (m, 2H), 7.38–7.34 (m, 2H), 7.33–7.25 (m, 13H), 7.25–7.20 (m, 2H), 7.19–7.14 (m, 3H), 6.92 (tt, J = 7.3, 1.1 Hz, 1H), 6.88–6.83 (m, 2H), 4.94 (d, J = 11.5 Hz, 1H), 4.81–4.70 (m, 2H), 4.60 (d, J = 9.6 Hz, 1H, H-1), 4.58 (d, J = 11.5 Hz, 1H), 4.48–4.38 (m, 2H), 4.16–4.05 (m, 4H), 3.94 (dd, J = 2.9, 0.8 Hz, 1H, H-4), 3.81 (t, J = 9.4 Hz, 1H, H-2), 3.67–3.58 (m, 3H, H-5, H-6), 3.56 (dd, J = 9.3, 2.7 Hz, 1H, H-3) ppm; 13C NMR (126 MHz, CDCl3): δC 159.0, 138.9, 138.5, 138.0, 134.3 (all quaternary), 131.6, 129.5, 128.9, 128.6, 128.6, 128.3, 128.0, 128.0, 127.9, 127.9, 127.8, 127.6, 127.2, 120.8, 114.7 (all aromatic), 87.9 (CH, C-1), 83.9 (CH, C-3), 78.4 (CH, C-2), 77.5 (CH, C-5), 74.6 (CH2), 73.9 (CH, C-4), 73.7, 73.1, 71.9 (all CH2), 68.9 (CH2, C-6), 67.5 (CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C41H42O6S: 685.25998, found [M + Na]+: 685.25861.
Phenyl 3,4,6-tri-O-benzyl-2-O-(((R)-tetrahydrofuran-2-yl)methoxy)-1-thio-β-D-glucopyranoside (7).
Using general procedure C starting from 1 (99 mg, 0.18 mmol) and (R)-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate (19), the crude product was purified by silica gel flash column chromatography (0% to 5% Et2O in toluene), affording (7) (63 mg, 54%) as colourless oil. TLC:Rf = 0.30 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.49–7.41 (m, 2H), 7.28–7.07 (m, 18H), 4.93 (d, J = 10.7 Hz, 1H), 4.72 (dd, J = 10.8, 2.4 Hz, 2H), 4.51 (d, J = 9.8 Hz, 1H, H-1), 4.49–4.45 (m, 2H), 4.41 (d, J = 11.9 Hz, 1H), 4.06–3.98 (m, 1H), 3.74 (dt, J = 8.4, 6.6 Hz, 1H), 3.68–3.63 (m, 4H, H-6), 3.61–3.55 (m, 2H, H-3, H-6), 3.49 (t, J = 9.4 Hz, 1H, H-4), 3.37 (ddd, J = 9.8, 4.9, 1.9 Hz, 1H, H-5), 3.23 (dd, J = 9.8, 8.7 Hz, 1H, H-2), 1.86 (dtd, J = 12.4, 7.1, 5.9 Hz, 1H), 1.79–1.72 (m, 2H), 1.53–1.46 (m, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 138.7, 138.4, 138.2, 134.1 (all quaternary), 132.0, 129.0, 128.5, 128.5, 128.4, 128.1, 128.0, 127.9, 127.8, 127.7, 127.6, 127.5 (all aromatic), 87.6 (CH, C-1), 86.7 (CH, C-3), 81.7 (CH, C-2), 79.2 (CH, C-5), 78.0 (CH, C-4), 77.8 (CH), 76.2, 75.8, 75.2, 73.5 (all CH2), 69.2 (CH2, C-6), 68.3, 28.3, 25.6 (all CH2) ppm. HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C38H42O6S: 649.25998, found [M + Na]+: 649.25857.
Phenyl 3,4,6-tri-O-benzyl-2-O-(((R)-tetrahydrofuran-2-yl)methoxy)-1-thio-β-D-galactopyranoside (8).
Using general procedure C starting from 2 (97 mg, 0.18 mmol) and (R)-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate (19), the crude product was purified by silica gel flash column chromatography (0% to 5% Et2O in toluene), affording (8) (95 mg, 83%) as white solid. TLC:Rf = 0.18 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.56–7.50 (m, 2H), 7.41–7.36 (m, 2H), 7.35–7.23 (m, 13H), 7.20–7.15 (m, 3H), 4.93 (d, J = 11.5 Hz, 1H), 4.81 (d, J = 11.6 Hz, 1H), 4.72 (d, J = 11.6 Hz, 1H), 4.61–4.54 (m, 2H, H-1), 4.47–4.37 (m, 2H), 4.11 (qd, J = 7.0, 4.4 Hz, 1H, H-5), 3.93 (d, J = 2.8 Hz, 1H), 3.86–3.70 (m, 4H, H-2, H-6), 3.67–3.53 (m, 5H, H-3, H-4), 1.97–1.87 (m, 1H), 1.87–1.79 (m, 2H), 1.61–1.57 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3) δC 138.0, 138.6, 138.0, 134.4 (all quaternary), 131.6, 128.9, 128.5, 128.5, 128.3, 128.0, 128.0, 127.9, 127.7, 127.7, 127.6, 127.1 (all aromatic), 87.9 (CH, C-1), 84.2 (CH, C-4), 78.2 (CH, C-2), 78.0 (CH, C-5), 77.4 (CH, C-3), 76.3, 74.5 (both CH2), 73.7 (CH), 73.0, 67.0 (both CH2), 68.3 (CH2, C-6), 29.8, 28.2, 25.6 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C38H42O6S: 649.25998, found [M + Na]+: 649.25849.
Phenyl 3,4,6-tri-O-benzyl-2-O-(((S)-tetrahydrofuran-2-yl)methoxy)-1-thio-β-D-glucopyranoside (9).
Using general procedure C starting from 1 (85 mg, 0.16 mmol) and (S)-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate (20), the crude product was purified by silica gel flash column chromatography (0% to 5% Et2O in toluene), affording 9 (71 mg, 72%) as colourless oil. TLC:Rf = 0.37 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CHCl3): δH 7.60–7.51 (m, 2H), 7.39–7.14 (m, 18H), 4.96 (d, J = 10.7 Hz, 1H), 4.82 (dd, J = 10.8, 7.9 Hz, 2H), 4.62 (d, J = 9.8 Hz, 1H, H-1), 4.60–4.54 (m, 2H), 4.52 (d, J = 12.0 Hz, 1H), 4.06 (qd, J = 6.8, 4.0 Hz, 1H), 3.93–3.84 (m, 2H), 3.75 (ddd, J = 12.7, 8.3, 1.9 Hz, 2H, H-6), 3.72–3.63 (m, 3H, H-3, H-6), 3.59 (t, J = 9.4 Hz, 1H, H-5), 3.48 (ddd, J = 9.6, 4.8, 2.0 Hz, 1H), 3.33 (dd, J = 9.7, 8.6 Hz, 1H, H-2), 1.96–1.75 (m, 3H), 1.66 (ddt, J = 11.9, 8.7, 7.3 Hz, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 138.7, 138.4, 138.2, 134.0 (all quaternary), 132.0, 128.9, 128.5, 128.4, 128.0, 127.9, 127.7, 127.6, 127.5 (all aromatic), 87.5 (CH, C-1), 86.7 (CH, C-3), 81.6 (CH, C-2), 79.1 (CH, C-5), 78.0 (CH), 77.8 (CH, C-4), 77.9, 75.8, 75.1, 73.5 (all CH2), 69.2 (CH2, C-6), 68.4, 28.0, 25.8 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C38H42O6S: 649.25998, found [M + Na]+: 649.25998.
Phenyl 3,4,6-tri-O-benzyl-2-O-(((S)-tetrahydrofuran-2-yl)methoxy)-1-thio-β-D-galactopyranoside (10).
Using general procedure C starting from 2 (100 mg, 0.18 mmol) and (S)-(tetrahydrofuran-2-yl)methyl 4-methylbenzenesulfonate (20), the crude product was purified by silica gel flash column chromatography (0% to 5% Et2O in toluene), affording 10 (76 mg, 66%) as colourless oil. TLC:Rf = 0.27 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.49–7.44 (m, 2H), 7.32–7.16 (m, 16H), 7.10 (p, J = 3.6 Hz, 3H), 4.86 (d, J = 11.6 Hz, 1H), 4.69 (d, J = 11.7 Hz, 1H), 4.65 (d, J = 11.7 Hz, 1H), 4.51 (t, J = 10.4 Hz, 2H, H-1), 4.38 (d, J = 11.7 Hz, 1H), 4.33 (d, J = 11.7 Hz, 1H), 3.98 (qd, J = 6.7, 4.3 Hz, 1H), 3.86 (d, J = 2.8 Hz, 1H, H-5), 3.82–3.73 (m, 2H), 3.70–3.63 (m, 2H, H-2, H-3), 3.59 (dd, J = 9.8, 4.4 Hz, 1H), 3.57–3.50 (m, 3H, H-4), 3.48 (dd, J = 9.2, 2.9 Hz, 1H), 1.85–1.69 (m, 3H), 1.57 (ddt, J = 11.3, 8.0, 7.0 Hz, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 138.9, 138.6, 138.0, 134.4 (all quaternary), 131.6, 128.8, 128.5, 128.5, 128.2, 128.0, 128.0, 127.0, 127.8, 127.7, 127.6, 127.1 (all aromatic), 87.8 (CH, C-1), 84.1 (CH, C-4), 78.3 (CH, C-2), 78.1 (CH), 77.5 (CH, C-3), 76.0, 74.6 (both CH2), 73.9 (CH, C-5), 73.7, 73.0, 69.0 (all CH2), 68.3 (CH2, C-6), 28.0, 25.7 (both CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C38H42O6S: 649.25998, found [M + Na]+: 649.26015.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-dimethylacetamide)-1-thio-β-D-glucopyranoside (11).
Using general procedure C starting from 1 (101 mg, 0.19 mmol) and N,N-dimethylacetamide (17), the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 11 (68 mg, 58%) as white solid. TLC:Rf = 0.30 (Et2O/DCM, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.60–7.52 (m, 2H), 7.39–7.18 (m, 18H), 4.93 (d, J = 10.6 Hz, 1H), 4.88–4.80 (m, 2H), 4.68 (d, J = 9.8 Hz, 1H, H-1), 4.59 (dd, J = 12.1, 5.3 Hz, 3H), 4.53 (d, J = 11.9 Hz, 1H), 4.29 (d, J = 12.5 Hz, 1H), 3.76 (ddd, J = 10.2, 6.8, 1.4 Hz, 2H, H-3, H-6), 3.70 (ddd, J = 11.0, 4.6, 1.3 Hz, 1H, H-6), 3.65–3.58 (m, 1H, H-4), 3.52–3.46 (m, 1H, H-5), 3.39 (ddt, J = 9.9, 8.7, 1.1 Hz, 1H, H-2), 2.93 (d, J = 8.9 Hz, 6H). 13C NMR (126 MHz, CDCl3): δC 168.3, 138.5, 138.4, 138.1, 133.6 (all quaternary), 132.1, 129.0, 128.6, 128.5, 128.5, 128.3, 128.0, 127.9, 127.8, 127.7, 127.7, 127.7 (all aromatic), 86.9 (CH, C-1), 86.5 (CH, C-3), 81.5 (CH, C-2), 79.1 (CH, C-5), 77.8 (CH, C-4), 75.9, 75.2, 73.5, 72.0 (all CH2), 69.1 (CH2, C-6), 36.5, 35.5 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C37H41NO6S: 650.25523, found [M + Na]+: 650.25491.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-dimethylacetamide)-1-thio-β-D-galactopyranoside (12).
Using general procedure C starting from 2 (155 mg, 0.27 mmol) and N,N-dimethylacetamide (17), the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 12 (185 mg, 65%) as white solid. TLC:Rf = 0.59 (Et2O/DCM, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.57–7.51 (m, 2H), 7.39–7.23 (m, 15H), 7.17 (dd, J = 5.0, 1.9 Hz, 3H), 4.91 (d, J = 11.5 Hz, 1H), 4.76–4.68 (m, 3H, H-1), 4.57 (d, J = 11.5 Hz, 1H), 4.46 (d, J = 12.3 Hz, 2H), 4.40 (d, J = 11.7 Hz, 1H), 4.31 (d, J = 12.8 Hz, 1H), 3.96 (d, J = 2.6 Hz, 1H, H-4), 3.79 (t, J = 9.3 Hz, 1H, H-2), 3.70 (dd, J = 9.1, 2.7 Hz, 1H, H-3), 3.66–3.60 (m, 3H, H-5, H-6), 2.87 (s, 3H), 2.83 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.5, 138.7, 138.3, 137.8, 133.8 (all quaternary), 131.5, 128.8, 128.4, 128.4, 128.2, 127.9, 127.8, 127.8, 127.7, 127.6, 127.5, 127.2 (all aromatic), 86.9 (CH, C-1), 83.8 (CH, C-3), 78.1 (CH, C-2), 77.2 (CH, C-5), 74.5, 73.5 (both CH2), 73.5 (CH, C-4), 72.7, 71.9 (both CH2), 68.7 (CH2, C-6), 36.2, 35.3 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C37H41NO6S: 650.25523, found [M + Na]+: 650.25553.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-diphenylphosphine oxide)-1-thio-β-D-glucopyranoside (13).
Using general procedure C starting from 1 (90 mg, 0.17 mmol) and (bromomethyl)diphenylphosphine oxide (18), the crude product was purified by silica gel flash column chromatography (60% to 100% EtOAc in n-heptane), affording 13 (56 mg, 45%) as white sticky compound. TLC: Rf = 0.57 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.86 (dddd, J = 11.6, 8.3, 3.0, 1.3 Hz, 4H), 7.54–7.11 (m, 27H), 4.82 (dd, J = 12.2, 6.3 Hz, 1H), 4.73 (d, J = 10.8 Hz, 1H), 4.61–4.46 (m, 6H, H-1), 4.36 (d, J = 10.7 Hz, 1H), 3.75 (dd, J = 10.9, 2.0 Hz, 1H, H-6), 3.68 (dd, J = 10.9, 4.8 Hz, 1H, H-6), 3.61–3.54 (m, 2H, H-3, H-4), 3.45 (ddd, J = 9.3, 4.7, 2.0 Hz, 1H, H-5), 3.41–3.33 (m, 1H, H-2) ppm; 13C NMR (126 MHz, CDCl3): δC 138.3, 138.2, 138.0, 133.5 (all quaternary), 132.3, 132.3, 132.2, 132.2, 132.1, 132.0, 131.9 (all aromatic), 131.8 (quaternary), 131.7, 131.7 (both aromatic), 131.3, 131.0, 130.5 (all quaternary), 129.1, 128.7, 128.6, 128.5, 128.5, 128.4, 128.06, 127.9, 127.8, 127.7, 127.7, 127.7, 127.7 (all aromatic), 87.0 (CH, C-1), 86.4 (CH, C-3), 83.1, 83.0 (both CH, C-2), 79.0 (CH, C-5), 77.9 (CH, C-4), 75.4, 75.1, 73.5, 71.3, 70.6 (all CH2), 69.0 (CH2, C-6) ppm; 31P NMR (202 MHz, CDCl3): δP 27.3 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C46H45O6PS: 757.27527, found [M + H]+: 757.27581.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-diphenylphosphine oxide)-1-thio-β-D-galactopyranoside (14).
Using general procedure C starting from 2 (100 mg, 0.18 mmol) and (bromomethyl)diphenylphosphine oxide (18), the crude product was purified by silica gel flash column chromatography (60% to 100% EtOAc in n-heptane), affording 14 (63 mg, 45%) as colourless sticky compound. TLC: Rf = 0.49 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.83 (dddd, J = 11.5, 9.5, 8.2, 1.4 Hz, 4H), 7.54–7.43 (m, 2H), 7.42–7.12 (m, 26H), 4.84 (d, J = 11.4 Hz, 1H), 4.66 (dd, J = 12.3, 6.1 Hz, 1H), 4.58 (dd, J = 12.3, 9.5 Hz, 1H), 4.53 (d, J = 9.7 Hz, 1H, H-1), 4.51–4.43 (m, 3H), 4.39 (dd, J = 11.5, 5.3 Hz, 2H), 3.89 (d, J = 2.8 Hz, 1H, H-4), 3.77 (t, J = 9.4 Hz, 1H, H-2), 3.64–3.53 (m, 3H, H-5, H-6), 3.49 (dd, J = 9.1, 2.8 Hz, 1H, H-3) ppm; 13C NMR (126 MHz, CDCl3): δC 138.7, 138.0, 137.9, 133.8 (all quaternary), 132.2, 132.2, 132.1, 132.1, 132.1, 132.0, 131.8, 131.7 (all aromatic), 131.7 (quaternary), 131.6 (aromatic), 131.0, 130.9 (both quaternary), 129.0, 128.6, 128.6, 128.6, 128.5, 128.4, 128.3, 128.0, 127.9, 127.8, 127.6, 127.6, 127.4 (all aromatic), 87.3 (CH, C-1), 84.3 (CH, C-3), 79.7, 79.6 (both CH, C-2), 77.3 (CH, C-5), 74.6, 73.7 (both CH2), 73.3 (CH, C-4), 72.5, 71.3, 70.6 (all CH2), 68.8 ppm (CH2, C-6); 31P NMR (202 MHz, CDCl3): δP 27.59 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C46H45O6PS: 757.27527, found [M + H]+: 757.27564.
Methyl 3,4,6-tribenzyl-2-O-(2-methoxyethoxy)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (22).
Using general procedure D starting from 3 (30 mg, 0.050 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 20% EtOAc in n-heptane), affording 22 as an anomeric mixture (α/β = 1/1, 48 mg, 96%). TLC:Rf = 0.31 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CHCl3): δH 8.03–7.96 (m, 2H), 7.96–7.88 (m, 2H), 7.89–7.81 (m, 2H), 7.55–7.46 (m, 2H), 7.47–7.20 (m, 26H), 7.15 (dd, J = 7.4, 2.2 Hz, 2H), 6.15 (t, J = 9.8 Hz, 1H, H-3′), 5.47 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.26 (dd, J = 10.1, 3.6 Hz, 1H, H-2′), 5.22 (d, J = 3.6 Hz, 1H, H-1′), 5.01 (d, J = 10.8 Hz, 1H), 4.81 (d, J = 10.8 Hz, 1H), 4.76 (d, J = 10.9 Hz, 1H), 4.54–4.48 (m, 2H), 4.44 (d, J = 12.2 Hz, 1H), 4.39 (d, J = 7.8 Hz, 1H, H-1), 4.33 (ddd, J = 9.7, 7.0, 2.2 Hz, 1H, H-5′), 4.13 (ddd, J = 10.6, 5.5, 3.7 Hz, 1H, H-6), 4.07 (dd, J = 11.0, 2.2 Hz, 1H), 3.78 (qd, J = 6.9, 4.2 Hz, 2H, H-6, H-6′), 3.69–3.57 (m, 3H, H-3, H-6, H-6′), 3.58–3.50 (m, 3H, H-5), 3.46 (s, 3H), 3.39 (ddd, J = 9.7, 4.6, 2.2 Hz, 1H, H-4), 3.33 (s, 3H), 3.27 (dd, J = 9.0, 7.8 Hz, 1H, H-2) ppm. 13C NMR (126 MHz, CDCl3): δC 166.0, 165.9, 165.5, 139.0 (all quaternary), 139.0, 138.3, 138.3, 133.5, 133.5, 133.2, 130.0, 130.0(all aromatic), 129.8, 129.3, 129.1 (all quaternary), 128.6, 128.5, 128.5, 128.5, 128.4, 128.2, 128.1, 127.9, 127.7, 127.7 (all aromatic), 104.0 (CH, C-1), 97.0 (CH, C-1′), 84.5 (CH, C-3), 83.4 (CH, C-2), 77.7 (CH2), 75.7 (CH2), 75.1 (CH2), 75.0 (CH, C-4), 73.6 (CH2), 72.3 (CH, C-5), 72.3 (CH, C-2′), 72.0 (CH, C-6), 70.7 (CH, C-3′), 69.9 (CH, C-4′), 69.1 (CH, C-5′), 68.9 (CH, C-6′), 59.0 (CH3), 55.7 (CH3) ppm. HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C58H60O15: 1019.38299. Found [M + Na]+: 1019.38148.
Methyl 3,4,6-tribenzyl-2-O-(2-methoxyethoxy)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (23).
Using general procedure D starting from 4 (40 mg, 0.067 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 23 as an anomeric mixture (α/β = 3/2, 63 mg, 95%). TLC:Rf = 0.27 (Et2O/toluene, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.98 (dq, J = 7.0, 2.1, 1.7 Hz, 2H), 7.93–7.89 (m, 2H), 7.86–7.82 (m, 2H), 7.54–7.45 (m, 3H), 7.44–7.21 (m, 29H), 6.12 (t, J = 9.8 Hz, 1H, H-3′), 5.53 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.25 (dd, J = 10.2, 3.7 Hz, 1H, H-2′), 5.14 (d, J = 3.7 Hz, 1H, H-1′), 4.99 (d, J = 3.5 Hz, 1H, H-1), 4.92 (d, J = 11.5 Hz, 1H), 4.80 (d, J = 11.9 Hz, 1H), 4.70 (d, J = 11.9 Hz, 1H), 4.55 (d, J = 11.5 Hz, 1H), 4.39 (d, J = 11.9 Hz, 1H), 4.34–4.28 (m, 2H, H-5′), 4.01 (t, J = 6.5 Hz, 1H, H-5), 3.96 (dd, J = 10.0, 3.5 Hz, 1H, H-2), 3.93–3.86 (m, 3H, H-3, H-4, H-6′), 3.85–3.79 (m, 2H), 3.69 (dd, J = 11.2, 2.1 Hz, 1H, H-6′), 3.55–3.50 (m, 2H), 3.49–3.43 (m, 3H, H-6), 3.37 (s, 3H), 3.32 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 165.9, 165.5, 139.1, 138.3, 133.5 (all quaternary), 133.2, 130.1, 130.0, 129.8, 129.5 (all aromatic), 129.3, 129.2, 128.5 (all quaternary), 128.5, 128.5, 128.5, 128.4, 128.4, 128.4, 128.3, 128.3, 128.0, 127.9, 127.8, 127.7, 127.6, 127.5, 124.5, 123.6, 119.2, 119.0 (all aromatic), 98.0 (CH, C-1), 96.9 (CH, C-1′), 78.5 (CH, C-3), 77.8 (CH, C-2), 75.3 (CH, C-4), 74.9, 73.4, 73.0, 72.3 (all CH2), 72.3 (C-2′), 70.9 (C-3′), 70.7 (CH2), 69.7 (CH, C-4′), 69.4 (CH, C-5), 68.9 (CH, C-6), 68.7 (CH, C-5′), 66.8 (C-6′), 59.1, 55.6 (both CH3) ppm. HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C58H60O15: 1019.38299. Found [M + Na]+: 1019.37696.
Methyl 3,4,6-tribenzyl-2-O-(2-phenoxyethoxy)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (24).
Using general procedure D starting from 5 (40 mg, 0.060 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 20% EtOAc in n-heptane), affording 24 as an anomeric mixture (α/β = 1/1, 35 mg, 54%). TLC:Rf = 0.32 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CHCl3): δH 8.02–7.95 (m, 2H), 7.92 (dt, J = 8.4, 1.7 Hz, 2H), 7.84 (ddd, J = 8.5, 3.3, 1.4 Hz, 2H), 7.54–7.19 (m, 24H), 7.15 (ddt, J = 6.1, 4.2, 2.0 Hz, 2H), 6.95–6.89 (m, 1H), 6.86–6.79 (m, 1H), 6.61–6.56 (m, 1H), 6.15 (td, J = 9.8, 2.1 Hz, 1H, H-3′), 5.55–5.47 (m, 1H, H-4′), 5.30–5.24 (m, 1H, H-2′), 5.22 (d, J = 3.7 Hz, 1H, H-1′), 5.03 (d, J = 10.8 Hz, 1H), 4.98 (d, J = 10.9 Hz, 1H), 4.81 (dd, J = 10.8, 6.8 Hz, 1H), 4.76 (dd, J = 10.8, 4.9 Hz, 1H), 4.57–4.43 (m, 3H), 4.42–4.36 (m, 1H, H-1), 4.32 (dtd, J = 12.1, 6.9, 6.1, 4.0 Hz, 2H, H-5′), 4.19–3.99 (m, 4H, H-6, H-6′), 3.78 (dt, J = 11.3, 6.0 Hz, 1H, H-6′), 3.70–3.52 (m, 4H, H-5, H-6, H-3), 3.45 (s, 3H), 3.41 (dt, J = 6.8, 2.1 Hz, 1H, H-4), 3.38–3.30 (m, 1H, H-2) ppm; 13C NMR (126 MHz, CDCl3): δC 165.9, 165.5, 158.9, 138.8 (all quaternary), 138.3 (aromatic), 138.2 (quaternary), 135.5, 133.6, 133.5, 133.2, 130.1, 130.0, 129.8, 129.5 (all aromatic), 129.4, 129.4, 129.2, 129.2, 129.1 (all quaternary), 129.0, 128.6, 128.6, 128.5, 128.5, 128.4, 128.3, 128.3, 128.2, 128.1, 127.9, 127.8, 127.7, 127.7, 127.7, 125.8, 120.8, 117.1, 115.7, 114.6 (all aromatic), 103.8 (CH, C-1), 97.1 (CH, C-1′), 84.4 (CH, C-3), 83.2 (CH, C-2), 82.9 (CH2), 77.7 (CH, C-5), 75.7 (CH2), 75.1 (CH, C-4), 75.1 (CH2), 73.6 (CH2), 72.2 (CH, C-2′), 71.1 (CH2), 70.8 (CH, C-3′), 69.7 (CH, C-4′), 69.0 (CH, C-5′), 68.9 (CH2, C-6), 67.7 (CH2, C-6′), 55.7 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C63H62O15: 1081.39864, found [M + Na]+: 1081.39610.
Methyl 3,4,6-tribenzyl-2-O-(2-phenoxyethoxy)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (25).
Using general procedure D starting from 8 (40 mg, 0.067 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 25 as an anomeric mixture (α/β = 3/2, 49 mg, 77%). TLC:Rf = 0.34 (Et2O/toluene, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.96 (m, 2H), 7.92–7.88 (m, 2H), 7.86–7.82 (m, 2H), 7.55–7.17 (m, 27H), 6.93–6.82 (m, 2H), 6.11 (t, J = 9.8 Hz, 1H, H-3′), 5.51 (t, J = 9.9 Hz, 1H, H-4′), 5.23 (dd, J = 10.2, 3.7 Hz, 1H, H-2′), 5.11 (d, J = 3.6 Hz, 1H, H-1′), 4.99 (d, J = 3.6 Hz, 1H, H-1), 4.92 (d, J = 11.5 Hz, 1H), 4.79 (d, J = 11.8 Hz, 1H), 4.70 (d, J = 11.9 Hz, 1H), 4.56 (d, J = 11.5 Hz, 1H), 4.39 (d, J = 11.9 Hz, 1H), 4.34–4.25 (m, 2H, H-5′), 4.13–3.96 (m, 6H, H-2, H-5, H-6), 3.95–3.83 (m, 3H, H-3, H-4, H-6′), 3.66 (dt, J = 11.3, 2.7 Hz, 1H, H-6′), 3.51–3.41 (m, 2H), 3.34 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 165.9, 165.5, 159.0, 139.0, 138.9 (all quaternary), 138.3 (aromatic), 138.2 (quaternary), 133.5, 133.2, 130.1, 130.0, 129.8, 129.5 (all aromatic), 129.5, 129.3, 129.2 (all quaternary), 128.5, 128.5, 128.5, 128.5, 128.4, 128.3, 127.8, 127.8, 127.7, 127.6, 127.5, 120.9, 117.2 (all aromatic), 97.9 (CH, C-1), 96.9 (CH, C-1′), 78.4 (CH, C-3), 78.0 (CH, C-2), 75.3 (CH, C-4), 74.9, 73.4, 73.0 (all CH2), 72.3 (CH, C-2′), 70.8 (CH, C-3′), 70.2 (CH2, C-6′), 69.8 (CH, C-4′), 69.5 (CH, C-5), 68.8 (CH2), 68.6 (CH, C-5′), 67.5 (CH2), 66.8 (CH2, C-6′), 55.6 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C63H62O15: 1081.39864, found [M + Na]+: 1081.39683.
Methyl 3,4,6-tribenzyl-2-O-(((R)-tetrahydrofuran-2-yl)methoxy)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (26).
Using general procedure D starting from 7 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 26 as an anomeric mixture (α/β = 1/2, 56 mg, 85%). TLC:Rf = 0.26 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.95 (m, 2H), 7.94–7.89 (m, 2H), 7.87–7.83 (m, 2H), 7.55–7.47 (m, 2H), 7.45–7.20 (m, 20H), 7.17–7.13 (m, 2H), 6.15 (dd, J = 10.1, 9.4 Hz, 1H, H-3′), 5.47 (dd, J = 10.3, 9.4 Hz, 1H, H-4′), 5.25 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.21 (d, J = 3.6 Hz, 1H, H-1′), 5.06 (d, J = 10.8 Hz, 1H), 4.81 (d, J = 10.8 Hz, 1H), 4.76 (d, J = 10.8 Hz, 1H), 4.52 (d, J = 5.0 Hz, 1H), 4.49 (d, J = 3.7 Hz, 1H), 4.43 (d, J = 12.2 Hz, 1H), 4.37 (d, J = 7.8 Hz, 1H, H-1), 4.33 (ddd, J = 9.9, 7.0, 2.2 Hz, 1H, H-5′), 4.14–4.05 (m, 2H), 3.96 (dd, J = 9.7, 4.3 Hz, 1H), 3.83 (dt, J = 8.4, 6.7 Hz, 1H), 3.79–3.72 (m, 2H, H-6′), 3.69–3.58 (m, 4H, H-3, H-6), 3.53 (t, J = 9.3 Hz, 1H, H-4), 3.46 (s, 3H), 3.39 (ddd, J = 9.7, 4.6, 2.2 Hz, 1H, H-5), 3.29 (dd, J = 9.0, 7.8 Hz, 1H, H-2), 2.01–1.93 (m, 1H), 1.86 (dt, J = 9.5, 6.6 Hz, 2H), 1.62–1.57 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 166.0, 165.9, 139.0, 138.3 (all quaternary), 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.5, 129.3, 129.1 (all quaternary), 128.6, 128.5, 128.5, 128.4, 128.2, 128.1, 127.8, 127.7, 127.7 (all aromatic), 103.9 (CH, C-1), 97.0 (CH, C-1′), 84.6 (CH, C-3), 83.3 (CH, C-2), 78.1 (CH), 77.7 (CH, C-4), 75.8, 75.7, 75.1 (all CH2), 75.0 (CH, C-5), 73.5 (CH2), 72.3 (CH, C-2′), 70.8 (CH, C-3′), 69.9 (CH, C-4′), 69.0 (CH, C-5′), 68.9 (CH2, C-6), 68.8 (CH2, C-6′), 68.2 (CH2), 55.7 (CH3), 28.4, 25.8 (both CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C60H62O15: 1045.39864. Found [M + Na]+: 1045.39527.
Methyl 3,4,6-tribenzyl-2-O-(((R)-tetrahydrofuran-2-yl)methoxy)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (27).
Using general procedure D starting from 8 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 27 as an anomeric mixture (α/β = 1/1, 53 mg, 81%). TLC:Rf = 0.27 (Et2O/toluene, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.96 (m, 2H), 7.89 (ddd, J = 27.7, 8.4, 1.4 Hz, 3H), 7.55–7.19 (m, 25H), 6.12 (t, J = 9.9 Hz, 1H, H-3′), 5.56 (t, J = 9.9 Hz, 1H, H-4′), 5.25 (dd, J = 10.2, 3.7 Hz, 1H, H-2′), 5.14 (d, J = 3.7 Hz, 1H, H-1′), 4.99 (d, J = 3.4 Hz, 1H, H-1), 4.92 (d, J = 11.5 Hz, 1H), 4.82 (d, J = 11.9 Hz, 1H), 4.70 (d, J = 12.0 Hz, 1H), 4.55 (d, J = 11.5 Hz, 1H), 4.37 (d, J = 11.9 Hz, 1H), 4.32–4.24 (m, 2H, H-5′), 4.09–4.02 (m, 1H), 3.99–3.93 (m, 2H, H-2, H-5), 3.92–3.85 (m, 3H, H-3, H-4), 3.84–3.81 (m, 1H, H-6′), 3.76–3.68 (m, 2H, H-6), 3.64 (dd, J = 11.2, 2.0 Hz, 1H, H-6′), 3.58 (dd, J = 10.1, 5.1 Hz, 1H, H-6), 3.43 (d, J = 6.7 Hz, 2H), 3.37 (s, 3H), 1.98–1.77 (m, 3H), 1.72 (ddt, J = 11.3, 8.1, 6.5 Hz, 1H); 13C NMR (126 MHz, CDCl3): δC 165.8, 165.3, 139.0, 138.8, 138.1 (all quaternary), 133.3, 133.0, 129.9, 129.8, 129.7 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.2, 127.6, 127.6, 127.5, 127.5, 127.4, 124.4, 123.5 (all aromatic), 97.9 (CH, C-1), 96.8 (CH, C-1′), 78.3 (CH, C-3), 77.7 (CH), 77.5 (CH, C-2), 75.1 (CH, C-4), 74.7, 73.5, 73.2, 72.9 (all CH2), 72.2 (CH, C-2′), 70.7 (CH, C-3′), 69.4 (CH, C-4′), 69.3 (CH, C-5), 68.8 (CH2), 68.6 (CH, C-5′), 68.3 (CH2, C-6), 66.6 (CH2, C-6), 55.4 (CH3), 29.7, 28.2, 25.7 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C60H62O15: 1045.39864. Found [M + Na]+: 1045.39502.
Methyl 3,4,6-tribenzyl-2-O-(((S)-tetrahydrofuran-2-yl)methoxy)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (28).
Using general procedure D starting from 9 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 28 as an anomeric mixture (α/β = 0.7/1, 35 mg, 55%). TLC:Rf = 0.20 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.96 (m, 2H), 7.94–7.91 (m, 2H), 7.87–7.83 (m, 2H), 7.53–7.47 (m, 2H), 7.44–7.20 (m, 20H), 7.14 (dd, J = 7.4, 2.1 Hz, 2H), 6.16 (t, J = 9.8 Hz, 1H, H-3′), 5.45 (t, J = 9.9 Hz, 1H, H-5′), 5.26 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.21 (d, J = 3.6 Hz, 1H, H-1′), 5.01 (d, J = 10.9 Hz, 1H), 4.80 (d, J = 10.8 Hz, 1H), 4.76 (d, J = 10.9 Hz, 1H), 4.54–4.48 (m, 2H), 4.45–4.38 (m, 2H, H-1), 4.34 (ddd, J = 10.0, 7.3, 2.2 Hz, 1H, H-5′), 4.05 (ddt, J = 14.5, 9.6, 4.3 Hz, 3H, H-6′), 3.84 (dt, J = 8.3, 6.6 Hz, 1H, H-6), 3.81–3.69 (m, 2H, H-6, H-6′), 3.67–3.52 (m, 5H, H-3, H-4), 3.47 (s, 3H), 3.39 (ddd, J = 9.7, 4.3, 2.3 Hz, 1H, H-5), 3.26 (dd, J = 8.9, 7.7 Hz, 1H, H-2), 1.96–1.78 (m, 3H), 1.64–1.58 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 166.0, 165.9, 165.6, 139.0, 138.3, 138.3 (all quaternary), 133.5, 133.5, 133.2, 130.1, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.3, 129.1 (all quaternary), 128.5, 128.5, 128.5, 128.4, 128.4, 128.1, 128.1, 127.8, 127.7 (all aromatic), 104.1 (CH, C-1), 96.9 (CH, C-1′), 84.6 (CH, C-3), 83.4 (CH, C-2), 78.1 (CH), 77.7 (CH, C-4), 75.8, 75.6, 75.1 (all CH2), 75.0 (CH, C-5), 73.6 (CH2), 72.3 (CH, C-2′), 70.7 (CH, C-3′), 70.1 (CH, C-4′), 69.1 (CH, C-5′), 68.9 (CH2, C-6′), 68.3 (CH2, C-6), 55.7 (CH3), 28.2, 25.9 (both CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C60H62O15: 1045.39864, found [M + Na]+: 1045.39864.
Methyl 3,4,6-tribenzyl-2-O-(((S)-tetrahydrofuran-2-yl)methoxy)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (29).
Using general procedure D starting from 10 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (0% to 15% Et2O in toluene), affording 29 as an anomeric mixture (α/β = 1/1, 41 mg, 73%). TLC:Rf = 0.30 (Et2O/toluene, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 8.01–7.96 (m, 2H), 7.93–7.89 (m, 2H), 7.87–7.83 (m, 2H), 7.55–7.20 (m, 24H), 6.12 (t, J = 9.9 Hz, 1H, H-3′), 5.54 (t, J = 9.9 Hz, 1H, H-4′), 5.24 (dd, J = 10.2, 3.7 Hz, 1H, H-2′), 5.14 (d, J = 3.7 Hz, 1H, H-1′), 5.03 (d, J = 3.5 Hz, 1H, H-1), 4.92 (d, J = 11.5 Hz, 1H), 4.81 (d, J = 11.9 Hz, 1H), 4.70 (d, J = 11.9 Hz, 1H), 4.55 (d, J = 11.5 Hz, 1H), 4.38 (d, J = 11.9 Hz, 1H), 4.34–4.26 (m, 2H, H-5′), 4.05 (qd, J = 6.8, 5.1 Hz, 1H, H-3), 4.02–3.96 (m, 2H, H-2, H-5), 3.91–3.86 (m, 2H, H-4, H-6′), 3.81 (dt, J = 8.4, 6.6 Hz, 1H, H-6), 3.73–3.61 (m, 4H, H-6, H-6′), 3.44 (dd, J = 6.6, 2.7 Hz, 2H), 3.37 (s, 3H), 1.96–1.75 (m, 3H), 1.63 (m, 2H) ppm; 13C NMR (126 MHz, CDCl3): δC 165.9, 165.4, 139.1, 138.9, 138.6 (all quaternary), 133.4, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.5, 129.3, 129.2 (all quaternary), 128.5, 128.5, 128.5, 128.4, 128.4, 128.4, 128.3, 127.8, 127.7, 127.6, 127.6, 127.5 (all aromatic), 97.9 (CH, C-1), 96.9 (CH, C-1′), 78.6 (CH), 78.4 (CH, C-3), 77.9 (CH, C-2), 75.3 (CH, C-4), 74.9, 74.1, 73.4, 73.1 (all CH2), 72.3 (CH, C-2′), 70.9 (CH, C-3′), 69.7 (CH, C-4′), 69.4 (CH, C-5), 68.9 (CH2, C-6), 68.7 (CH, C-5′), 68.3 (CH2), 66.8 (CH2, C-6′), 55.6 (CH3), 28.4, 25.7 (both CH2) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C60H62O15: 1045.39864, found [M + Na]+: 1045.39704.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide)-β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (30).
Using general procedure D starting from 11 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 30 as pure β-anomer (38 mg, 57%). TLC:Rf = 0.18 (EtOAc/heptane, 50/50 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.96 (m, 2H), 7.91 (dd, J = 8.3, 1.4 Hz, 2H), 7.86–7.83 (m, 2H), 7.54–7.47 (m, 2H), 7.44–7.21 (m, 22H), 7.18–7.12 (m, 2H), 6.15 (t, J = 9.8 Hz, 1H, H-3′), 5.47 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.25 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.21 (d, J = 3.6 Hz, 1H, H-1′), 5.00 (d, J = 11.0 Hz, 1H), 4.80 (dd, J = 10.9, 5.7 Hz, 2H), 4.70 (d, J = 13.0 Hz, 1H), 4.55–4.49 (m, 2H), 4.48–4.42 (m, 2H, H-1), 4.34 (ddd, J = 9.9, 7.0, 2.3 Hz, 1H, H-5′), 4.26 (d, J = 13.0 Hz, 1H), 4.06 (dd, J = 11.0, 2.3 Hz, 1H, H-6′), 3.77 (dd, J = 11.1, 7.0 Hz, 1H, H-6′), 3.69 (t, J = 9.0 Hz, 1H, H-3), 3.64–3.61 (m, 2H, H-6), 3.56 (t, J = 9.4 Hz, 1H, H-4), 3.46 (s, 3H), 3.40 (ddd, J = 9.8, 4.2, 2.5 Hz, 1H, H-5), 3.33 (dd, J = 9.0, 7.8 Hz, 1H, H-2), 2.94 (d, J = 10.3 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.8, 166.0, 165.9, 165.5, 138.8, 138.2 (all quaternary), 133.5, 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.5, 128.5, 128.5, 128.4, 128.4, 128.2, 128.1, 127.8, 127.8, 127.7, 127.6 (all aromatic), 103.4 (CH, C-1), 97.0 (CH, C-1′), 84.2 (CH, C-3), 83.3 (CH, C-2), 77.6 (CH, C-4), 75.7, 75.1 (both CH2), 75.0 (CH, C-5), 73.5 (CH2), 72.2 (CH, C-2′), 71.5 (CH2), 70.7 (CH, C-3′), 69.9 (CH, C-4′), 69.0 (CH, C-5′), 68.8 (CH2, C-6), 68.7 (CH2, C-6′), 55.7, 36.4, 35.5 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C59H61NO15: 1024.41194, found [M + H]+: 1024.41336.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide) -α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (31).
Using general procedure D starting from 12 (40 mg, 0.064 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 31 as an anomeric mixture (α/β = 1/4, 28.1 mg, 43%). TLC:Rf = 0.65 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.99–7.95 (m, 2H), 7.91–7.87 (m, 2H), 7.86–7.82 (m, 2H), 7.55–7.45 (m, 2H), 7.44–7.22 (m, 20H), 7.22–7.18 (m, 2H), 6.13 (dd, J = 10.1, 9.5 Hz, 1H, H-3′), 5.38 (dd, J = 10.4, 9.4 Hz, 1H, H-4′), 5.22 (dd, J = 10.1, 3.6 Hz, 1H, H-2′), 5.18 (d, J = 3.6 Hz, 1H, H-1′), 4.88 (d, J = 11.6 Hz, 1H), 4.78 (d, J = 11.8 Hz, 1H), 4.72 (d, J = 11.8 Hz, 1H), 4.64 (d, J = 12.7 Hz, 1H), 4.56 (d, J = 11.6 Hz, 1H), 4.42 (d, J = 7.5 Hz, 1H, H-1), 4.38–4.26 (m, 4H, H-5′), 4.01 (dd, J = 10.9, 2.2 Hz, 1H, H-6′), 3.84 (d, J = 2.9 Hz, 1H, H-4), 3.73 (dd, J = 10.9, 7.9 Hz, 1H, H-6′), 3.66 (dd, J = 9.6, 7.6 Hz, 1H, H-2), 3.55 (dd, J = 9.6, 2.9 Hz, 1H, H-3), 3.53–3.45 (m, 3H, H-5, H-6), 3.44 (s, 3H), 2.92 (d, J = 9.4 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 169.0, 166.0, 165.9, 165.6, 138.7, 138.7, 138.0 (all quaternary), 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.6, 128.5, 128.5, 128.4, 128.4, 128.3, 128.0, 127.9, 127.8, 127.7, 127.6 (all aromatic), 103.7 (CH, C-1), 96.8 (CH, C-1′), 81.7 (CH, C-3), 80.6 (CH, C-2), 74.7, 73.6 (both CH2), 73.6 (CH, C-4), 73.5 (CH, C-5), 73.2 (CH2), 72.3 (CH, C-2′), 72.0 (CH2), 70.7 (CH, C-3′), 70.1 (CH, C-4′), 69.0 (CH, C-5′), 68.9 (CH2, C-6′), 68.7 (CH2, C-6), 55.7, 36.5, 35.5 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C49H61NO15: 1024.41194, found [M + H]+: 1024.41045.
Methyl 3,4,6-tribenzyl-2-O-(methyldiphenylphosphine-oxide)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (32).
Using general procedure D starting from 13 (40 mg, 0.053 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 32 as an anomeric mixture (α/β = 1/8, 41 mg, 67%). TLC:Rf = 0.64 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.95 (m, 2H), 7.92–7.79 (m, 8H), 7.56 (td, J = 7.4, 1.4 Hz, 1H), 7.54–7.45 (m, 4H), 7.44–7.31 (m, 8H), 7.31–7.18 (m, 13H), 7.15–7.09 (m, 2H), 7.09–7.05 (m, 2H), 6.13 (t, J = 9.8 Hz, 1H, H-3′), 5.31 (dd, J = 10.1, 9.3 Hz, 1H, H-4′), 5.19 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.04 (dd, J = 12.6, 6.3 Hz, 1H), 4.98 (d, J = 3.5 Hz, 1H, H-1′), 4.71 (d, J = 10.8 Hz, 1H), 4.51–4.41 (m, 4H), 4.40–4.30 (m, 4H, H-1, H-5′), 4.01 (dd, J = 10.4, 2.2 Hz, 1H, H-6′), 3.63 (dd, J = 10.5, 8.7 Hz, 1H), 3.57 (d, J = 3.2 Hz, 2H, H-6), 3.53 (d, J = 9.4 Hz, 1H, H-4), 3.48 (t, J = 8.9 Hz, 1H, H-3), 3.33 (ddt, J = 7.8, 5.7, 2.5 Hz, 2H, H-2, H-5), 3.28 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 165.9, 165.8, 165.8, 138.5, 138.1, 138.1 (all quaternary), 133.6, 133.5, 133.2, 132.3, 132.3, 132.2, 132.2, 131.9, 131.8, 131.8, 131.7 (all aromatic), 131.0, 130.9 (both quaternary), 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 128.9 (all quaternary), 128.7, 128.6, 128.6, 128.6, 128.5, 128.5, 128.4, 128.4, 128.4, 128.0, 127.9, 127.8, 127.8, 127.7, 127.6 (all aromatic), 103.5 (CH, C-1), 96.7 (CH, C-1′), 84.8 (CH, C-2), 84.0 (CH, C-3), 77.5 (CH, C-4), 75.3, 75.0 (both CH2), 74.9 (CH, C-5), 73.6 (CH2), 72.1 (CH, C-2′), 70.5 (CH, C-3′), 70.3 (CH, C-4′), 69.8 (CH2), 69.5 (CH2, C-6′), 68.8 (CH, C-5′), 68.5 (CH2, C-6), 55.7 (CH3) ppm; 31P NMR (202 MHz, CDCl3): δP 27.35 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C68H65O15P: 1153.41393, found [M + H]+: 1153.41312.
Methyl 3,4,6-tribenzyl-2-O-(methyldiphenylphosphine-oxide) -α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (33).
Using general procedure D starting from 14 (48 mg, 0.063 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (33), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 33 as an anomeric mixture (α/β = 1/8, 50 mg, 68%). TLC:Rf = 0.48 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.91–7.88 (m, 2H), 7.82–7.70 (m, 9H), 7.55–7.04 (m, 29H), 6.03 (t, J = 9.8 Hz, 1H), 5.24–5.17 (m, 1H), 5.12 (dd, J = 10.2, 3.6 Hz, 1H), 4.95–4.87 (m, 2H), 4.76 (d, J = 11.6 Hz, 1H), 4.43 (d, J = 11.6 Hz, 1H), 4.41–4.33 (m, 3H), 4.27–4.21 (m, 3H), 4.17 (d, J = 11.8 Hz, 1H), 3.92 (dd, J = 10.3, 2.1 Hz, 1H), 3.68 (d, J = 2.9 Hz, 1H), 3.61 (dd, J = 9.6, 7.6 Hz, 1H), 3.50 (dd, J = 10.3, 9.0 Hz, 1H), 3.38–3.30 (m, 3H), 3.30–3.26 (m, 1H), 3.17 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δC 165.8, 165.8, 165.7, 138.5, 138.4, 137.9 (all quaternary), 133.5, 133.4, 133.2, 132.7, 132.7, 132.2, 132.2 (all aromatic), 131.9 (quaternary), 131.9, 131.8, 131.7, 131.6 (all aromatic), 131.1, 131.1 (both quaternary), 130.0, 130.0, 129.7 (all aromatic), 129.4, 129.2 (both quaternary), 129.0 (aromatic), 128.9 (quaternary), 128.9, 128.6, 128.6, 128.5, 128.5, 128.4, 128.4, 128.4, 128.3, 127.9, 127.9, 127.7, 127.6, 127.5 (all aromatic), 103.7 (CH, C-1), 96.6 (CH, C-1′), 82.1 (CH, C-2), 81.4 (CH, C-3), 74.7, 73.6 (both CH2), 73.5 (CH, C-5), 73.4 (CH, C-4), 73.0 (CH2), 72.1 (CH, C-2′), 70.7 (CH, C-3′), 70.5 (CH2), 70.3 (CH, C-4′), 70.0 (CH2), 69.4 (CH2, C-6′), 68.7 (CH, C-5′), 68.6 (CH, C-5′), 55.6 (CH3) ppm; 31P NMR (202 MHz, CDCl3): δP 27.73 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C68H65O15P: 1153.41393, found [M + H]+: 1153.41352.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-diethylacetamide)-1-thio-β-D-glucopyranoside (34).
Using general procedure C starting from 1 (106 mg, 0.20 mmol) and 2-chloro-N,N-diethylacetamide, the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 34 (84 mg, 66%) as sticky yellowish compound. TLC:Rf = 0.68 (Et2O/DCM, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.59–7.51 (m, 2H), 7.40–7.19 (m, 18H), 4.99 (d, J = 10.4 Hz, 1H), 4.84 (t, J = 10.7 Hz, 2H), 4.67 (d, J = 9.8 Hz, 1H, H-1), 4.65–4.57 (m, 3H), 4.52 (d, J = 12.0 Hz, 1H), 4.25 (d, J = 12.1 Hz, 1H), 3.80–3.74 (m, 2H, H-3, H-6), 3.71 (dd, J = 10.9, 4.7 Hz, 1H, H-6), 3.61 (t, J = 9.4 Hz, 1H, H-4), 3.50 (ddd, J = 9.9, 4.7, 1.9 Hz, 1H, H-5), 3.44–3.32 (m, 3H, H-2), 3.26 (hept, J = 7.5 Hz, 2H), 1.12 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 167.3, 138.4, 138.1, 133.5 (all quaternary), 132.0, 129.0, 128.5, 128.5, 128.4, 128.4, 128.0, 127.9, 127.8, 127.7, 127.6, 127.6 (all aromatic), 86.8 (CH, C-1), 86.4 (CH, C-3), 81.6 (CH, C-2), 79.1 (CH, C-5), 77.7 (CH, C-4), 75.9, 75.1, 73.4, 72.1 (all CH2), 69.1 (CH2, C-6), 41.3, 40.2 (both CH2), 14.4, 13.0 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C39H45NO6S: 656.30458, found [M + H]+: 656.30491.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-diethylacetamide)-1-thio-β-D-galactopyranoside (35).
Using general procedure C starting from 2 (105 mg, 0.19 mmol) and 2-chloro-N,N-diethylacetamide, the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 35 (86 mg, 68%) as sticky white compound. TLC:Rf = 0.76 (Et2O/DCM, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.58–7.49 (m, 2H), 7.42–7.07 (m, 18H), 4.93 (d, J = 11.5 Hz, 1H), 4.77 (d, J = 11.5 Hz, 1H), 4.73 (d, J = 11.5 Hz, 1H), 4.66 (d, J = 9.6 Hz, 1H, H-1), 4.57 (d, J = 11.6 Hz, 1H), 4.50 (d, J = 12.3 Hz, 1H), 4.46 (d, J = 11.7 Hz, 1H), 4.41 (d, J = 11.7 Hz, 1H), 4.29 (d, J = 12.4 Hz, 1H), 3.95 (d, J = 2.8 Hz, 1H, H-4), 3.78 (t, J = 9.3 Hz, 1H, H-2), 3.69–3.59 (m, 4H, H-3, H-5, H-6), 3.35 (q, J = 7.1 Hz, 2H), 3.21 (qd, J = 7.2, 2.9 Hz, 2H), 1.08 (dt, J = 28.1, 7.0 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 167.6, 138.9, 138.4, 138.0, 134.0 (all quaternary), 131.6, 128.9, 128.6, 128.5, 128.3, 128.0, 128.0, 127.9, 127.9, 127.8, 127.6, 127.3 (all aromatic), 87.2 (CH, C-1), 83.8 (CH, C-3), 78.3 (CH, C-2), 77.4 (CH, C-5), 74.6 (CH2), 73.8 (CH, C-4), 73.7, 73.0, 72.2 (all CH2), 68.9 (CH2, C-6), 41.2, 40.1 (both CH2), 14.4, 13.1 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C39H45NO6S: 656.30458, found [M + H]+: 656.30448.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-diphenylacetamide)-1-thio-β-D-glucopyranoside (36).
Using general procedure C starting from 1 (106 mg, 0.20 mmol) and 2-chloro-N,N-diphenylacetamide, the crude product was purified by silica gel flash column chromatography (0% to 30% EtOAc in n-heptane), affording 36 (83 mg, 56%) as sticky yellow compound. TLC:Rf = 0.29 (EtOAc/heptane, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.43–7.38 (m, 2H), 7.36–7.06 (m, 28H), 4.99 (d, J = 10.7 Hz, 1H), 4.86 (d, J = 10.7 Hz, 1H), 4.81 (d, J = 10.8 Hz, 1H), 4.64 (d, J = 9.7 Hz, 1H, H-1), 4.59–4.55 (m, 2H), 4.52–4.44 (m, 2H), 4.10 (d, J = 14.6 Hz, 1H), 3.80–3.73 (m, 2H, H-3, H-6), 3.70 (dd, J = 10.8, 4.3 Hz, 1H, H-6), 3.55 (t, J = 9.5 Hz, 1H, H-4), 3.47 (ddd, J = 9.8, 4.3, 2.0 Hz, 1H, H-5), 3.21 (t, J = 9.2 Hz, 1H, H-2) ppm; 13C NMR (126 MHz, CDCl3): δC 168.2, 138.7, 138.4, 138.1, 132.7 (all quaternary), 132.6, 128.9, 128.5, 128.4, 128.1, 128.0, 127.9, 127.8, 127.6, 127.6, 127.6 (all aromatic), 86.3 (CH, C-3), 86.3 (CH, C-1), 81.3 (CH, C-2), 79.0 (CH, C-5), 77.5 (CH, C-4), 75.8, 75.1, 73.4, 72.1 (all CH2), 69.0 (CH2, C-6) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C47H45NO6S: 752.30458, found [M + H]+: 752.30680.
Phenyl 3,4,6-tri-O-benzyl-2-O-(methyl-N,N-diphenylacetamide)-1-thio-β-D-galactopyranoside (37).
Using general procedure C starting from 2 (102 mg, 0.19 mmol) and 2-chloro-N,N-diphenylacetamide, the crude product was purified by silica gel flash column chromatography (0% to 30% EtOAc in n-heptane), affording 37 (45 mg, 32%) as sticky white compound. TLC:Rf = 0.29 (EtOAc/heptane, 40/60 v/v); 1H NMR (500 MHz, CDCl3): δH 7.45–7.04 (m, 30H), 4.90 (d, J = 11.6 Hz, 1H), 4.79 (d, J = 11.5 Hz, 1H), 4.75 (d, J = 11.5 Hz, 1H), 4.64 (d, J = 9.4 Hz, 1H, H-1), 4.54 (d, J = 11.6 Hz, 1H), 4.48–4.37 (m, 3H), 4.11 (d, J = 14.7 Hz, 1H), 3.92 (d, J = 2.8 Hz, 1H, H-4), 3.69 (dd, J = 6.3, 2.8 Hz, 1H, H-3), 3.65–3.57 (m, 4H, H-2, H-5, H-6) ppm; 13C NMR (126 MHz, CDCl3): δC 168.6, 138.9, 138.7, 138.0, 133.4 (all quaternary), 132.0, 128.8, 128.5, 128.5, 128.3, 128.0, 127.9, 127.7, 127.7, 127.5, 127.2 (all aromatic), 86.8 (CH, C-1), 83.7 (CH, C-3), 78.4 (CH, C-5), 77.2 (CH, C-2), 74.6 (CH2), 74.0 (CH, C-4), 73.7, 73.1, 72.2 (all CH2), 68.7 (CH2, C-6) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C47H45NO6S: 752.30458, found [M + H]+: 752.30458.
Phenyl 3,4,6-tri-O-benzyl-2-O-((piperidin-1-yl)ethan-1-one)-1-thio-β-D-glucopyranoside (38).
Using general procedure C starting from 1 (101 mg, 0.19 mmol) and 2-chloro-1-(piperidin-1-yl)ethan-1-one, the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 38 (95 mg, 77%) as white solid. TLC:Rf = 0.68 (Et2O/DCM, 20/80 v/v); 1H NMR (500 MHz, CDCl3): δH 7.57–7.53 (m, 2H), 7.39–7.18 (m, 18H), 4.95 (d, J = 10.5 Hz, 1H), 4.85 (d, J = 4.5 Hz, 1H), 4.83 (d, J = 4.0 Hz, 1H), 4.67 (d, J = 9.8 Hz, 1H, H-1), 4.59 (dd, J = 12.0, 1.8 Hz, 3H), 4.52 (d, J = 11.9 Hz, 1H), 4.30 (d, J = 12.0 Hz, 1H), 3.79–3.74 (m, 2H, H-3, H-6), 3.70 (dd, J = 10.9, 4.7 Hz, 1H, H-6), 3.60 (t, J = 9.5 Hz, 1H, H-4), 3.57–3.47 (m, 3H, H-5), 3.41–3.30 (m, 3H, H-2), 1.62 (q, J = 6.2 Hz, 2H), 1.57–1.50 (m, 3H), 1.50–1.43 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 166.5, 138.4, 138.2, 133.6 (all quaternary), 132.1, 129.1, 128.6, 128.6, 128.5, 128.4, 128.0, 128.0, 127.9, 127.5, 127.7, 127.7 (all aromatic), 87.0 (CH, C-1), 86.5 (CH, C-3), 81.5 (CH, C-2), 79.1 (CH, C-5), 77.8 (CH, C-4), 76.0, 75.2, 73.5, 72.3 (all CH2), 69.1 (CH2, C-6), 46.2, 43.0, 26.6, 25.7, 24.7 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C40H45NO6S: 668.30458, found [M + H]+: 668.30591.
Phenyl 3,4,6-tri-O-benzyl-2-O-((piperidin-1-yl)ethan-1-one)-1-thio-β-D-galactopyranoside (39).
Using general procedure C starting from 2 (120 mg, 0.22 mmol) and 2-chloro-1-(piperidin-1-yl)ethan-1-one, the crude product was purified by silica gel flash column chromatography (0% to 10% Et2O in DCM), affording 39 (77 mg, 52%) as white solid. TLC:Rf = 0.78 (Et2O/DCM, 30/70 v/v); 1H NMR (500 MHz, CDCl3): δH 7.59–7.49 (m, 2H), 7.42–7.22 (m, 15H), 7.22–7.13 (m, 3H), 4.92 (d, J = 11.5 Hz, 1H), 4.73 (s, 2H), 4.66 (d, J = 9.6 Hz, 1H, H-1), 4.57 (d, J = 11.6 Hz, 1H), 4.46 (dd, J = 12.0, 2.4 Hz, 2H), 4.41 (d, J = 11.7 Hz, 1H), 4.33 (d, J = 12.2 Hz, 1H), 3.96 (d, J = 2.8 Hz, 1H, H-4), 3.77 (t, J = 9.4 Hz, 1H, H-2), 3.68–3.58 (m, 4H, H-3, H-5, H-6), 3.51 (tq, J = 13.2, 7.6, 5.9 Hz, 2H), 3.37–3.23 (m, 2H), 1.72–1.33 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 166.8, 138.9, 138.4, 138.0, 134.0 (all quaternary), 131.7, 128.9, 128.6, 128.5, 128.3, 128.1, 127.9, 127.9, 127.9, 127.8, 127.6, 127.3 (all aromatic), 87.2 (CH, C-1), 84.0 (CH, C-3), 78.1 (CH, C-2), 77.3 (CH, C-5), 74.6, 73.7 (both CH2), 73.6 (CH, C-4), 72.9, 72.4 (both CH2), 68.9 (CH2, C-6), 46.1, 42.9, 26.5, 25.6, 24.7 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C40H45NO6S: 668.30458, found [M + H]+: 668.30369.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-diethylacetamide)-β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (40).
Using general procedure D starting from 34 (40 mg, 0.061 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 40 as pure β-anomer (27 mg, 43%). TLC:Rf = 0.53 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.96 (m, 2H), 7.93–7.89 (m, 2H), 7.87–7.82 (m, 2H), 7.54–7.47 (m, 2H), 7.45–7.21 (m, 30H), 7.15 (dd, J = 7.4, 2.0 Hz, 2H), 6.15 (t, J = 9.8 Hz, 1H, H-3′), 5.45 (t, J = 9.8 Hz, 1H, H-4′), 5.25 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.20 (d, J = 3.6 Hz, 1H, H-1′), 5.03 (d, J = 10.8 Hz, 1H), 4.82 (d, J = 10.9 Hz, 1H), 4.78 (d, J = 10.8 Hz, 1H), 4.72 (d, J = 12.8 Hz, 1H), 4.53 (d, J = 4.0 Hz, 1H), 4.51 (d, J = 2.6 Hz, 1H), 4.44 (d, J = 6.9 Hz, 2H, H-1), 4.34 (ddd, J = 9.8, 7.2, 2.3 Hz, 1H, H-5′), 4.23 (d, J = 12.8 Hz, 1H), 4.04 (dd, J = 11.1, 2.3 Hz, 1H, H-6′), 3.78 (dd, J = 11.1, 7.1 Hz, 1H, H-6′), 3.69 (t, J = 9.0 Hz, 1H, H-3), 3.64–3.62 (m, 2H, H-6), 3.55 (t, J = 9.3 Hz, 1H, H-4), 3.45 (s, 3H), 3.43–3.35 (m, 3H, H-5), 3.34–3.20 (m, 3H, H-2), 1.15 (dt, J = 16.9, 7.1 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 167.9, 166.0, 165.9, 165.5, 138.8, 138.3 (all quaternary), 133.5, 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.6, 128.5, 128.5, 128.4, 128.4, 128.1, 127.9, 127.8, 127.7, 127.7 (all aromatic), 103.6 (CH, C-1), 97.0 (CH, C-1′), 84.2 (CH, C-3), 83.6 (CH, C-2), 77.6 (CH, C-4), 75.7, 75.1 (both CH2), 75.0 (CH, C-5), 73.5 (CH2), 72.2 (CH, C-2′), 71.6 (CH2), 70.7 (CH, C-3′), 69.9 (CH, C-4′), 69.1 (CH, C-5′), 68.9 (C-6, C-6′), 55.7 (CH3), 41.3, 40.2 (both CH2), 14.5, 13.1 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C61H65NO15: 1052.44324, found [M + H]+: 1052.44311.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-diethylacetamide)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (41).
Using general procedure D starting from 35 (40 mg, 0.061 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 41 as an anomeric mixture (α/β = 1/5, 28 mg, 44%). TLC:Rf = 0.63 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.95 (m, 2H), 7.89 (dd, J = 8.3, 1.4 Hz, 2H), 7.86–7.81 (m, 2H), 7.53–7.45 (m, 2H), 7.45–7.35 (m, 5H), 7.34–7.23 (m, 19H), 7.23–7.18 (m, 2H), 6.13 (t, J = 9.8 Hz, 1H, H-3′), 5.38 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.23 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.17 (d, J = 3.7 Hz, 1H, H-1′), 4.89 (d, J = 11.6 Hz, 1H), 4.81 (d, J = 11.8 Hz, 1H), 4.72 (d, J = 11.8 Hz, 1H), 4.66 (d, J = 12.6 Hz, 1H), 4.56 (d, J = 11.6 Hz, 1H), 4.42 (d, J = 7.6 Hz, 1H, H-1), 4.39–4.32 (m, 2H, H-5′), 4.30 (d, J = 11.8 Hz, 1H), 4.26 (d, J = 12.7 Hz, 1H), 4.00 (dd, J = 11.0, 2.2 Hz, 1H, H-6′), 3.84 (d, J = 2.9 Hz, 1H, H-4), 3.75 (dd, J = 11.0, 8.0 Hz, 1H, H-6′), 3.65 (dd, J = 9.6, 7.5 Hz, 1H, H-2), 3.55 (dd, J = 9.6, 3.0 Hz, 1H, H-3), 3.52–3.44 (m, 3H, H-5, H-6), 3.43 (s, 3H), 3.39–3.33 (m, 2H), 3.24 (ddq, J = 28.6, 14.0, 7.1, 6.7 Hz, 2H), 1.12 (t, J = 7.1 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.1, 166.0, 165.9, 165.6, 138.8, 138.8, 138.0 (all quaternary), 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.6, 128.5, 128.4, 128.4, 128.4, 128.3, 128.0, 127.9, 127.9, 127.6, 127.6 (all aromatic), 103.8 (CH, C-1), 96.8 (CH, C-1′), 81.7 (CH, C-3), 80.7 (CH, C-2), 74.7 (CH2), 73.7 (CH, C-4), 73.6 (CH2), 73.5 (CH, C-5), 73.3 (CH2), 72.3 (CH, C-2′), 72.0 (CH2), 70.7 (CH, C-3′), 70.1 (CH, C-4′), 69.1 (CH, C-5′), 68.9 (CH2, C-6′), 68.7 (CH, C-6), 55.7 (CH3), 41.2, 40.1 (both CH2), 14.5, 13.1 (both CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C61H65NO15: 1052.44324, found [M + H]+: 1052.44158.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-diphenylacetamide) -β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (42).
Using general procedure D starting from 36 (40 mg, 0.053 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 42 as pure β-anomer (53 mg, 86%). TLC:Rf = 0.31 (EtOAc/heptane, 40/60 v/v); 1H NMR (500 MHz, CDCl3): δH 8.03–7.98 (m, 2H), 7.89 (dd, J = 8.3, 1.4 Hz, 2H), 7.87–7.78 (m, 5H), 7.62–7.57 (m, 1H), 7.54–7.46 (m, 5H), 7.42–7.19 (m, 27H), 7.18–7.14 (m, 2H), 6.14 (t, J = 9.9 Hz, 1H, H-3′), 5.52 (t, J = 9.9 Hz, 1H, H-4′), 5.26 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.21 (d, J = 3.6 Hz, 1H, H-1′), 5.03 (d, J = 10.9 Hz, 1H), 4.80 (dd, J = 10.9, 2.9 Hz, 2H), 4.59 (d, J = 14.8 Hz, 1H), 4.56–4.46 (m, 3H), 4.36 (d, J = 7.8 Hz, 1H, H-1), 4.24 (ddd, J = 10.3, 5.4, 2.4 Hz, 1H, H-5′), 4.11 (d, J = 14.8 Hz, 1H), 4.01 (dd, J = 11.2, 2.5 Hz, 1H, H-6′), 3.81 (d, J = 5.8 Hz, 1H), 3.73 (dd, J = 11.2, 5.5 Hz, 1H, H-6′), 3.70–3.63 (m, 2H, H-3, H-6), 3.60 (dd, J = 10.9, 4.8 Hz, 1H, H-6), 3.50–3.44 (m, 1H, H-4), 3.43 (s, 3H), 3.37 (ddd, J = 9.9, 4.8, 2.0 Hz, 1H, H-5), 3.14 (dd, J = 9.1, 7.8 Hz, 1H, H-2) ppm; 13C NMR (126 MHz, CDCl3): δC 168.6, 165.9, 165.8, 165.2, 138.9, 138.2, 138.1 (all quaternary), 133.4, 133.3, 133.0, 132.6, 132.6, 131.5, 131.5 (all aromatic), 130.5 (quaternary), 130.0, 129.9 (both aromatic), 129.6 (quaternary), 129.6 (aromatic), 129.4, 129.1, 129.1 (both quaternary), 128.8, 128.4, 128.4, 128.3, 128.3, 128.2, 128.1, 128.0, 127.7, 127.6, 127.5, 127.5 (all aromatic), 103.1 (CH, C-1), 97.0 (CH, C-1′), 84.2 (CH, C-3), 83.0 (CH, C-2), 77.4 (CH, C-4), 75.6, 74.9 (both CH2), 74.9 (CH, C-5), 73.3 (CH2), 72.0 (CH, C-2′), 72.0 (CH2), 70.8 (CH, C-3′), 69.3 (CH, C-4′), 68.8 (CH2, C-6), 68.7 (CH, C-5), 68.2 (CH2, C-6′), 55.6 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C69H65NO15: 1148.44324, found [M + H]+: 1148.44728.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-diphenylacetamide)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (43).
Using general procedure D starting from 37 (20 mg, 0.027 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 43 as an anomeric mixture (α/β = 1/8, 22 mg, 71%). TLC:Rf = 0.76 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 8.02–7.97 (m, 2H), 7.86 (ddd, J = 16.8, 8.3, 1.4 Hz, 4H), 7.54–7.49 (m, 1H), 7.48–7.44 (m, 1H), 7.42–7.20 (m, 32H), 6.11 (t, J = 9.8 Hz, 1H, H-3′), 5.42 (t, J = 9.9 Hz, 1H, H-4′), 5.22 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.17 (d, J = 3.6 Hz, 1H, H-1′), 4.88 (d, J = 11.6 Hz, 1H), 4.83 (d, J = 11.7 Hz, 1H), 4.73 (d, J = 11.7 Hz, 1H), 4.56–4.48 (m, 2H), 4.40–4.31 (m, 3H, H-1), 4.25 (ddd, J = 9.5, 6.7, 2.3 Hz, 1H, H-5′), 4.17 (d, J = 14.8 Hz, 1H), 3.94 (dd, J = 11.2, 2.4 Hz, 1H, H-6′), 3.81 (d, J = 2.9 Hz, 1H, H-4), 3.72 (dd, J = 11.2, 6.7 Hz, 1H, H-6′), 3.56 (dd, J = 9.6, 3.0 Hz, 1H, H-3), 3.53–3.44 (m, 4H, H-2, H-5, H-6), 3.39 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 169.1, 166.0, 166.0, 165.5, 139.0, 138.9, 138.0 (all quaternary), 133.5, 133.4, 133.1 (all aromatic), 130.1, 130.0, 129.7 (all quaternary), 129.5, 129.3, 129.2, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.9, 127.8, 127.6, 127.6 (all aromatic), 103.7 (CH, C-1), 96.9 (CH, C-1′), 81.9 (CH, C-3), 80.6 (CH, C-2), 74.7 (CH2), 74.1 (CH, C-4), 73.6, 73.5 (both CH2), 73.4 (CH, C-5), 72.3 (CH, C-2′), 72.1 (CH, C-2′), 70.9 (CH2), 69.7 (CH, C-4′), 69.0 (CH, C-5′), 68.7 (CH2, C-6), 68.6 (CH2, C-6′), 55.7 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C69H65NO15: 1148.44324, found [M + H]+: 1148.44155.
Methyl 3,4,6-tribenzyl-2-O-((piperidin-1-yl)ethan-1-one)-α/β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (44).
Using general procedure D starting from 38 (40 mg, 0.060 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 44 as an anomeric mixture (α/β = 1/30, 26 mg, 40%). TLC:Rf = 0.56 (EtOAc/heptane, 40/60 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.95 (m, 2H), 7.93–7.90 (m, 2H), 7.84 (dd, J = 8.4, 1.4 Hz, 2H), 7.54–7.47 (m, 2H), 7.44–7.20 (m, 20H), 7.18–7.13 (m, 2H), 6.15 (t, J = 9.8 Hz, 1H, H-3′), 5.46 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.26 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.21 (d, J = 3.6 Hz, 1H, H-1′), 5.00 (d, J = 10.8 Hz, 1H), 4.80 (dd, J = 12.2, 10.9 Hz, 2H), 4.72 (d, J = 12.5 Hz, 1H), 4.53–4.50 (m, 2H), 4.46–4.41 (m, 2H, H-1), 4.34 (ddd, J = 9.8, 7.0, 2.3 Hz, 1H, H-5′), 4.24 (d, J = 12.6 Hz, 1H), 4.06 (dd, J = 11.0, 2.3 Hz, 1H, H-6′), 3.77 (dd, J = 11.0, 7.1 Hz, 1H, H-6′), 3.68 (t, J = 9.0 Hz, 1H, H-3), 3.62 (t, J = 3.1 Hz, 2H), 3.59–3.51 (m, 3H, H-4, H-6), 3.46 (s, 3H), 3.40 (ddd, J = 9.9, 4.3, 2.5 Hz, 1H, H-5), 3.31 (dd, J = 9.0, 7.8 Hz, 1H, H-2), 1.75–1.57 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 167.1, 166.0, 165.9, 165.5, 138.8, 138.3 (all quaternary), 133.5, 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.2, 129.1 (all quaternary), 128.6, 128.5, 128.5, 128.5, 128.4, 128.3, 128.1, 127.9, 127.8, 127.7, 127.7 (all aromatic), 103.5 (CH, C-1), 97.0 (CH, C-1′), 84.2 (CH, C-3), 83.3 (CH, C-2), 77.4 (CH, C-4), 75.7, 75.1 (both CH2), 75.0 (CH, C-5), 73.5 (CH2), 72.2 (CH, C-2′), 71.8 (CH2), 70.7 (CH, C-3′), 69.9 (CH, C-4′), 69.0 (CH, C-5′), 68.8 (CH2, C-6), 68.8 (CH2, C-6′), 55.7 (CH3), 46.1, 42.9, 26.6, 25.7, 24.7 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C62H65NO15: 1064.44324, found [M + H]+: 1064.44448.
Methyl 3,4,6-tribenzyl-2-O-((piperidin-1-yl)ethan-1-one)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzoyl-α-D-glucopyranoside (45).
Using general procedure D starting from 39 (30 mg, 0.045 mmol) and methyl 2,3,4 tri-O-benzoyl-α-D-glycopyranose (21), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 45 as an anomeric mixture (α/β = 1/4, 20 mg, 42%). TLC:Rf = 0.69 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 8.00–7.93 (m, 2H), 7.91–7.86 (m, 2H), 7.86–7.81 (m, 2H), 7.53–7.45 (m, 2H), 7.43–7.35 (m, 5H), 7.34–7.22 (m, 15H), 7.22–7.17 (m, 2H), 6.13 (t, J = 9.8 Hz, 1H, H-3′), 5.38 (dd, J = 10.3, 9.5 Hz, 1H, H-4′), 5.24 (dd, J = 10.2, 3.6 Hz, 1H, H-2′), 5.18 (d, J = 3.7 Hz, 1H, H-1′), 4.88 (d, J = 11.6 Hz, 1H), 4.79 (d, J = 11.8 Hz, 1H), 4.72 (d, J = 11.8 Hz, 1H), 4.65 (d, J = 12.4 Hz, 1H), 4.55 (d, J = 11.6 Hz, 1H), 4.40 (d, J = 7.6 Hz, 1H, H-1), 4.37–4.25 (m, 4H, H-6′), 4.01 (dd, J = 10.9, 2.2 Hz, 1H, H-6′), 3.83 (d, J = 2.9 Hz, 1H, H-4), 3.73 (dd, J = 10.9, 7.9 Hz, 1H), 3.64 (dd, J = 9.6, 7.6 Hz, 1H, H-2), 3.60–3.18 (m, 11H, H-3, H-5, H-6), 1.57–1.41 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 167.2, 166.0, 165.9, 165.6, 138.7, 138.7, 138.0 (all quaternary), 133.5, 133.2, 130.1, 130.0, 129.8 (all aromatic), 129.4, 129.3, 129.1 (all quaternary), 128.5, 128.5, 128.5, 128.4, 128.3, 128.0, 127.9, 127.8, 127.7, 127.6 (all aromatic), 103.7 (CH, C-1), 96.8 (CH, C-1′), 81.7 (CH, C-3), 80.5 (CH, C-2), 74.7, 73.6 (both CH2), 73.6 (CH, C-5), 73.5 (CH, C-4), 73.2, 72.3 (both CH2), 72.2 (CH, C-2′), 70.7 (CH, C-3′), 70.1 (CH, C-4′), 69.0 (CH, C-5′), 68.9 (CH2, C-6′), 68.7 (CH2, C-6), 55.7 (CH3), 46.1, 42.9, 26.6, 25.7, 24.7 (all CH2) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C62H65NO15: 1064.44324, found [M + H]+: 1064.43913.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide)-β-D-glucopyranosyl-(1 → 6)-2,3,4-O-tribenzyl-α-D-glucopyranoside (50).
Using general procedure D starting from 12 (50 mg, 0.080 mmol) and methyl 2,3,4 tri-O-benzyl-α-D-glycopyranose (46), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 50 as pure β-anomer (30 mg, 39%). TLC:Rf = 0.54 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 7.29 (tdt, J = 18.9, 8.6, 3.4 Hz, 28H), 7.17–7.14 (m, 2H), 4.97 (dd, J = 11.0, 2.6 Hz, 2H), 4.85–4.75 (m, 5H), 4.66 (d, J = 12.2 Hz, 1H), 4.62–4.51 (m, 6H, H-1′), 4.40 (d, J = 7.8 Hz, 1H, H-1), 4.24 (d, J = 13.3 Hz, 1H), 4.11 (dd, J = 10.9, 2.1 Hz, 1H, H-6′), 3.99 (t, J = 9.2 Hz, 1H, H-4′), 3.84 (ddd, J = 10.2, 5.5, 2.0 Hz, 1H, H-5′), 3.73–3.61 (m, 4H, H-3, H-6, H-6′), 3.56 (t, J = 9.4 Hz, 1H, H-4), 3.48 (dd, J = 9.6, 3.6 Hz, 1H, H-2′), 3.45–3.33 (m, 6H, H-2, H-5, H-3′), 2.79 (s, 3H), 2.75 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.5, 138.9, 138.9, 138.4, 138.3, 138.2, 138.2 (all quaternary), 128.6, 128.5, 128.5, 128.5, 128.5, 128.5, 128.5, 128.4, 128.2, 128.2, 128.1, 128.1, 127.9, 127.9, 127.8, 127.7, 127.7, 127.6 (all aromatic), 103.4 (CH, C-1), 98.0 (CH, C-1′), 84.3 (CH, C-3), 83.0 (CH, C-5), 82.0 (CH, C-4′), 79.9 (CH, C-2′), 78.4 (CH, C-3′), 77.8 (CH, C-4), 75.9, 75.6 (both CH2), 75.1 (CH, C-5), 75.0, 75.0, 73.5, 73.34, 71.3 (all CH2), 70.0 (CH, C-5′), 69.1 (CH2, C-6), 68.9 (CH2, C-6′), 55.4, 36.1, 35.3 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C59H67NO12: 1004.45609, found [M + Na]+: 1004.45386.
3,4,6-Tribenzyl-2-O-(methyl-N,N-diphenylacetamide)-α/β-D-glucopyranosyl-(1 → 6)-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (51).
Using general procedure D starting from 11 (30 mg, 0.048 mmol) and 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (47), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 51 as an anomeric mixture (α/β = 1/9, 23 mg, 61%). TLC:Rf = 0.33 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.42–7.37 (m, 2H), 7.35–7.23 (m, 11H), 7.14 (dd, J = 7.5, 2.1 Hz, 2H), 5.48 (d, J = 5.0 Hz, 1H, H-1′), 5.09 (d, J = 10.9 Hz, 1H), 4.84 (d, J = 10.8 Hz, 1H), 4.81 (d, J = 2.6 Hz, 1H), 4.78 (d, J = 5.0 Hz, 1H), 4.61–4.56 (m, 2H, H-3′), 4.50 (dd, J = 11.5, 4.7 Hz, 2H), 4.43 (d, J = 7.8 Hz, 1H, H-1), 4.29 (dd, J = 5.0, 2.4 Hz, 1H, H-2′), 4.23–4.21 (m, 1H), 4.20 (d, J = 2.1 Hz, 1H, H-4′), 4.11 (dd, J = 10.7, 2.9 Hz, 1H, H-6′), 4.01 (dt, J = 8.0, 2.4 Hz, 1H, H-5′), 3.73–3.63 (m, 4H, H-3, H-6, H-6′), 3.63–3.56 (m, 1H, H-4), 3.42 (ddd, J = 9.9, 4.4, 2.1 Hz, 1H, H-5), 3.36 (dd, J = 9.0, 7.8 Hz, 1H, H-2), 2.96 (s, 3H), 2.93 (s, 3H), 1.49 (s, 3H), 1.43 (s, 3H), 1.30 (d, J = 4.9 Hz, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 169.2, 138.9, 138.3, 138.3 (all quaternary), 128.5, 128.5, 128.4, 128.1, 128.0, 127.8, 127.7, 127.6 (all aromatic), 109.5, 108.6 (both quaternary), 103.8 (CH, C-1), 96.4 (CH, C-1′), 84.1 (CH, C-3), 83.5 (CH, C-2), 77.6 (CH, C-4), 75.6, 75.1 (both CH2), 74.8 (CH, C-5), 73.6 (CH2), 71.6 (CH, C-4′), 71.3 (CH2), 70.9 (CH, C-3′), 70.5 (CH, C-2), 69.9 (CH2, C-6′), 68.9 (CH2, C-6), 67.6, 36.5, 35.5, 26.2, 26.1, 25.1, 24.6 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C43H55NO12: 778.38025, found [M + H]+: 778.38084.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide)-α/β-D-glucopyranosyl-(1 → 4)-2,3,6-O-benzyl-α-D-glucopyranoside (52).
Using general procedure D starting from 12 (30 mg, 0.048 mmol) and methyl 2,3,6-tri-O-benzyl-α-D-glucopyranoside (48), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 52 as an anomeric mixture (α/β = 1/2, 10 mg, 30%). TLC:Rf = 0.46 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.42–7.18 (m, 28H), 7.16 (dd, J = 7.5, 2.0 Hz, 2H), 5.03 (d, J = 11.4 Hz, 1H), 4.88 (d, J = 11.1 Hz, 1H), 4.84 (d, J = 11.2 Hz, 1H), 4.79–4.74 (m, 3H), 4.63–4.60 (m, 2H), 4.59–4.50 (m, 4H, H-1′), 4.45 (d, J = 7.9 Hz, 1H, H-1), 4.41 (d, J = 7.1 Hz, 1H), 4.36 (d, J = 13.4 Hz, 1H), 4.29 (d, J = 13.1 Hz, 1H), 4.01 (t, J = 9.4 Hz, 1H, H-5′), 3.98–3.94 (m, 1H, H-6′), 3.84 (t, J = 9.2 Hz, 1H, H-3′), 3.73–3.68 (m, 2H, H-4′, H-6′), 3.66 (dd, J = 11.0, 1.9 Hz, 1H, H-6), 3.60 (t, J = 9.3 Hz, 1H, H-4), 3.55–3.47 (m, 3H, H-2′, H-3′, H-6), 3.36 (s, 3H), 3.28–3.21 (m, 2H, H-2, H-5), 2.86 (s, 3H), 2.78 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 176.1, 168.6, 139.7, 138.9, 138.6, 138.5, 138.4, 138.3 (all quaternary), 128.6, 128.5, 128.5, 128.5, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.1, 127.9, 127.9, 127.9, 127.8, 127.8, 127.7, 127.7, 127.6, 127.6, 127.5, 127.5, 127.4, 127.4, 127.2, 127.2, 127.0 (all aromatic), 101.9 (CH, C-1), 98.5 (CH, C-1′), 84.6 (CH, C-3), 83.8 (CH, C-2), 80.4 (CH, C-3′), 79.2 (CH, C-2′), 78.2 (CH, C-4), 76.3 (CH, C-5′), 75.5, 75.4 (both CH2), 75.2 (CH, C-5), 74.9, 73.8, 73.5, 73.4, 71.6 (all CH2), 70.2 (CH, C-4′), 68.9 (CH2, C-6), 68.6 (CH2, C-6′), 55.6, 36.3, 35.4 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C59H67NO12: 1004.45609, found [M + Na]+: 1004.45235.
1-Adamantanyl-2-O-(methyl-N,N-dimethylacetamide)-3,4,6-tribenzyl-α/β-D-glucopyranoside (53).
Using general procedure D starting from 11 (30 mg, 0.048 mmol) and adamantanol (49), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 53 as an anomeric mixture (α/β = 1/1, 11 mg, 34%). TLC:Rf = 0.36 (Et2O/toluene, 50/50 v/v); 1H NMR (500 MHz, CDCl3): δH 7.38–7.34 (m, 2H), 7.33–7.23 (m, 12H), 7.19 (dd, J = 7.7, 1.9 Hz, 2H), 4.99 (d, J = 11.0 Hz, 1H), 4.83 (d, J = 10.9 Hz, 2H), 4.71 (d, J = 7.8 Hz, 1H, H-1), 4.64 (d, J = 13.2 Hz, 1H), 4.60–4.50 (m, 3H), 4.28 (d, J = 13.2 Hz, 1H), 3.73–3.68 (m, 2H, H-3, H-6), 3.60 (dd, J = 10.7, 5.5 Hz, 1H, H-6), 3.50 (dd, J = 9.9, 8.6 Hz, 1H, H-4), 3.45 (ddd, J = 9.8, 5.5, 1.9 Hz, 1H, H-5), 3.30 (dd, J = 9.2, 7.8 Hz, 1H, H-2), 2.92 (s, 3H), 2.90 (s, 3H), 2.14 (p, J = 3.1 Hz, 3H), 1.92–1.87 (m, 3H), 1.81 (dp, J = 11.2, 1.9 Hz, 3H), 1.67–1.60 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.9, 139.0, 138.5, 138.3 (all quaternary), 128.5, 128.4, 128.4, 128.1, 128.1, 127.9, 127.7, 127.6 (all aromatic), 96.1 (CH, C-1), 84.7 (CH, C-3), 83.6 (CH, C-2), 78.3 (CH, C-4), 75.7, 75.0 (both CH2), 74.6 (CH, C-5), 73.5, 71.6 (both CH2), 69.6 (CH2, C-6), 42.9 (CH2), 36.4 (CH3 and CH2), 30.9 (CH) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C41H51NO7: 692.35632, found [M + Na]+: 692.35509.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide)-α/β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzyl-α-D-glucopyranoside (54).
Using general procedure D starting from 12 (30 mg, 0.048 mmol) and methyl 2,3,4 tri-O-benzyl-α-D-glycopyranose (46), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 54 as mixture of anomers (α/β = 1/3, 20 mg, 40%). TLC:Rf = 0.54 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 7.72 (dd, J = 5.7, 3.3 Hz, 2H), 7.53 (dd, J = 5.7, 3.3 Hz, 2H), 7.41–7.17 (m, 35H), 4.95 (d, J = 11.2 Hz, 1H), 4.89 (d, J = 11.5 Hz, 1H), 4.80–4.74 (m, 4H), 4.65 (d, J = 12.2 Hz, 1H), 4.57–4.55 (m, 3H, H-1′), 4.42 (d, J = 11.8 Hz, 1H), 4.37 (m, 4H, H-1), 4.30 (d, J = 13.1 Hz, 1H), 4.08 (dd, J = 10.8, 2.1 Hz, 1H, H-6′), 3.96 (t, J = 9.2 Hz, 1H, H-3′), 3.88 (d, J = 2.9 Hz, 1H, H-4), 3.83 (ddd, J = 10.2, 6.0, 2.1 Hz, 1H, H-5′), 3.70 (dd, J = 9.6, 7.6 Hz, 1H, H-2), 3.62–3.46 (m, 6H, H-5, H-6, H-2′, H-4′, H-6), 3.38–3.32 (m, 4H, H-3), 2.84–2.68 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 168.9, 139.0, 138.8, 138.7, 138.5, 138.3, 138.0 (all quaternary), 131.1, 129.0, 128.6, 128.6, 128.5, 128.5, 128.5, 128.3, 128.3, 128.2, 128.2, 128.0, 128.0, 128.0, 127.7, 127.7, 127.7, 127.6, 127.6 (all aromatic), 103.7 (CH, C-1′), 97.9 (CH, C-1), 82.1 (CH, C-3′), 82.0 (CH, C-5), 80.3 (CH, C-2), 80.0 (CH, C-2′), 78.6 (CH, C-3), 75.9, 75.0, 74.8 (all CH2), 73.7 (CH, C-4), 73.4 (CH, C-4′), 73.4, 73.0, 71.7 (all CH2), 70.0 (CH, C-5′), 69.0 (CH2, C-6′) 68.7 (CH2, C-6), 55.4, 36.2, 35.3 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C59H67NO12: 1004.45609, found [M + Na]+: 1004.45410.
3,4,6-Tribenzyl-2-O-(methyl-N,N-diphenylacetamide)-α/β-D-galactopyranosyl-(1 → 6)-1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (55).
Using general procedure D starting from 12 (40 mg, 0.063 mmol) and 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose (47), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 55 as an anomeric mixture (α/β = 1/4, 28 mg, 56%). TLC:Rf = 0.40 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.35–7.32 (m, 2H), 7.31–7.15 (m, 18H), 5.40 (d, J = 5.0 Hz, 1H, H-1′), 4.84 (dd, J = 16.0, 11.7 Hz, 2H), 4.70 (t, J = 12.5 Hz, 2H), 4.54 (d, J = 11.7 Hz, 1H), 4.49 (dd, J = 7.9, 2.4 Hz, 1H, H-3′), 4.38–4.30 (m, 2H, H-1), 4.20 (dd, J = 5.0, 2.4 Hz, 1H, H-2′), 4.17 (d, J = 13.2 Hz, 1H), 4.10 (dd, J = 8.0, 1.9 Hz, 1H, H-4′), 4.00 (dd, J = 10.7, 2.9 Hz, 1H, H-6′), 3.92 (dt, J = 8.0, 2.5 Hz, 1H, H-5′), 3.78 (d, J = 2.9 Hz, 1H, H-4′), 3.62 (dd, J = 9.7, 7.6 Hz, 1H, H-2), 3.56–3.45 (m, 4H, H-3, H-6, H-6′), 3.45–3.40 (m, 1H, H-5), 2.87 (s, 3H), 2.85 (s, 3H), 1.40 (s, 3H), 1.35 (s, 3H), 1.22 (s, 3H), 1.22 (s, 4H) ppm; 13C NMR (126 MHz, CDCl3): δC 169.4, 138.9, 138.8, 138.0 (all quaternary), 128.6, 128.6, 128.4, 128.3, 128.2, 128.1, 128.0, 127.9, 127.9, 127.7, 127.6 (all aromatic), 109.5, 108.6 (both quaternary), 104.0 (CH, C-1), 96.4 (CH, C-1′), 81.3 (CH, C-3), 80.9 (CH, C-2), 74.0 (CH, C-4), 73.6, 73.5 (both CH2), 73.4 (CH, C-5), 71.7 (CH2), 71.7 (CH, C-4′), 70.9 (CH, C-3′), 70.5 (CH, C-2′), 69.8 (CH2, C-6′), 68.8 (CH2, C-6), 67.6 (CH, C-5′), 36.5, 35.5, 26.2, 26.1, 25.2 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C43H55NO12: 778.38025, found [M + H]+: 778.38075.
Methyl 3,4,6-tribenzyl-2-O-(methyl-N,N-dimethylacetamide)-α/β-D-galactopyranosyl-(1 → 4)-2,3,6-O-benzyl-α-D-glucopyranoside (56).
Using general procedure D starting from 12 (40 mg, 0.063 mmol) and methyl 2,3,6-tri-O-benzyl-α-D-glucopyranoside (48), the crude product was purified by silica gel flash column chromatography (30% to 80% EtOAc in n-heptane), affording 56 as an anomeric mixture (α/β = 1/1, 14 mg, 22%). TLC:Rf = 0.69 (EtOAc/heptane, 80/20 v/v); 1H NMR (500 MHz, CDCl3): δH 7.40–7.19 (m, 27H), 7.18–7.11 (m, 3H), 5.00 (d, J = 10.7 Hz, 1H), 4.91 (d, J = 11.4 Hz, 1H), 4.80 (d, J = 12.0 Hz, 1H), 4.75–4.70 (m, 2H), 4.67–4.58 (m, 2H), 4.57 (d, J = 3.7 Hz, 1H, H-1′), 4.54–4.51 (m, 2H), 4.50–4.46 (m, 1H), 4.43 (d, J = 7.7 Hz, 1H, H-1), 4.36 (d, J = 6.7 Hz, 1H), 4.33 (d, J = 2.2 Hz, 1H), 4.23 (d, J = 11.9 Hz, 1H), 4.02–3.97 (m, 1H, H-6′), 3.95 (d, J = 9.4 Hz, 1H, H-4′), 3.91–3.88 (m, 1H, H-4), 3.82 (t, J = 9.3 Hz, 1H, H-3′), 3.75–3.70 (m, 2H, H-5′, H-6′), 3.61 (dd, J = 9.7, 7.6 Hz, 1H, H-2), 3.52 (t, J = 8.7 Hz, 1H, H-6), 3.48 (dd, J = 9.6, 3.8 Hz, 1H, H-2′), 3.42–3.32 (m, 6H, H-3, H-6), 3.29 (dd, J = 8.6, 5.0 Hz, 1H, H-5), 2.83 (s, 3H), 2.77 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 175.9, 169.3, 168.9, 139.6, 139.1, 138.6, 138.6, 138.3, 137.6 (all quaternary), 128.6, 128.6, 128.5, 128.5, 128.5, 128.4, 128.4, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.1, 128.1, 128.1, 128.0, 128.0, 128.0, 127.9, 127.9, 127.8, 127.8, 127.7, 127.6, 127.6, 127.5, 127.5, 127.4, 127.4, 127.1, 127.1, 126.7 (all aromatic), 102.4 (CH, C-1), 98.5 (CH, C-1′), 82.5 (CH, C-3), 80.9 (CH, C-2), 80.5 (CH, C-3′), 79.3 (CH, C-2′), 76.7 (CH, C-4′), 75.5, 74.9, 73.8 (all CH2), 73.5 (CH, C-4), 73.4, 73.1 (both CH2), 73.1 (CH, C-5′), 72.3, 72.0 (both CH2), 68.7 (CH2, C-6′), 68.2 (CH2, C-6), 55.5, 36.6, 36.3 (all CH3) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C59H67NO12: 982.47415, found [M + H]+: 982.47593.
1-Adamantanyl-2-O-(methyl-N,N-dimethylacetamide)-3,4,6-tribenzyl-α/β-D-galactopyranoside (57).
Using general procedure D starting from 12 (40 mg, 0.063 mmol) and adamantanol (49), the crude product was purified by silica gel flash column chromatography (30% to 60% EtOAc in n-heptane), affording 57 as an anomeric mixture (α/β = 2/3, 12 mg, 28%). TLC:Rf = 0.47 (EtOAc/heptane, 60/40 v/v); 1H NMR (500 MHz, CDCl3): δH 7.37–7.17 (m, 15H), 4.90 (d, J = 11.6 Hz, 1H), 4.77–4.70 (m, 2H), 4.67 (d, J = 7.4 Hz, 1H), 4.61 (d, J = 10.2 Hz, 1H), 4.58 (d, J = 8.8 Hz, 1H), 4.44 (d, J = 11.7 Hz, 1H), 4.39 (d, J = 11.7 Hz, 1H), 4.34 (d, J = 7.0 Hz, 1H), 3.85 (d, J = 2.8 Hz, 1H), 3.64 (dd, J = 9.7, 7.5 Hz, 1H), 3.59 (t, J = 3.1 Hz, 1H), 3.56 (d, J = 7.1 Hz, 2H), 3.52–3.50 (m, 1H), 2.90 (s, 3H), 2.89–2.88 (s, 3H), 2.11 (s, 3H), 1.87 (d, J = 12.2 Hz, 3H), 1.78 (dd, J = 11.1, 2.6 Hz, 3H), 1.64–1.59 (m, 6H) ppm; 13C NMR (126 MHz, CDCl3): δC 169.1, 138.8, 138.8, 138.3, 138.2 (all quaternary), 128.5, 128.5, 128.44, 128.3, 128.3, 128.0, 127.9, 127.8, 127.8, 127.8, 127.7, 127.6, 127.6, 127.5, 127.5, 127.5 (all aromatic), 96.3 (CH, C-1), 82.4 (CH, C-3), 80.7 (CH, C-2), 74.6 (CH2), 73.8 (CH, C-4), 73.7 (CH2), 73.3 (CH2), 73.2 (CH, C-5), 72.1 (CH2), 69.4 (CH2, C-6), 42.8 (CH2), 36.4 (CH3 and CH2), 30.8 (CH) ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C41H51NO7: 670.37438, found [M + H]+: 670.37506.
3,4,6-Tribenzyl-β-D-galactopyranosyl-(1 → 6)-2,3,4-O-tribenzyl-α-D-glucopyranoside (58).
25 mg (0.025 mmol, 1.0 eq.) of 50 was dissolved in THF (250 μL) and 1.0 μL (0.056 mmol, 2.2 eq.) water was added. To the mixture was added 150 μL of a 1.0 M t-BuOK solution (0.15 mmol, 5.9 eq.). The reaction mixture was stirred at room temperature for 18 h. The reaction was quenched with ice until two layers were formed. A solution of 2 M KHSO4 was added until the pH was 1. The solution was taken up in water (5 mL). The mixture was extracted with Et2O (5 × 10 mL) and the combined organic layers were dried over anhydrous MgSO4, filtrated and evaporated in vacuo to yield the carboxylic acid. The carboxylic acid was dissolved in dry DMF (40 μL). 5.3 μL DIPEA (0.031 mmol, 1.2 eq.) was added and the mixture was stirred on ice. To the cold solution was added 11 μL (0.027 mmol, 1.1 eq.) of a DPPA in dry DMF solution (1.1/1 v/v) and the solution was allowed to warm up to room temperature and was stirred for 2 h. The solution was cooled on ice and 1.4 μL (0.012 mmol, 0.5 eq.) of an acetic acid in dry DMF solution (1/1 v/v) was added to quench the reaction. At ambient temperature was added 21 μL (0.23 mmol, 9 eq.) of t-BuOH and the mixture was heated to 100 °C for 1 h. The solution was cooled on ice and 92 μL of a 2 M HCl solution was added. The mixture was taken up in water (5 mL) and extracted with toluene (3 × 10 mL). The combined toluene layers were washed with 1 M NaOH (10 mL), water (10 mL), brine (10 mL), dried over Na2SO4 (anhydrous), filtrated and evaporated in vacuo. The residue was dissolved in ethanol (10 μL), THF (30 μL) and 1 M NaOH was added (30 μL). The mixture was heated to 60 °C and for 3 h, heating was removed and the reaction was stirred for 14 h at ambient temperature. The reaction mixture was taken up in water (10 mL) and was extracted with toluene (3 × 10 mL). The combined toluene layers were washed with water (2 × 15 mL), washed with brine (15 mL), dried over Na2SO4 (anhydrous), filtrated and evaporated in vacuo to yield the crude product. The product was purified by silica gel flash column chromatography (30 to 60% EtOAc in n-heptane) to afford the product 58 (11.3 mg, 50%). TLC:Rf = 0.48 (EtOAc/heptane, 40/60 v/v); 1H NMR (500 MHz, CDCl3): δH 7.37–7.23 (m, 27H), 7.18–7.10 (m, 3H), 4.98 (d, J = 10.8 Hz, 1H), 4.90 (t, J = 11.7 Hz, 2H), 4.84–4.76 (m, 4H), 4.65 (d, J = 12.1 Hz, 1H), 4.63–4.60 (m, 2H, H-1′), 4.58 (d, J = 12.2 Hz, 1H), 4.54–4.50 (m, 2H), 4.22 (d, J = 6.7 Hz, 1H, H-1), 4.14 (dd, J = 11.1, 2.3 Hz, 1H, H-6′), 3.99 (t, J = 9.3 Hz, 1H, H-3), 3.82 (ddd, J = 10.1, 5.1, 2.2 Hz, 1H, H-5), 3.72 (dd, J = 10.9, 2.0 Hz, 1H, H-6), 3.67 (ddd, J = 10.9, 4.9, 2.0 Hz, 2H, H-6, H-6′), 3.59–3.47 (m, 5H, H-2, H-3, H-4, H-2′, H-4′), 3.44 (dq, J = 7.1, 2.1 Hz, 1H, H-5), 3.37 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): δC 138.7, 138.6, 138.2, 138.2, 138.1, 138.1 (all quaternary), 129.4, 128.5, 128.4, 128.4, 128.3, 128.1, 128.0, 128.0, 127.9, 127.8, 127.8, 127.7, 127.7, 127.7, 127.6, 127.6, 124.4, 121.3 (all aromatic), 103.5 (CH, C-1′), 98.1 (CH, C-1), 84.5 (CH, C-2), 82.0 (CH, C-3), 79.7 (CH, C-4), 78.0 (CH, C-4′), 77.5 (CH, C-2′), 75.8 (CH2), 75.3 (CH, C-5), 75.1, 75.0, 75.0 (all CH2), 74.5 (CH, C-3), 73.5, 73.4 (both CH2), 69.8 (CH, C-5′), 69.0 (CH2, C-6), 68.8 (CH2, C-6′), 55.3 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C55H60O11: 919.40333, found 919.40370.
(R)-(Tetrahydrofuran-2-yl)methanol.
In a three neck round bottom flask equipped with cooler, thermometer and dropping funnel was dissolved 0.95 gram (25 mmol, 1.6 eq.) LiAlH4 at 0 °C under an inert atmosphere in dry THF (24 mL). The LiAlH4 solution was stirred for 15 minutes. 1.5 mL (15 mmol, 1.0 eq.) (2R)-tetrahydro-2-furancarboxylic acid was dissolved in dry THF (15 mL). The carboxylic acid in THF solution was added dropwise to the LiAlH4 solution. The solution was allowed to reach a maximum temperature of 40 °C during addition of carboxylic acid solution in THF. The mixture was stirred at room temperature for 16 hours. The reaction was quenched by slow addition of saturated ammonium chloride (130 mL) at 0 °C. EtOAc (90 mL) was added to the mixture. The mixture was filtrated and the organic layer was separated from the aqueous layer. The aqueous layer was washed with EtOAc (5 × 30 mL). The combined organic layers were dried over MgSO4, filtrated and evaporated in vacuo to afford the pure product as colourless liquid (300 mg, 19%). TLC: Rf = 0.25 (EtOH/DCM, 1/20 v/v); 1H NMR (500 MHz, CDCl3): δH 4.01 (qd, J = 6.8, 3.3 Hz, 1H), 3.90–3.84 (m, 1H), 3.83–3.75 (m, 1H), 3.66 (dd, J = 11.7, 3.3 Hz, 1H), 3.50 (dd, J = 11.6, 6.2 Hz, 1H), 2.60 (s, 1H), 1.98–1.85 (m, 3H), 1.70–1.60 (m, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 79.5 (CH), 68.2, 64.9, 27.1, 26.0 (all CH2) ppm.
(S)-(Tetrahydrofuran-2-yl)methanol.
In a three neck round bottom flask equipped with cooler, thermometer and dropping funnel was dissolved 1.13 gram (30 mmol, 1.9 eq.) LiAlH4 at 0 °C under an inert atmosphere in dry THF (10 mL). The LiAlH4 solution was stirred for 15 minutes. 1.24 mL (13 mmol, 1.0 eq.) (2S)-tetrahydro-2-furancarboxylic acid was dissolved in dry THF (5 mL). The carboxylic acid in THF solution was added dropwise to the LiAlH4 solution. The solution was allowed to reach a maximum temperature of 40 °C during addition of carboxylic acid in THF. The mixture was stirred at room temperature for 16 hours. The reaction was quenched by slow addition of saturated ammonium chloride (130 mL) at 0 °C. EtOAc (90 mL) was added to the mixture. The mixture was filtrated and the organic layer was separated from the aqueous layer. The aqueous layer was washed with EtOAc (5 × 30 mL). The combined organic layers were dried over MgSO4, filtrated and evaporated in vacuo to obtain the pure product as yellow liquid (530 mg, 33%). TLC: Rf = 0.40 (EtOH/DCM, 1/20 v/v); 1H NMR (500 MHz, CDCl3): δH 4.01 (qd, J = 6.7, 3.3 Hz, 1H), 3.91–3.83 (m, 1H), 3.82–3.75 (m, 1H), 3.66 (dd, J = 11.6, 3.3 Hz, 1H), 3.50 (dd, J = 11.6, 6.2 Hz, 1H), 2.69 (s, 1H), 2.04–1.84 (m, 3H), 1.73–1.58 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 79.6 (CH), 68.3 (CH2), 64.9 (CH2), 27.2 (CH2), 26.1 (CH2) ppm.
(R)-(Tetrahydrofuran-2-yl)methyl 4-methylbenzene-sulfonate (19), method based on literature.34 To a solution of 0.88 gram (4.6 mmol, 1.6 eq.) tosylchloride and 0.7 mL (8.8 mmol, 3.0 eq.) pyridine in DCM (10 mL) was added 300 mg (2.94 mmol, 1.0 eq.) 56-(R) in DCM (10 mL). The reaction was stirred for 16 hours. Subsequently, water (20 mL) and DCM (20 mL) were added. The organic layer was separated from the aqueous layer and the organic layer was washed with 10% HCl (40 mL) and brine (40 mL). The organic layer was dried over Na2SO4, filtrated and evaporated in vacuo. The remaining oil was purified using silica gel flash column chromatography (0% to 20% EtOAc in n-heptane) to yield the product 17-(R) (545 mg, 73%). TLC: Rf = 0.30 (EtOAc/heptane, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.83–7.77 (m, 2H), 7.37–7.33 (m, 2H), 4.11–4.06 (m, 1H), 4.04–3.96 (m, 2H), 3.81–3.70 (m, 2H), 2.45 (s, 3H), 2.02–1.93 (m, 1H), 1.87 (dddd, J = 10.0, 8.0, 6.1, 3.0 Hz, 2H), 1.66 (ddt, J = 12.1, 8.2, 6.8 Hz, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 144.9, 133.1 (both quaternary carbon), 129.9, 128.1 (both aromatic carbon), 76.0 (CH), 71.6, 68.7, 28.0, 25.7 (all CH2), 21.8 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06612.
7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.83–7.77 (m, 2H), 7.37–7.33 (m, 2H), 4.11–4.06 (m, 1H), 4.04–3.96 (m, 2H), 3.81–3.70 (m, 2H), 2.45 (s, 3H), 2.02–1.93 (m, 1H), 1.87 (dddd, J = 10.0, 8.0, 6.1, 3.0 Hz, 2H), 1.66 (ddt, J = 12.1, 8.2, 6.8 Hz, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 144.9, 133.1 (both quaternary carbon), 129.9, 128.1 (both aromatic carbon), 76.0 (CH), 71.6, 68.7, 28.0, 25.7 (all CH2), 21.8 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06612.
(S)-(Tetrahydrofuran-2-yl)methyl 4-methylbenzene-sulfonate (20), method based on literature.34 To a solution of 0.88 gram (4.6 mmol, 1.7 eq.) tosylchloride and 0.7 mL (8.7 mmol, 3.3 eq.) pyridine in DCM (10 mL) was added 271 mg (2.65 mmol, 1.0 eq.) 56-(S) in DCM (10 mL). The reaction was stirred for 16 hours. Subsequently, water (20 mL) and DCM (20 mL) were added. The organic layer was separated from the aqueous layer and the organic layer was washed with 10% HCl (40 mL) and brine (40 mL). The organic layer was dried over Na2SO4, filtrated and evaporated in vacuo. The remaining oil was purified using silica gel flash column chromatography (0% to 20% EtOAc in n-heptane) to afford the product 17-(S) (410 mg, 60%). TLC: Rf = 0.30 (EtOAc/heptane, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.87–7.73 (m, 2H), 7.40–7.30 (m, 2H), 4.12–4.06 (m, 1H), 4.04–3.95 (m, 2H), 3.76 (ddt, J = 30.0, 8.3, 6.7 Hz, 2H), 2.45 (s, 3H), 2.04–1.94 (m, 1H), 1.87 (dtt, J = 8.0, 6.7, 3.2 Hz, 2H), 1.71–1.64 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 144.91, 133.13 (both quaternary), 129.94, 128.10 (both aromatic), 76.05 (CH), 71.60, 68.75, 27.99, 25.71 (all CH2), 21.78 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06504.
7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.87–7.73 (m, 2H), 7.40–7.30 (m, 2H), 4.12–4.06 (m, 1H), 4.04–3.95 (m, 2H), 3.76 (ddt, J = 30.0, 8.3, 6.7 Hz, 2H), 2.45 (s, 3H), 2.04–1.94 (m, 1H), 1.87 (dtt, J = 8.0, 6.7, 3.2 Hz, 2H), 1.71–1.64 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 144.91, 133.13 (both quaternary), 129.94, 128.10 (both aromatic), 76.05 (CH), 71.60, 68.75, 27.99, 25.71 (all CH2), 21.78 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06504.
(Hydroxymethyl)diphenylphosphine oxide, method based on literature.35 To a solution consisting of 6.2 mL 37% aqueous formaldehyde (0.083 mmol, 25 eq.) and 6.5 mL concentrated hydrochloric acid was added 0.6 mL (0.003 mmol, 1.0 eq.) chlorodiphenylphosphine. The solution was heated to 100 °C and was stirred for 16 hours. After 16 hours the reaction mixture was neutralised using NaHCO3. The neutralised solution was extracted with DCM (3 × 40 mL), dried over MgSO4, filtrated and evaporated. The product was without further purification as white solid (660 mg, 85%). TLC:Rf = 0.57 (MeOH/EtOAc, 10/90 v/v); 1H NMR (500 MHz, CDCl3): δH 7.82–7.72 (m, 4H), 7.59–7.42 (m, 6H), 5.23 (s, 1H), 4.42 (dd, J = 11.0, 3.8 Hz, 2H) ppm; 13C NMR (126 MHz, CDCl3): δC 132.2, 131.41, 131.3 (all quaternary), 130.9, 130.1 (both quaternary), 128.8, 128.7 (both aromatic), 61.67, 61.0 (both CH2) ppm; 31P NMR (202 MHz, CDCl3): δP 30.77 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C13H13O2P: 233.07314, found [M + H]+: 233.07190.
(Bromomethyl)diphenylphosphine oxide (18), method based on literature.36 A suspension of 500 mg (2.15 mmol, 2.5 eq.) 56 and molecular sieves (4 Å) in benzene (2 mL) was heated to reflux. While refluxing, 0.1 mL (1.1 mmol, 1.2 eq.) PBr3 was added dropwise and the solution started to boil heavily. After stirring for 10 minutes an orange sticky precipitate was formed. The solution was refluxed for 5 h. The solution (orange) was cooled to room temperature, chloroform (5 mL) was added, the mixture was stirred for 15 minutes and the precipitate was scratched of the flask surface, stirred for 1 h and filtrated. The organic layer was washed with water (15 mL), saturated aqueous NaHCO3 solution (15 mL) and water (15 mL). The organic layer was dried over Na2SO4 and filtrated. The solvent was evaporated and the remaining crude product was purified by silica gel flash column chromatography (60% to 100% EtOAc in n-heptane) to afford 91 (375 mg, 59%). TLC:Rf = 0.57 (EtOAc/heptane, 90/10 v/v); 1H NMR (500 MHz, CDCl3): δH 7.85–7.76 (m, 4H), 7.64–7.56 (m, 2H), 7.56–7.48 (m, 4H), 3.81 (d, J = 5.8 Hz, 2H) ppm; 13C NMR (126 MHz, CDCl3): δC 132.8, 131.6 (both aromatic), 130.6, 129.8 (both quaternary), 128.9 (aromatic), 23.9, 23.4 (both CH2) ppm; 31P NMR (202 MHz, CDCl3): δP 27.05 ppm; HRMS (ESI-TOF) (m/z): [M + H]+ calculated for C13H12BrOP: 294.98874, found [M + H]+: 294.98977.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We gratefully acknowledge the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) for the NWO Vidi grant VI.VIDI.192.070 awarded to TJB.
References
- T. J. Boltje, T. Buskas and G. J. Boons, Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research, Nat. Chem., 2009, 1(8), 611–622 CrossRef CAS.
- X. Zhu and R. R. Schmidt, New principles for glycoside-bond formation, Angew. Chem., Int. Ed., 2009, 48(11), 1900–1934 CrossRef CAS PubMed.
- J. T. Smoot and A. V. Demchenko, How the Arming Participating Moieties can Broaden the Scope of Chemoselective Oligosaccharide Synthesis by Allowing the Inverse Armed− Disarmed Approach, J. Org. Chem., 2008, 73(22), 8838–8850 CrossRef CAS PubMed.
- K. Le Mai Hoang and X. Liu, The intriguing dual-directing effect of 2-cyanobenzyl ether for a highly stereospecific glycosylation reaction, Nat. Commun., 2014, 5, 5051 CrossRef.
- M. Karak, Y. Joh, M. Suenaga, T. Oishi and K. Torikai, 1,2- trans Glycosylation via Neighboring Group Participation of 2- O-Alkoxymethyl Groups: Application to One-Pot Oligosaccharide Synthesis, Org. Lett., 2019, 21(4), 1221–1225 CrossRef CAS.
- R. A. Mensink, H. Elferink, P. B. White, N. Pers, F. P. J. T. Rutjes and T. J. Boltje, A Study on Stereoselective Glycosylations via Sulfonium Ion Intermediates, Eur. J. Org. Chem., 2016, 2016(27), 4656–4667 CrossRef CAS.
- M. A. Fascione, C. A. Kilner, A. G. Leach and W. B. Turnbull, Do glycosyl sulfonium ions engage in neighbouring-group participation? A study of oxathiane glycosyl donors and the basis for their stereoselectivity, Chemistry, 2012, 18(1), 321–333 CrossRef CAS PubMed.
- T. Fang, Y. Gu, W. Huang and G. J. Boons, Mechanism of Glycosylation of Anomeric Sulfonium Ions, J. Am. Chem. Soc., 2016, 138(9), 3002–3011 CrossRef CAS PubMed.
- J.-H. Kim, H. Yang, J. Park and G.-J. Boons, A general strategy for stereoselective glycosylations, J. Am. Chem. Soc., 2005, 127(34), 12090–12097 CrossRef CAS PubMed.
- T. J. Boltje, J.-H. Kim, J. Park and G.-J. Boons, Stereoelectronic effects determine oxacarbenium vs β-sulfonium ion mediated glycosylations, Org. Lett., 2010, 13(2), 284–287 CrossRef PubMed.
- S. J. Moons, R. A. Mensink, J. P. J. Bruekers, M. L. A. Vercammen, L. M. Jansen and T. J. Boltje, alpha-Selective Glycosylation with beta-Glycosyl Sulfonium Ions Prepared via Intramolecular Alkylation, J. Org. Chem., 2019, 84(7), 4486–4500 CrossRef CAS PubMed.
- D. J. Cox, G. P. Singh, A. J. A. Watson and A. J. Fairbanks, Neighbouring Group Participation During Glycosylation: Do 2-Substituted Ethyl Ethers Participate?, Eur. J. Org. Chem., 2014, 2014(21), 4624–4642 CrossRef CAS.
- S. S. Nigudkar and A. V. Demchenko, Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry, Chem. Sci., 2015, 6(5), 2687–2704 RSC.
- C. S. Chao, C. Y. Lin, S. Mulani, W. C. Hung and K. K. Mong, Neighboring-group participation by C-2 ether functions in glycosylations directed by nitrile solvents, Chemistry, 2011, 17(43), 12193–12202 CrossRef CAS.
- W. Gilbert, Z. Youlin and H. Xuefei, Pre-activation based stereoselective glycosylations: Stereochemical control by additives and solvent, Sci. China: Chem., 2011, 54(1), 66–73 CrossRef.
- H. Satoh, H. S. Hansen, S. Manabe, W. F. van Gunsteren and P. H. Hünenberger, Theoretical investigation of solvent effects on glycosylation reactions: stereoselectivity controlled by preferential conformations of the intermediate oxacarbenium-counterion complex, J. Chem. Theory Comput., 2010, 6(6), 1783–1797 CrossRef CAS PubMed.
- J. Park, S. Kawatkar, J.-H. Kim and G.-J. Boons, Stereoselective glycosylations of 2-azido-2-deoxy-glucosides using intermediate sulfonium ions, Org. Lett., 2007, 9(10), 1959–1962 CrossRef CAS PubMed.
- Y. Kobashi and T. Mukaiyama, Highly α-Selective Glycosylation with Glycosyl Acetate via Glycosyl Phosphonium Iodide, Chem. Lett., 2004, 33(7), 874–875 CrossRef CAS.
- L. Wang, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codee, Reagent Controlled Stereoselective Synthesis of alpha-Glucans, J. Am. Chem. Soc., 2018, 140(13), 4632–4638 CrossRef CAS.
- A. B. Ingle, C.-S. Chao, W.-C. Hung and K.-K. T. Mong, Tuning Reactivity of Glycosyl Imidinium Intermediate for 2-Azido-2-deoxyglycosyl Donors in α-Glycosidic Bond Formation, Org. Lett., 2013, 15(20), 5290–5293 CrossRef CAS PubMed.
- A.-H. A. Chu, S. H. Nguyen, J. A. Sisel, A. Minciunescu and C. S. Bennett, Selective synthesis of 1, 2-cis-α-glycosides without directing groups. Application to iterative oligosaccharide synthesis, Org. Lett., 2013, 15(10), 2566–2569 CrossRef CAS PubMed.
- S. N. Lam and J. Gervay-Hague, Solution-Phase Hexasaccharide Synthesis Using Glucosyl Iodides, Org. Lett., 2002, 4(12), 2039–2042 CrossRef CAS PubMed.
- S. R. Lu, Y. H. Lai, J. H. Chen, C. Y. Liu and K. K. Mong, Dimethylformamide: an unusual glycosylation modulator, Angew. Chem., Int. Ed., 2011, 50(32), 7315–7320 CrossRef CAS PubMed.
- P. Cheshev, A. Marra and A. Dondoni, Direct epoxidation of d-glucal and d-galactal derivatives with in situ generated DMDO, Carbohydr. Res., 2006, 341(16), 2714–2716 CrossRef CAS PubMed.
- C. H. Marzabadi and C. D. Spilling, Stereoselective Glucal Epoxide Formation, J. Org. Chem., 1993, 58, 3761–3766 CrossRef CAS.
- G. Veeneman, S. Van Leeuwen and J. Van Boom, Iodonium ion promoted reactions at the anomeric centre. II An efficient thioglycoside mediated approach toward the formation of 1, 2-trans linked glycosides and glycosidic esters, Tetrahedron Lett., 1990, 31(9), 1331–1334 CrossRef CAS.
- J.-H. Kim, H. Yang, V. Khot, D. Whitfield and G.-J. Boons, Stereoselective Glycosylations Using (R)- or (S)-(Ethoxycarbonyl)benzyl Chiral Auxiliaries at C-2 of Glycopyranosyl Donors, Eur. J. Org. Chem., 2006, 2006(22), 5007–5028 CrossRef.
- D. M. Smith and K. A. Woerpel, Electrostatic interactions in cations and their importance in biology and chemistry, Org. Biomol. Chem., 2006, 4(7), 1195–1201 RSC.
- H. Koskela, I. Kilpelainen and S. Heikkinen, Some aspects of quantitative 2D NMR, J. Magn. Reson., 2005, 174(2), 237–244 CrossRef CAS.
- S. Heikkinen, M. M. Toikka, P. T. Karhunen and I. A. Kilpeläinen, Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: application to wood lignin, J. Am. Chem. Soc., 2003, 125(14), 4362–4367 CrossRef CAS PubMed.
- P. G. Gassman, Base-promoted hydrolysis of amides at ambient temperatures, J. Am. Chem. Soc., 1976, 98(5), 1275 CrossRef CAS.
- Y.-i. Ichikawa, H. Kubota, K. i. Fujita, T. Okauchi and K. Narasaka, Stereoselective β-C-and β-S-glycosylation of 2-deoxyribofuranose derivatives controlled by the 3-hydroxy protective group, Bull. Chem. Soc. Jpn., 1989, 62(3), 845–852 CrossRef CAS.
-
T. Motomura, H. Nagamori, K. Suzawa, H. Ito, T. Morita, S. Kobayashi and H. Shinkai, Fluorene compound and pharmaceutical use thereof, US Pat., 8343994, 2013 Search PubMed.
- S. Seo, X. Yu and T. J. Marks, Intramolecular hydroalkoxylation/cyclization of alkynyl alcohols mediated by lanthanide catalysts. Scope and reaction mechanism, J. Am. Chem. Soc., 2008, 131(1), 263–276 CrossRef PubMed.
- E. M. Schuster, M. Botoshansky and M. Gandelman, Pincer click ligands, Angew. Chem., Int. Ed., 2008, 47(24), 4555–4558 CrossRef CAS PubMed.
- A. N. Yarkevich, L. N. Petrova and S. O. Bachurin, Synthesis and biological activity of dialkylamino-substituted phosphine oxides, Russ. J. Gen. Chem., 2012, 82(10), 1659–1664 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ob02700a |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ,
Paul B.
White
and
Thomas J.
Boltje
,
Paul B.
White
and
Thomas J.
Boltje
 *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.83–7.77 (m, 2H), 7.37–7.33 (m, 2H), 4.11–4.06 (m, 1H), 4.04–3.96 (m, 2H), 3.81–3.70 (m, 2H), 2.45 (s, 3H), 2.02–1.93 (m, 1H), 1.87 (dddd, J = 10.0, 8.0, 6.1, 3.0 Hz, 2H), 1.66 (ddt, J = 12.1, 8.2, 6.8 Hz, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 144.9, 133.1 (both quaternary carbon), 129.9, 128.1 (both aromatic carbon), 76.0 (CH), 71.6, 68.7, 28.0, 25.7 (all CH2), 21.8 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06612.
7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.83–7.77 (m, 2H), 7.37–7.33 (m, 2H), 4.11–4.06 (m, 1H), 4.04–3.96 (m, 2H), 3.81–3.70 (m, 2H), 2.45 (s, 3H), 2.02–1.93 (m, 1H), 1.87 (dddd, J = 10.0, 8.0, 6.1, 3.0 Hz, 2H), 1.66 (ddt, J = 12.1, 8.2, 6.8 Hz, 1H) ppm. 13C NMR (126 MHz, CDCl3): δC 144.9, 133.1 (both quaternary carbon), 129.9, 128.1 (both aromatic carbon), 76.0 (CH), 71.6, 68.7, 28.0, 25.7 (all CH2), 21.8 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06612.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.87–7.73 (m, 2H), 7.40–7.30 (m, 2H), 4.12–4.06 (m, 1H), 4.04–3.95 (m, 2H), 3.76 (ddt, J = 30.0, 8.3, 6.7 Hz, 2H), 2.45 (s, 3H), 2.04–1.94 (m, 1H), 1.87 (dtt, J = 8.0, 6.7, 3.2 Hz, 2H), 1.71–1.64 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 144.91, 133.13 (both quaternary), 129.94, 128.10 (both aromatic), 76.05 (CH), 71.60, 68.75, 27.99, 25.71 (all CH2), 21.78 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06504.
7 v/v); 1H NMR (500 MHz, CDCl3): δH 7.87–7.73 (m, 2H), 7.40–7.30 (m, 2H), 4.12–4.06 (m, 1H), 4.04–3.95 (m, 2H), 3.76 (ddt, J = 30.0, 8.3, 6.7 Hz, 2H), 2.45 (s, 3H), 2.04–1.94 (m, 1H), 1.87 (dtt, J = 8.0, 6.7, 3.2 Hz, 2H), 1.71–1.64 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3): δC 144.91, 133.13 (both quaternary), 129.94, 128.10 (both aromatic), 76.05 (CH), 71.60, 68.75, 27.99, 25.71 (all CH2), 21.78 (CH3) ppm; HRMS (ESI-TOF) (m/z): [M + Na]+ calculated for C12H16O4S: 279.06670, found [M + Na]+: 279.06504.







