Transforming inorganic layered montmorillonite into inorganic–organic hybrid materials for various applications: a brief overview
G. Bishwa Bidita
Varadwaj
a,
Kulamani
Parida
*b and
Vincent O.
Nyamori
*a
aSchool of Chemistry and Physics, University of KwaZulu-Natal, Westville Campus, Durban-4000, South Africa. E-mail: nyamori@ukzn.ac.za
bCentre for Nano Science and Nano Technology, Institute of Technical Education and Research, Siksha ‘O’ Anusandhan University, Bhubaneswar-751030, India. E-mail: kulamaniparida@soauniversity.ac.in
First published on 19th July 2016
Abstract
The covalent grafting of organic components over the surface of montmorillonite clay forming inorganic–organic hybrid materials is reviewed. The synthesis strategies based on the reaction of silanol groups on the clay surface with organosilane reagents and the roles of various reaction parameters in the formulation of these inorganic–organic hybrid materials have been discussed in detail. The multiple potential applications of these hybrid materials in technological applications, such as adsorption of environmental pollutants, nanocomposite synthesis, health care and catalysis, are overviewed briefly in this review.
1. Introduction
Thoughtful efforts to synergize the favourable physico-chemical properties of inorganic and organic components in a single material paved the way for the formulation of inorganic–organic hybrid materials. Recent developments in materials chemistry have enabled researchers to develop various inorganic–organic functional materials by modifying inorganic hosts with organic functional molecules.1–3 The field of inorganic–organic hybrid materials has become one of the most attractive and emergent themes in materials science. The combination of different organic and inorganic moieties allows the preparation of hybrid materials featuring unique properties for multiple potential applications as advanced materials. The fabrication of such hybrid materials aims to combine the advantages of both the inorganic and organic components within the same solid, making possible designing of materials with high mechanical, structural and hydrothermal stability of inorganic materials, with the flexibility and functionality typical of organic compounds. In this regard, clay minerals constitute a versatile source of inorganic support for the fabrication of nanostructured advanced inorganic–organic hybrid materials due to their remarkable structural diversities as well as different chemical compositions.Clay minerals are a class of inorganic layered compounds.4 The basic structure of clays can be obtained through the stacking of two types of structural sheets. These are tetrahedral and octahedral sheets, sometimes separated by an interlayer. Different clay minerals are formed by different combination of these two units. The combination of basic clay units and interlayers produces many clay mineral species. These different clay minerals have quite different properties.
Among the wide variety of clay minerals available in nature, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type clay montmorillonite, having the general formula (Na, Ca)0.33 (Al, Mg)2 (Si4O10), is the most common and familiar member of the smectite group of clay minerals in materials chemistry (Fig. 1).5 It basically consists of 1 nm thick aluminosilicate layers, where one octahedral alumina sheet is sandwiched between two tetrahedral silica sheets. These layers are weakly bound by van der Waals forces. Partial isomorphous substitution of Si4+ ions by trivalent metal cations and Al3+ ions by divalent metal cations in the aluminosilicate layers causes a charge deficit. To balance this charge deficit, a number of exchangeable hydrated alkali and alkaline earth metal cations are electrostatically fixed in the interlayer space of montmorillonite.
1 type clay montmorillonite, having the general formula (Na, Ca)0.33 (Al, Mg)2 (Si4O10), is the most common and familiar member of the smectite group of clay minerals in materials chemistry (Fig. 1).5 It basically consists of 1 nm thick aluminosilicate layers, where one octahedral alumina sheet is sandwiched between two tetrahedral silica sheets. These layers are weakly bound by van der Waals forces. Partial isomorphous substitution of Si4+ ions by trivalent metal cations and Al3+ ions by divalent metal cations in the aluminosilicate layers causes a charge deficit. To balance this charge deficit, a number of exchangeable hydrated alkali and alkaline earth metal cations are electrostatically fixed in the interlayer space of montmorillonite.
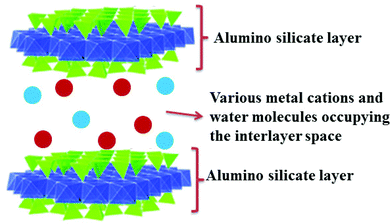 | ||
| Fig. 1 Schematic representation of montmorillonite layers and the cations along with water molecules present in its interlayer space. | ||
Montmorillonite is sometimes activated prior to its use. Acid activation on montmorillonite is a widely used approach to make it a more attractive material with high surface area and acidity. During acid activation, the edges of the crystals are opened, and the Al3+ and Mg2+ cations in the octahedral sheet (from the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layers) are exposed to the acid and leach out; the surface pore diameter expands; the degree of crystallinity of the clay mineral is reduced; and the specific surface area increases. The protons exchanged for interlamellar ions and the leached hydrated alumina occupying the cation exchange sites result in enhanced acidity in clay. The conditions maintained during acid activation such as concentration of acid and temperature influence the final acidity and result in different products. The varieties of commercially available acid activated montmorillonite are KSF, K10, K20, KP10 etc.6
1 layers) are exposed to the acid and leach out; the surface pore diameter expands; the degree of crystallinity of the clay mineral is reduced; and the specific surface area increases. The protons exchanged for interlamellar ions and the leached hydrated alumina occupying the cation exchange sites result in enhanced acidity in clay. The conditions maintained during acid activation such as concentration of acid and temperature influence the final acidity and result in different products. The varieties of commercially available acid activated montmorillonite are KSF, K10, K20, KP10 etc.6
In the context of preparing inorganic–organic hybrid materials, montmorillonite plays an important role as a parent inorganic host, with a determined crystal structure, morphology and chemical composition, resulting in a huge variety of host–guest assemblies and nanoarchitectures with versatile physical and chemical properties. Tailoring the functions of montmorillonite based inorganic–organic hybrid materials is a relatively new, yet rapidly evolving area, with a significant increase in the number of publications over the last few years.
The research on organic modification of montmorillonite in its early stage involved synthesis strategies based on cation exchange or adsorption of many organic moieties like cationic surfactants, metal complexes possessing organic ligands with the clay surface. However, at a later stage, the need for materials formed by strong bonding between the organic and inorganic components has triggered the covalent combination of structural organic and inorganic fragments. Covalent attachment of functional organic groups on the montmorillonite surface has advantages over physisorption and cation exchange. Such compounds would benefit from a much higher chemical stability as the guest species would be irreversibly bound to the structure (Fig. 2).
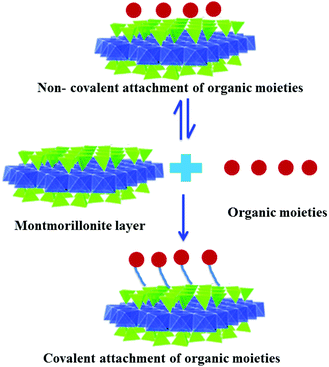 | ||
| Fig. 2 Schematic representation of covalent and non-covalent attachment of organic moieties over the surface of montmorillonite. | ||
Grafting is the most viable route for the covalent immobilization of organic functional groups over the surface of montmorillonite. In the process of grafting, the surface hydroxyl groups act as convenient anchoring points for organic functionalization. Organic functionalization by grafting is most commonly carried out by silylation.
2. Silylation/silane grafting over the surface of montmorillonite
Successful silylation of clay mineral surfaces strongly depends on the reactivity of clay mineral surfaces, including internal surfaces, external surfaces and “broken” edges. The molecular bond of the silane with the clay mineral can occur on the external surface of the clay mineral without altering the basal spacing, or it may occur in the interlayer space and/or edges of the clay mineral, providing expansion of the basal spacing.7,8 The chemical modification of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phyllosilicates by grafting with silanes occurs mainly on the hydroxyl groups of the external surface or broken edges of the clay.9–11 However, in the case of montmorillonite, the broken edges are the most reactive sites for grafting. Hydroxyl groups are known to be on the edges of clay particles. But in the case of montmorillonite, it is conjectured that a number of these may also be present in the interlamellar space due to structural defects and irregularities. This is particularly expected when montmorillonite is acid activated. During acid activation the interlayer cations are exchanged with H+ ions. These sites are susceptible to grafting via a hydrolysis reaction in acid activated montmorillonite. That is why the coexistence of phases of interlayer grafted and externally grafted montmorillonite could be observed when the montmorillonite is acidified before the grafting process.12 The successful grafting of the silylating agents onto the clay surfaces can established by 29Si NMR spectroscopic techniques, where two T3 and T2 peaks are usually recorded in the case of grafted clays along with the characteristic Q peak of Si atoms in the clays (Fig. 3).13
1 phyllosilicates by grafting with silanes occurs mainly on the hydroxyl groups of the external surface or broken edges of the clay.9–11 However, in the case of montmorillonite, the broken edges are the most reactive sites for grafting. Hydroxyl groups are known to be on the edges of clay particles. But in the case of montmorillonite, it is conjectured that a number of these may also be present in the interlamellar space due to structural defects and irregularities. This is particularly expected when montmorillonite is acid activated. During acid activation the interlayer cations are exchanged with H+ ions. These sites are susceptible to grafting via a hydrolysis reaction in acid activated montmorillonite. That is why the coexistence of phases of interlayer grafted and externally grafted montmorillonite could be observed when the montmorillonite is acidified before the grafting process.12 The successful grafting of the silylating agents onto the clay surfaces can established by 29Si NMR spectroscopic techniques, where two T3 and T2 peaks are usually recorded in the case of grafted clays along with the characteristic Q peak of Si atoms in the clays (Fig. 3).13
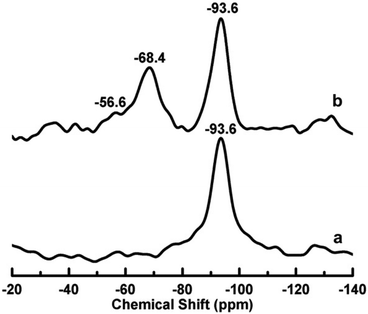 | ||
| Fig. 3 29Si CP/MAS NMR spectra of montmorillonite (a) and silylated montmorillonite (b) showing Q peaks at −93.6 ppm and T3 and T2 peaks at −68.4 ppm and −56.6 ppm, respectively, in the case of silylated montmorillonite.13 | ||
The thermal analysis also gives an idea of the orientation of the organic functionalities over the surface of montmorillonite.7 As can be seen from Fig. 4, the mass losses below 200 °C in both the materials are ascribed to the loss of physically adsorbed water molecules. It can also be seen that there is no mass loss in between 300 and 600 °C in the case of montmorillonite, whereas a three step mass loss in between this temperature range can be seen in the case of silylated montmorillonite. The three peaks observed in the DTG curves can be ascribed to the corresponding degradation of silane molecules adsorbed, intercalated/grafted.
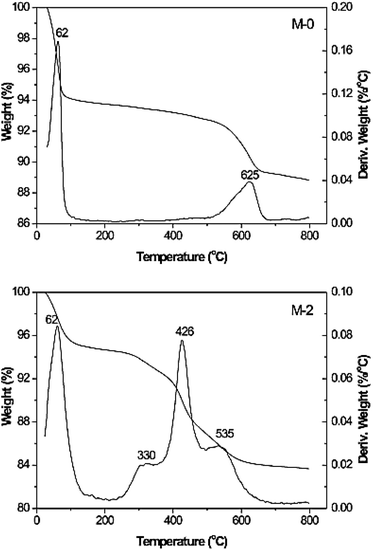 | ||
| Fig. 4 TGA and DTG curves of natural montmorillonite (M-0) and silylated montmorillonite (M-2).7 | ||
In interlayer grafting, the silylation reaction and the interlayer microstructure of the grafting products strongly depend on the clay materials. The properties like ion exchange and swelling ability of clay minerals have significant effects on the interaction between clays and the silylating agents. He et al. reported a grafting reaction between a trifunctional silylating agent, 3-aminopropyltriethoxysilane (APTES), and two kinds of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type layered silicates, i.e., natural montmorillonite and synthetic fluorohectorite.7 The arrangements of the silylating agents in the interlayer space were found to be different in both the clays. From XRD spectra, the basal spacings for montmorillonite and fluorohectorite were found to be 1.18 and 1.21 nm, respectively, whereas the basal spacing increased to 1.77 and 1.45 nm respectively after grafting (Fig. 5). The increase in basal spacing was more in the case of functionalized montmorillonite. Therefore, they concluded that in the natural montmorillonite, APTES adopted a parallel-bilayer arrangement, while it adopted a parallel-monolayer arrangement in the synthetic fluorohectorite. These different silane arrangements have a prominent effect on the mechanism of the condensation reaction within the clay gallery.
1 type layered silicates, i.e., natural montmorillonite and synthetic fluorohectorite.7 The arrangements of the silylating agents in the interlayer space were found to be different in both the clays. From XRD spectra, the basal spacings for montmorillonite and fluorohectorite were found to be 1.18 and 1.21 nm, respectively, whereas the basal spacing increased to 1.77 and 1.45 nm respectively after grafting (Fig. 5). The increase in basal spacing was more in the case of functionalized montmorillonite. Therefore, they concluded that in the natural montmorillonite, APTES adopted a parallel-bilayer arrangement, while it adopted a parallel-monolayer arrangement in the synthetic fluorohectorite. These different silane arrangements have a prominent effect on the mechanism of the condensation reaction within the clay gallery.
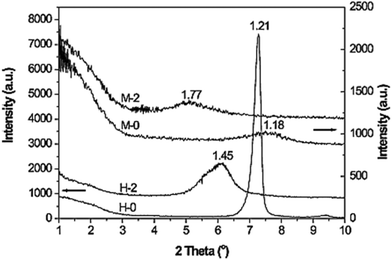 | ||
| Fig. 5 XRD patterns of montmorillonite (M-0) and fluorohectorite (H-0) before and after grafting.7 | ||
Like the different varieties of clays that behave differently towards the silylation, it is the grafting methods and media, which impart variation in the synthesis of the silylated clays, largely affecting the interlayer microstructures of the parent clay. Shen et al. reported silane grafting on Na+ montmorillonite taking two different grafting reaction systems: an ethanol–water mixture and vapor of silane using two different silylating agents: APTES and trimethylchlorosilane.14 The configuration of the silanes showed different roles in intercalation. Again, they found that the products prepared by vapor deposition have a larger basal spacing than that obtained from solution because of the diverse extents of silane hydrolysis in various grafting systems. In the case of the ethanol–water system, the silane molecules hydrolyse and condense among themselves before entering the interlayer space of montmorillonite, forming various sized polymers. Therefore, only those polymers with appropriate size and configuration can intercalate into the clay layers. However, in the case of vapour, the silanes enter the clay galleries and then hydrolysis and condensation occur there. The more prominent silylation for vapour of silane than for the ethanol–water system was supported by thermal analysis and FTIR spectroscopy.
In ethanol–water medium, the silanes and polymerized silanes enter the clay galleries. The interlayer water hydrolyses the silanes in the interlayer space into silanols, which condense among themselves or with the adjacent layers of the clay. Simultaneous condensation of the silane moiety with the two adjacent layers of the clay fixes the gallery height of the clay, which is also known as the “locking effect” (Fig. 6).13 This locking effect fixes the interlayer height of the silylated montmorillonite, which does not further change on intercalation of other species, thereby decreasing the swelling ability of the clay. As can be seen from Fig. 7, the interlayer distance of pristine montmorillonite changes upon CTAB intercalation, but due to the locking effect in the case of silylated montmorillonite, the interlayer distance got fixed.
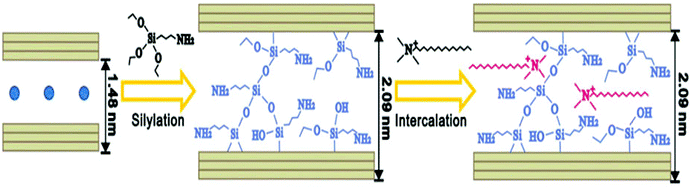 | ||
| Fig. 6 Silylation of montmorillonite with APTES and subsequent intercalation with CTAB.13 | ||
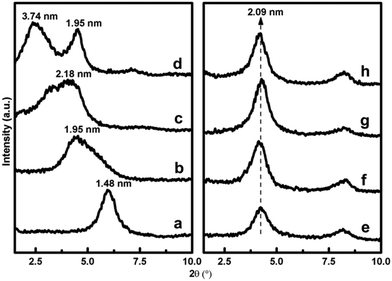 | ||
| Fig. 7 XRD spectra of pristine montmorillonite (a), silylated montmorillonite (b), CTAB intercalated pristine montmorillonite at different concentrations of CTAB (b)–(d); CTAB intercalated silylated montmorillonite at different concentrations of CTAB (f)–(h).13 | ||
The swelling behavior of the silylated clays is an important factor for the intercalation of guest molecules and exfoliation of the silylated clays layers for several industrial applications. The solvents play a pivotal role in controlling the swelling ability of the silylated clays. Grafting of silane onto the broken edge platelets or between the clay platelets depends on the solvent surface energy. The solvents having lower or equal surface energies than clay mineral can wet the clay surface easily, enabling the silane interaction with the hydroxyl groups of the clay edges, whereas solvents with high surface energy make the silane diffuse into the clay mineral layers because the interaction between the silane and edge clay platelets is insignificant due to low wetting phenomena.15 More silanes are diffused with increasing solvent surface energy. The surface area of the resulting material increases when prepared in a solvent having high surface energy.8
Again, the polarity of the solvent is a significant factor during silylation. The solvation properties of solvents influence the amount of silane loading. In the case of polar protic solvents, the solvent molecules solvate the silanol groups of the clay when it gets close to the clay surface. They form a solvent cage outside silanols on clay surfaces, making them less available to react with silanes. This results in a low loading of silanes on the clay surface, whereas in the case of nonpolar solvents, a lack of the solvation ability facilitates a greater amount of silane loading. Again, the swelling property also depends on the polarity of the solvents. Su et al. reported different swelling behaviors of the grafted products in polar and nonpolar solvents during the grafting of APTES onto the surface of montmorillonite.16 They observed that silylated products prepared in polar-protic solvents display a swelling ability, whereas silylated products prepared in non-polar solvents lead to a loss of swelling ability of the silylated products. Nonpolar solvents with low dielectric constant enable the intercalation, hydrolysis, and condensation of silane molecules and the formation of polysiloxane oligomers in the interlayer space of montmorillonite. However, in the case of polar-protic solvents with a high dielectric constant, a low loading of silane and a low degree of condensation among silane molecules occurs (Fig. 8).
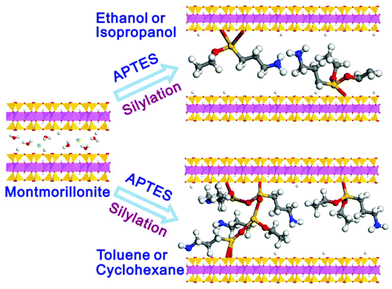 | ||
| Fig. 8 Schematic illustration of montmorillonite silylation with APTES in various solvents.16 | ||
Motokura et al. also carried out a detailed investigation on the effect of polar and non-polar solvents during the functionalization of acid activated montmorillonite with APTES.17 They carried out a detailed investigation and studied the effect of various solvents on basal spacing of the functionalized montmorillonite (Table 1). They found two types of acidic sites over the surface of acid activated montmorillonite, i.e., strong and weak acid sites.
| Samples (solvents) | Interlayer distance (Å) |
|---|---|
| Acid activated montmorillonite –NH2 [heptane] | 7.7 |
| Acid activated montmorillonite –NH2 [THF] | 7.9 |
| Acid activated montmorillonite –NH2 [toluene] | 9.6 |
| Acid activated montmorillonite –NH2 [DMSO] | 8.4 |
| Acid activated montmorillonite –NH2 [acetonitrile] | 5.4 |
| Acid activated montmorillonite –NH2 [water] | 7.3 |
| Acid activated montmorillonite | 2.6 |
During functionalization in nonpolar solvents, the amine groups in APTES readily interact with strong acid sites; then the silane-coupling reaction proceeds with the neighboring silanol groups. During silylation, the strongest acid sites remain connected to the nitrogen atom, whereas the weak acid sites leave the amine group, resulting in the formation of both protonated and unprotonated primary amines. The covalent bonding due to grafting increases the distance between the amine group and the acid sites geometrically. This suppresses the undesired acid-amine neutralization. However, in polar solvents, the immobilized propyl amine adopts a bent structure by interacting with the acid sites (Fig. 9). Therefore, the grafted clay synthesized in non-polar solvents has a larger basal spacing than polar solvents.
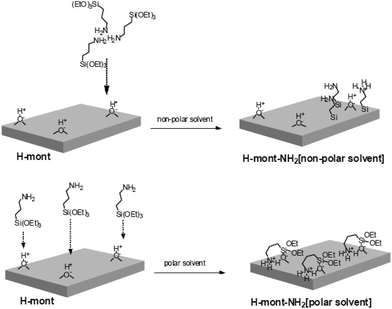 | ||
| Fig. 9 Immobilization of silylating agents over the surface of acid activated montmorillonite in polar and non-polar solvents.17 | ||
Again, the basal spacing also varies with the chain length of the organic silane moieties. By combining experimental and computational techniques, Piscitelli et al. reported that increasing the chain length of the silane moieties resulted in a decrease of the basal spacing of the grafted material.18 Organic silanes with short chain lengths are attracted by the surface of the clay. During its flattening onto the clay surface, a screening between the charges of the clay layers favours the weakening of interlayer attraction, which results in a larger d spacing value. However in the case of long chains containing one or more –NH groups, they have a tendency to interact among themselves because of both intermolecular hydrogen bonding and hydrophobic interactions. Therefore, their flattening onto the clay surface is reduced. As a result of this, the charge distribution on the clay surface is less screened. Therefore the increase in basal spacing is less pronounced as compared to the silanes with low chain lengths.
Studies investigating the modification of the varieties of montmorillonite with different types of silanes and different routes of synthesis have been performed by several researchers.19–24 Some researchers used the ultrasonic synthesis technique for grafting aminosilanes on the surface of montmorillonite.25,26 Usually montmorillonite consists of different silicate particles of various sizes like crystallites (0.001–0.01 μm), primary particles (1–10 μm) and agglomerated particles (100–1000 μm). Ultrasonic treatment results in dispersion of the agglomerated montmorillonite particles, turning them into lower sized primary particles or individual crystallites. After this, grafting of aminosilanes on the surface of clay is performed with the hydroxyl end groups.
Some reports also exist where montmorillonite was modified with many surfactants and pillaring agents prior to grafting with silylating agents for various reasons.27–29 Montmorillonite is often transformed into pillared interlayered clays, to increase their thermal and hydrothermal stability for its use in specific applications.30–32 The process involves exchange of cations with the pillaring agents (metal polycations), which upon calcination results in metal oxide pillars propping apart the clay layers, making the materials more stable. Zhu et al. reported that pillaring with large Al oligomeric cations increased the number of reactive –OH groups in the interlayer, which increased the silylation on the clay surface.27 The d001 value increased on pillaring and remained unchanged on further silylation. Based on this and other experimental parameters, they deduced a mechanism of the whole process that, after pillaring, some of the silylating agents also react with the –OH group of the pillaring agents.
Recently, Stevens et al. reported a water aided exfoliation/grafting method to introduce amine groups on the surface of montmorillonite and CTAB intercalated montmorillonite.33 The ultrasonic treatment of montmorillonite/CTAB intercalated montmorillonite led to a higher amine grafting rate and less pore blockage, where N-2-aminoethyl-3-aminopropyl trimethoxysilane (AAPTMS) was used as a silylating agent.
Most of the studies discussed above involved the functionalization of montmorillonite with silylating agents containing mainly amine groups. Apart from amine functionalization, many studies have been devoted to the functionalization of montmorillonite with 3-mercaptopropyltrimethoxy silane containing the sulfur group, which is subsequently converted to sulfonic acid by oxidation with H2O2 for its application in various fields.34–36 However, the conventional method is associated with certain drawbacks, for example, the oxidation of sulfur to sulfonic acid is highly exothermic and some amount of grafted silanes containing sulfur species gets lost during the process. Therefore, Kim et al. reported a simplified one-step grafting approach to render the organic sulfonic acid (HSO3−) functionality over the clay surface by refluxing organic sultones and a perfluorinated sultone with montmorillonite.37 From XPS analysis, they observed only one kind of sulfur species with a binding energy of 168.0 eV, which is associated with S6+ species of the SO3H group.
To introduce SO3H groups with a longer chain length, in another study, the same group reported a one-step synthesis of SO3H functionalized montmorillonite by the condensation reaction of the surface hydroxyl group of montmorillonite with the thiol group of 3-mercaptopropyltrimethoxy silane, and simultaneously attaching the sulfonic acid group by a ring opening reaction of 1,3-propane sultone.38 The new material is more thermally stable and showed a higher ion exchange capacity than montmorillonite grafted by a two-step method, which involves 3-mercaptopropyltrimethoxy silane condensation followed by oxidation with H2O2 (Fig. 10). The –SO3H groups with a long chain length increases the interlayer space of the functionalized montmorillonite and the high ion exchange capacity promotes the intercalation of various guest species.
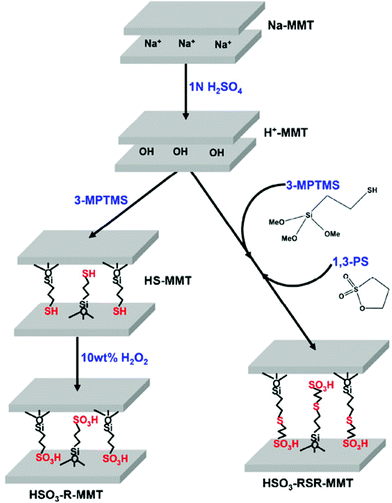 | ||
| Fig. 10 A schematic representation for the sulfonic acid functionalization steps of montmorillonites.38 | ||
Various other silylating agents reported in the literature for the functionalization of montmorillonite include 3-methyl acryloxy propyltrimethoxysilane, 2-acrylamido-2-methylpropanesulfonic acid, vinyltriethoxysilane, [3-(glycidyloxy)propyl]-trimethoxysilane aminoalkyldimethoxysilane (3-bromopropyl)trimethoxysilane(pentafluorophenyl)trimethoxysilane and (3-isocyanatopropyl)triethoxysilane and phenyltrimethoxysilane.39–44
Anirudhan et al. immobilized 3-methylacryloxypropyltrimethoxysilane onto the surface of Na-montmorillonite using ethanol/water solvent under a N2 atmosphere.45 They reported an interlayer grafting of the silylating agent on the clay surface. The functionalization occurred in two steps. The first step involved the hydrolysis of the silane molecule followed by silanol condensation as the second step.
Realizing the importance of organically functionalized montmorillonite, we have also synthesized many inorganic–organic hybrid materials using K10 montmorillonite as the inorganic moiety. K10 montmorillonite is a commercially available acid activated montmorillonite. Acid activation causes the leaching of Al3+ ions from the octahedral layers of montmorillonite. This delamination causes the formation of large voids in the montmorillonite structure, increasing the surface area and the formation of additional OH groups in the clay skeleton. We have reported the synthesis of bifunctional materials containing both acidic and basic sites. We carried out mono and diamine immobilization on the surface of K10 montmorillonite using APTES and AAPTMS.46 In another study, the surface of K10 montmorillonite was grafted with binary acid–base functionalities, such as sulfonic acid (–SO3H) and amine (–NH2) groups using 3-mercaptopropyltrimethoxy silane and APTES as the silylating agents respectively.47 The –SH group of the 3-mercaptopropyltrimethoxy silane was oxidized to a –SO3H group with the help of H2O2. The successful immobilization of both the silane moieties and the successful formation of a –SO3H group were confirmed by characterization techniques like NMR and XPS.
2.1 Auxiliary modifications on the silylated montmorillonite surface
The chemical modification of montmorillonite using silane coupling agents enables the introduction of functional groups that make the material suitable for interaction with different species. The modification becomes more flexible, especially when the silylating species contains an amine group. The metal ions can easily coordinate with the N atoms of the amine groups. In this regard, Pereira et al. reported the anchoring of a copper(II) acetylacetonate complex onto amine functionalized K10 montmorillonite and LAPONITE®, by the Schiff condensation between the amine groups of the anchored silylating agent and the carbonyl groups of the acetylacetonate ligand.48 They also immobilized the same metal complex onto the neat clays directly and found that the clay functionalization enhanced the complex immobilisation.Our group also did auxiliary modification on organically functionalized K10 montmorillonite. In one of our studies, we coordinated Cu2+ and Ni2+ metal ions with the N atom of the amine moiety of the amine functionalized montmorillonite.49 The metal ion incorporation onto the amine functionalized montmorillonite was done by a simple wetness impregnation method. Varying Cu and Ni ratios we prepared different samples. Various characterizations were employed to confirm the formation of the material. It was confirmed from the NMR spectrum that the amine moiety was covalently grafted on the clay surface and the +2 oxidation states of the Cu and Ni were confirmed from the XPS spectra.
In our previous work, we successfully immobilized Pd(II) over the surface of amine functionalized K10 montmorillonite by the same incipient wetness impregnation method.50 However, metals in their metallic states are garnering more attention in many applications because of their high surface-to-volume ratio. However a suitable support is needed to inhibit their aggregation for their effective utilization for many applications. Therefore, we functionalized K10 montmorillonite with two organic functional moieties, i.e., APTES and phenyltrimethoxysilane. After the organic functionalization, we loaded Pd(II) onto its surface, which was further reduced to Pd(0) using NaBH4 as a reducing agent. The organic amine group containing N acts as a linker, thereby binding Pd nanoparticles covalently through a Pd–N bond; decorating and stabilizing 3–5 nm sized Pd(0) nanoparticles through the organic amine moieties resulting in the formation of Pd(0) nanoparticle immobilized amine and phenyl group functionalized acid activated montmorillonite (Pd(0) NH2, Ph@MMT).51 The covalent immobilization of the phenyl and amine groups was confirmed from the NMR spectrum while the metallic state of Pd was observed from TEM, XRD and XPS studies.
3. Applications of organically modified montmorillonite
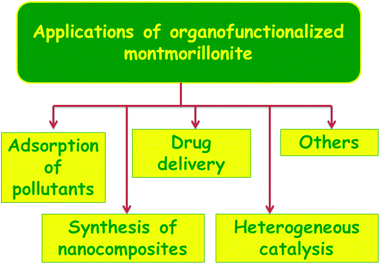
Montmorillonite clays functionalized with various organic groups served as an attractive class of inorganic–organic layered materials with remarkable properties and applications.52–55 In silylated montmorillonite, the interesting properties arise due to the combination of the nanoscopic space with the rich surface chemistry of the functional groups that are covalently linked to the montmorillonite clay. With the rapid progress of fabrication of montmorillonite, the research focus has been gradually shifted to practical applications with the potential of influencing our daily living conditions. Few such applications of organically functionalized montmorillonite are discussed in this section (Fig. 11). This section will not be so elaborative and will provide the readers a brief idea of the various applications of silylated montmorillonite, which will be helpful for them to carry out research in their area of interest.3.1 Adsorption of pollutants
Environmental pollution has become a severe problem that the globe is facing these days. It is increasing with each passing year, causing grave and irreparable damage to the earth. Water and air pollution are the most harmful forms of pollution in our environment. For removal of water and air pollutants, adsorption is the especially popular and frequently used technique due to its cleanliness, low cost and superior efficiency. Over the recent decades, adsorption has boomed owing to its economical application to many pollutants.56–59Clays are widely researched and applied owing to their high adsorption efficiency and cheapness.60–62 Extensive studies have shown that the sorptive capabilities of clay minerals can be modified substantially by incorporation of organic moieties into the clay interlamellar structure, i.e., in terms of maximum loading capacity as well as selectivity. The incorporation of an organic ligand can significantly increase both the retention capacity and the adsorption kinetics of the clay.
During adsorption of heavy metal cations, covalently attached organic groups over the surface of montmorillonite show advantages over physically adsorbed organic species such as surfactants on the clay surface, because their connection to the surface of montmorillonite is usually by weaker linkages like electrostatic interactions or van der Waals forces. Therefore, the surfactants are prone to desorption from the organically functionalized montmorillonite during the processes of sorption from aqueous solution or water-involved regeneration, which may cause a secondary pollution in aqueous medium.
The silylated montmorillonite showed better efficacy towards adsorption of heavy metal cations than raw montmorillonite. In the case of silylated montmorillonite, by introducing special functional groups in the organosilane agents used, the adsorption selectivity of the resultant materials to contaminants could be greatly improved. Organic species bearing a metal chelating thiol (–SH) functional group when grafted on the montmorillonite surface show efficacy towards both the binding affinity for heavy metals like Pb(II), Hg(II), Ni(II) etc. and the capability of being regenerated.9,12,20
Similarly, montmorillonite grafted with amino functionalities also showed efficacy towards various metal cations. Wu et al. studied the adsorption of Sr(II) employing neat Ca montmorillonite as well as three different samples of organically modified montmorillonite.23 They found that the adsorption capacity of APTES functionalized montmorillonite is much higher than that of neat clay as well as surfactant immobilized montmorillonite. The adsorption occurs on neat montmorillonite by simple ion exchange, on surfactant modified montmorillonite by surface adsorption and on APTES functionalized montmorillonite by ligand adsorption, respectively.
The amino organosilane intercalated montmorillonite forming cube-like structures in the interlamellar space of montmorillonite synthesized by Balomenou et al. bearing one polyamino tail at each cube apex adsorbed various metal ions in a monodentate fashion resulting in a maximization of metal binding efficiency.19 Our group also reported excellent adsorption capacity of sulfonic acid and amine group grafted K10 montmorillonite towards various metal cations like Pb2+, Hg2+ and Cd2+. We concluded from the results that the presence of S and N atoms in the organic functional groups is responsible for making the material highly adsorptive.47
3.2 Nanocomposite synthesis
Organically modified montmorillonite can be used to develop polymer–clay nanocomposites. In the last few years, polymer–clay nanocomposites have fascinated research and industry worldwide.71 The amalgamation of clay layers in polymer matrices is known to improve the properties of the resulting composites similarly to the naturally occurring polymer nanocomposites like bone, nacre and wood. The improvement is in various aspects like enhancement of mechanical properties, ionic conductivity, gas barrier properties, and reduction of thermal expansion.The nanocomposite of a partially sulfonated poly(ether ketone) polymer and organically modified montmorillonite provides proton exchange membranes.40 These nanocomposite membranes containing organically modified montmorillonite showed higher proton conductivity and membrane selectivity in comparison with that for neat Na montmorillonite. Various other organically modified montmorillonite–polymer nanocomposites prepared using various methods are: organically functionalized montmorillonite/polystyrene/polyacrylate/epoxy/poly(vinyl alcohol) (PVA)/Nafion® nanocomposites.21,22,24,26,28,35,37,38,72–74
Yu et al. prepared stable octadecenylsuccinic anhydride (ODSA) pickering emulsions using γ-methacryloxy propyltrimethoxysilane functionalized montmorillonite as particulate emulsifiers of ODSA and water at room temperature.39
3.3 Drug delivery
Over the last few years, inorganic layered materials, suitably modified with biologically active species, have gained growing attention as drug delivery agents. Clays, due to their chemical inertness, high adsorption capacity and good biocompatibility, give rise to persistent interest in their development for biological purposes.53,75 Drug delivery is one of the most important applications of organically functionalized montmorillonite, which allows ideally continuous, controlled, and/or targeted delivery of drugs along with improving their therapeutic effects. Utilizing the host–guest interaction of cyclodextrin molecules and the controlled release properties of silylated montmorillonite, Anirudhan et al. prepared a novel composite hydrogel MACD-g-MPTMS/MMT for colon specific tetracycline hydrochloride drug delivery.45 They demonstrated that this system can effectively deliver to the colon without the loss of the drug in the stomach, and could be a good candidate for an orally administered drug delivery system. Though there is not much work performed in this direction, this work can pave the way for researchers to carry out research on using organically functionalized montmorillonite as effective drug carriers.3.4 Heterogeneous catalysis
In heterogeneous catalysis, surface functionalization is a powerful means to modify the interactions between the support and the active phase.76 It is also capable of fine-tuning various properties of the support like electron donor–acceptor properties and acid–base properties and makes the catalyst stable for several catalytic cycles. In silylated montmorillonites, the covalently attached organic groups are sufficiently stable for recycling and reuse and can be easily modified to create a variety of catalytic sites. The covalent bonding between organic components and clay minerals enables a durable immobilization of the organic moieties in the resultant products and prevents their leaching into the surrounding solutions, in heterogeneous catalysis. In this regard, Moraes et al. reported bentonite functionalized propyl sulfonic acid as an effective catalyst towards the esterification reaction of acetic acid and 1-propanol.36Again, acid–base dual-activation catalysis has received much attention in the last few years. Many types of homogeneous reaction systems with acid–base dual-activation have been established, but apart from the recovery and reuse associated with homogeneous catalysis, it was also observed that strongly acidic and basic species in a single reactor induce neutralization immediately, thus affording inactive salts. A possible solution to these problems is the utilization of heterogeneous catalysts that possess both acidic and basic functions on their surfaces, without being neutralized. Amine group immobilization on the acid activated montmorillonite provides such a bifunctional system. Motokura et al. reported a one-pot deacetalization–Knoevenagel condensation reaction using organic primary amine grafted acid activated montmorillonite.17 In this direction, our group also studied Knoevenagel condensation between various alcohols and substrates containing active methylene groups using monoamine and diamine functionalized K10 montmorillonite.46 At room temperature and in the absence of solvents, diamine functionalized montmorillonite exhibited high efficiency in promoting the reaction. When appropriate organic moieties like amines are introduced on the surface of K10 montmorillonite, the resultant catalyst showed excellent activity towards the C–C bond forming Knoevenagel condensation reaction.
Moreover, the presence of additional organic functionalities gives rise to enhanced activities by cooperative effects. These multifunctional catalysts can enable one-pot reaction sequences, which save energy, time and the amounts of solvents used and are of extreme importance.77,78 Therefore, we prepared a bifunctionalized catalyst by grafting sulfonic acid (–SO3H) and amine (–NH2) on the surface of K10 montmorillonite through silylation.47 We studied the co-operative activity of this bifunctionalized catalyst in one-pot Henry reaction, which is one of the most useful C–C bond forming reactions and is usually catalyzed by organic and inorganic bases.
The functional groups, which are covalently tethered to the clay structure, offer strong capping ability for metal ions and nanoparticles, making them active catalysts for various organic reactions. In this regard, we reported organically functionalized montmorillonite supported Cu and Ni catalysts towards C–S coupling reaction and supported Pd(0) nanoparticles towards C–C coupling reactions.49,51 Pd(II) loaded K10 montmorillonite was also successfully applied for copper and phosphine free carbonylative Sonogashira reaction of aryl and hetero aryl iodides with terminal alkynes.50 In a recent report, Dutta et al. evaluated the success of rhodium complex immobilized amine functionalized montmorillonite towards carbonylation of methanol to acetic acid. The reaction was carried out in the vapour phase and they observed a catalytic conversion of up to 99.9%.79
3.5 Other applications
Besides the applications discussed above, organically functionalized montmorillonites are also used for various other applications. These applications include the production of functionalized cotton textiles by their derivatization with silylated montmorillonites and adsorption of organic dye.29,444. Conclusions and future prospects
This review summarizes recent progress in the study of using montmorillonite as an inorganic host to produce inorganic–organic hybrids upon grafting with organosilane reagents, their auxiliary modifications and the manifold applications of these fabricated materials. The integration of different organic functional sites onto the surface of montmorillonite combines the advantages of montmorillonite as well as the organic functionalities in a single material. Some exciting research contributions are highlighted in this review to illustrate the unique properties and important applications of the organically functionalized montmorillonite. Particularly, the synthesis and applications of these materials is a new and emerging area, and it is worth doing further research in this direction. However, there is a great deal of room for researchers to investigate in this area. Insights obtained in this study are of high importance to understand the mechanism of silylation of clay mineral surfaces and develop an efficient silylating technology. Mostly these materials are used in synthesis of nanocomposites, as adsorbents and heterogeneous catalysts. Furthermore, they also showed their efficacy in drug delivery and textiles. Future research should focus on closely linking the material's physicochemical properties with practical applications.Acknowledgements
GBBV and VON acknowledge the financial support from the University of KwaZulu-Natal (UKZN), Westville Campus, Durban, South Africa and the National Research Foundation, South Africa. They are also thankful to the UKZN Nanotechnology Platform for its support.References
- S. Wang, L. Tan, C. Zhang, I. Hussain and B. Tan, J. Mater. Chem. A, 2015, 3, 6542 CAS.
- A. Li, H. Shen, H. Ren, C. Wang, D. Wu, R. A. Martin and D. Qiu, J. Mater. Chem. B, 2015, 3, 1379 RSC.
- X. Liu, W. Zhao, H. Cui, Y. Xie, Y. Wang, T. Xu and F. Huang, Inorg. Chem. Front., 2015, 2, 315 RSC.
- G. W. Brindley, X-ray Identification and Crystal Structures of Clay Minerals, London, 1951 Search PubMed.
- G. B. B. Varadwaj and K. M. Parida, RSC Adv., 2013, 3, 13583 RSC.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed.
- H. He, J. Duchet, J. Galy and J. F. Gerard, J. Colloid Interface Sci., 2005, 288, 171 CrossRef CAS PubMed.
- A. M. Shanmugharaj, K. Y. Rhee and S. H. Ryu, J. Colloid Interface Sci., 2006, 298, 854 CrossRef CAS PubMed.
- I. K. Tonle, E. Ngameni, D. Njopwouo, C. Carteret and A. Walcarius, Phys. Chem. Chem. Phys., 2003, 5, 4951 RSC.
- C. Maqueda, Clays Clay Miner., 1998, 46, 423 Search PubMed.
- G. Sheng, S. Xu and S. A. Boyd, Soil Sci. Soc. Am. J., 1999, 63, 73 CrossRef CAS.
- L. Mercier and C. Detellier, Environ. Sci. Technol., 1995, 29, 1318 CrossRef CAS PubMed.
- L. Su, Q. Tao, H. He, J. Zhu and P. Yuan, Mater. Chem. Phys., 2012, 136, 292 CrossRef CAS.
- W. Shen, H. He, J. Zhu, P. Yuan and R. L. Frost, J. Colloid Interface Sci., 2007, 313, 268 CrossRef CAS PubMed.
- P. T. Bertuoli, D. Piazza, L. C. Scienza and A. J. Zattera, Appl. Clay Sci., 2014, 87, 46 CrossRef CAS.
- L. Su, Q. Tao, H. He, J. Zhu, P. Yuan and R. Zhu, J. Colloid Interface Sci., 2013, 391, 16 CrossRef CAS PubMed.
- K. Motokura, M. Tada and Y. Iwasawa, J. Am. Chem. Soc., 2009, 131, 7944 CrossRef CAS PubMed.
- F. Piscitelli, P. Posocco, R. Toth, M. Fermeglia, S. Pricl, G. Mensitieri and M. Lavorgna, J. Colloid Interface Sci., 2010, 351, 108 CrossRef CAS PubMed.
- G. Balomenou, P. Stathi, A. Enotiadis, D. Gournis and Y. Deligiannakis, J. Colloid Interface Sci., 2008, 325, 74 CrossRef CAS PubMed.
- W. A. Carvalho, C. Vignado and J. Fontana, J. Hazard. Mater., 2008, 153, 1240 CrossRef CAS PubMed.
- R. Ianchis, L. O. Cinteza, D. Donescu, C. Petcu, M. C. Corobea, R. Somoghi, M. Ghiurea and C. Spataru, Appl. Clay Sci., 2011, 52, 96 CrossRef CAS.
- A. A. Silva, K. Dahmouche and B. G. Soares, Appl. Clay Sci., 2011, 54, 151 CrossRef CAS.
- P. Wu, Y. Dai, H. Long, N. Zhu, P. Li, J. Wu and Z. Dang, Chem. Eng. J., 2012, 191, 288 CrossRef CAS.
- M. Huskić, M. Žigon and M. Ivanković, Appl. Clay Sci., 2013, 85, 109 CrossRef.
- R. Ianchis, D. Donescu, C. Petcu, M. Ghiurea, D. F. Anghel, G. Stanga and A. Marcu, Appl. Clay Sci., 2009, 45, 164 CrossRef CAS.
- G.-B. Huang, C.-H. Ge and B.-J. He, Appl. Surf. Sci., 2011, 257, 7123 CrossRef CAS.
- L. Zhu, S. Tian, J. Zhu and Y. Shi, J. Colloid Interface Sci., 2007, 315, 191 CrossRef CAS PubMed.
- A. K. Mishra, S. Allauddin, R. Narayan, T. M. Aminabhavi and K. V. S. N. Raju, Ceram. Interfaces, 2012, 38, 929 CrossRef CAS.
- Z. Qin, P. Yuan, S. Yang, D. Liu, H. He and J. Zhu, Appl. Clay Sci., 2014, 99, 229 CrossRef CAS.
- G. B. B. Varadwaj, S. Sahu and K. Parida, Ind. Eng. Chem. Res., 2011, 50, 8973 CrossRef CAS.
- G. B. B. Varadwaj and K. M. Parida, Catal. Lett., 2011, 141, 1476 CrossRef CAS.
- A. C. Pradhan, G. B. B. Varadwaj and K. M. Parida, Dalton Trans., 2013, 42, 15139 RSC.
- L. Stevens, K. Williams, W. Y. Han, T. Drage, C. Snape, J. Wood and J. Wang, Chem. Eng. J., 2013, 215–216, 699 CrossRef CAS.
- K.-W. Park and O.-Y. Kwon, Bull. Korean Chem. Soc., 2004, 25, 965 CrossRef CAS.
- C. H. Rhee, H. K. Kim, H. Chang and J. S. Lee, Chem. Mater., 2005, 17, 1691 CrossRef CAS.
- D. S. Moraes, R. S. Angélica, C. E. F. Costa, G. N. R. Filho and J. R. Zamian, Appl. Clay Sci., 2011, 51, 209 CrossRef CAS.
- Y. Kim, J. S. Lee, C. H. Rhee, H. K. Kim and H. Chang, J. Power Sources, 2006, 162, 180 CrossRef CAS.
- Y. Kim, Y. Choi, H. K. Kim and J. S. Lee, J. Power Sources, 2010, 195, 4653 CrossRef CAS.
- D. Yu, Z. Lin and Y. Li, Colloids Surf., A, 2013, 422, 100 CrossRef CAS.
- M. F. Samberan, M. M. H. Sadrabadi, S. R. Ghaffarian and A. Alimadadi, Int. J. Hydrogen Energy, 2013, 38, 14076 CrossRef CAS.
- Y. Xia, M. Ghasemlou, M. Rubino, R. Auras and J. Baghdachi, ACS Appl. Mater. Interfaces, 2015, 7, 24944 CAS.
- W. Ren, A. K. Chaudhary and K. Jayaraman, Ind. Eng. Chem. Res., 2015, 54, 4264 CrossRef CAS.
- D. Romanzini, V. Piroli, A. Frache, A. J. Zattera and S. C. Amico, Appl. Clay Sci., 2015, 114, 550 CrossRef CAS.
- A. Monteiro, B. Jarrais, I. M. Rocha, C. Pereira, M. F. R. Pereira and C. Freire, Appl. Clay Sci., 2014, 101, 304 CrossRef CAS.
- T. S. Anirudhan, S. Sandeep and P. L. Divya, RSC Adv., 2012, 2, 9555 RSC.
- G. B. B. Varadwaj, S. Rana and K. M. Parida, Dalton Trans., 2013, 42, 5122 RSC.
- G. B. B. Varadwaj, S. Rana, K. Parida and B. B. Nayak, J. Mater. Chem. A, 2014, 2, 7526 CAS.
- C. Pereira, S. Patrício, A. R. Silva, A. L. Magalhães, A. P. Carvalho, J. Pires and C. Freire, J. Colloid Interface Sci., 2007, 316, 570 CrossRef CAS PubMed.
- G. B. B. Varadwaj, S. Rana and K. M. Parida, RSC Adv., 2013, 3, 7570 RSC.
- S. P. Chavan, G. B. B. Varadwaj, K. M. Parida and B. M. Bhanage, Appl. Catal., A, 2015, 506, 237 CrossRef CAS.
- G. B. B. Varadwaj, S. Rana and K. M. Parida, J. Phys. Chem. C, 2014, 118, 1640 CAS.
- D. M. Fox, P. H. Maupin, R. H. Harris Jr., J. W. Gilman, D. V. Eldred, D. Katsoulis, P. C. Trulove and H. C. De Long, Langmuir, 2007, 23, 7707 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, P. Aranda, M. Darder and G. Rytwo, J. Mater. Chem., 2010, 20, 9306 RSC.
- L. Bromberg, C. M. Straut, A. Centrone, E. Wilusz and T. A. Hatton, ACS Appl. Mater. Interfaces, 2011, 3, 1479 CAS.
- M. Boruah, P. Gogoi, A. K. Manhar, M. Khannam, M. Mandal and S. K. Dolui, RSC Adv., 2014, 4, 43865 RSC.
- E. Kumar, A. Bhatnagar, W. Hogland, M. Marques and M. Sillanpää, Chem. Eng. J., 2014, 241, 443 CrossRef CAS.
- S. Sen Gupta and K. G. Bhattacharyya, Phys. Chem. Chem. Phys., 2012, 14, 6698 RSC.
- H.-T. Fan, W. Sun, B. Jiang, Q.-J. Wang, D.-W. Li, C.-C. Huang, K.-J. Wang, Z.-G. Zhang and W.-X. Li, Chem. Eng. J., 2016, 286, 128 CrossRef CAS.
- M. Yusuf, F. M. Elfghi, S. A. Zaidi, E. C. Abdullah and M. A. Khan, RSC Adv., 2015, 5, 50392 RSC.
- J. Gao and J. A. Pedersen, Environ. Sci. Technol., 2005, 39, 9509 CrossRef CAS PubMed.
- S. Sen Gupta and K. G. Bhattacharyya, RSC Adv., 2014, 4, 28537 RSC.
- Y. Bentahar, C. Hurel, K. Draoui, S. Khairoun and N. Marmier, Appl. Clay Sci., 2016, 119, 385 CrossRef CAS.
- W. S. Wan Ngah and M. A. K. M. Hanafiah, Bioresour. Technol., 2008, 99, 3935 CrossRef CAS PubMed.
- P. Z. Ray and H. J. Shipley, RSC Adv., 2015, 5, 29885 RSC.
- I. Ghiloufi, J. El Ghoul, A. Modwi and L. El Mir, Mater. Sci. Semicond. Process., 2016, 42, 102 CrossRef CAS.
- V. K. Gupta, P. J. M. Carrott, M. M. L. R. Carrott and Suhas, Crit. Rev. Environ. Sci. Technol., 2009, 39, 783 CrossRef.
- A. Bhatnagar and M. Sillanpaa, Chem. Eng. J., 2010, 157, 277 CrossRef CAS.
- S. Kagawa, S. Suh, K. Hubacek, T. Wiedmann, K. Nansai and J. Minx, Global Environ. Change, 2015, 35, 486 CrossRef.
- J. E. C. Lerner, E. Y. Sanchez, J. E. Sambeth and A. A. Porta, Atmos. Environ., 2012, 55, 440 CrossRef.
- N. Ramirez, A. Cuadras, E. Rovira, F. Borrull and R. M. Marce, Environ. Int., 2012, 39, 200 CrossRef CAS PubMed.
- M. Kotal and A. K. Bhowmick, Prog. Polym. Sci., 2015, 51, 127 CrossRef CAS.
- T. F. Silva, B. G. Soares, S. C. Ferreira and S. Livi, Appl. Clay Sci., 2014, 99, 93 CrossRef CAS.
- X. Ge, M.-C. Li, X. X. Li and U. R. Cho, Appl. Clay Sci., 2015, 118, 265 CrossRef CAS.
- D. Romanzini, A. Frache, A. J. Zattera and S. C. Amico, J. Phys. Chem. Solids, 2015, 87, 9 CrossRef CAS.
- W. Ma, W. O. Yah, H. Otsuka and A. Takahara, J. Mater. Chem., 2012, 22, 11887 RSC.
- D. Rath, S. Rana and K. M. Parida, RSC Adv., 2014, 4, 57111 RSC.
- B. M. Choudary, N. S. Chowdari, S. Madhi and M. L. Kantam, J. Org. Chem., 2003, 68, 1736 CrossRef CAS PubMed.
- K. Motokura, N. Fujita, K. Mori, T. Mizugaki, K. Ebitani, K. Jitsukawa and K. Kaneda, Chem. – Eur. J., 2006, 12, 8228 CrossRef CAS PubMed.
- P. K. Saikia, P. P. Sarmah, B. J. Borah, L. Saikia and D. K. Dutta, J. Mol. Catal. A: Chem., 2016, 412, 27 CrossRef CAS.
| This journal is © the Partner Organisations 2016 |