Facile synthesis and photovoltaic applications of a new alkylated bismethano fullerene as electron acceptor for high open circuit voltage solar cells†
Derya Barana,
Sule Erten-Ela*bd,
Andreas Kratzerb,
Tayebeh Ameria,
Christoph J. Brabecac and
Andreas Hirsch*b
aInstitute of Materials for Electronics and Energy Technology, Department of Materials Science and Engineering, Friedrich-Alexander-University Erlangen-Nurnberg, Martensstrasse 7, 91058, Erlangen, Germany
bDepartment of Chemistry and Pharmacy, Interdisciplinary Center of Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg, Henkestrasse 42, 91054 Erlangen, Germany. E-mail: andreas.hirsch@fau.de; Tel: +49 9131 85 22 537
cBavarian Center for Applied Energy Research (ZAE Bayern), Haberstrasse 2, 91058, Erlangen, Germany
dInstitute of Solar Energy, Ege University, Izmir, 35100, Turkey. E-mail: suleerten@yahoo.com; sule.erten@ege.edu.tr; Tel: +90 232 3111231
First published on 21st July 2015
Abstract
It is crucial to control the lowest unoccupied molecular orbital (LUMO) of electron accepting materials for producing efficient charge transfer in bulk heterojunction (BHJ) solar cells. Due to their high LUMO level, soluble bis-adducts of C60 are of high interest for improving the Voc in BHJ solar cells. In this work, we have developed a novel bis-4-propylpentyl[6,6]methanofullerene bis-adduct, NCBA, using a alkyl solubilizing group. The optoelectronic, electrochemical and photovoltaic properties of this bis-product are investigated. NCBA is successfully applied as the electron acceptor with poly(3-hexylthiophene) (P3HT) in a BHJ solar cell showing a high Voc of 0.73 V.
Introduction
The power conversion efficiencies of organic photovoltaic devices have been swiftly increased in the last years to 11% for single junction polymer:fullerene cells.1–3 Poly(3-hexylthiophene) (P3HT) is the widely used commercial conjugated polymer donor material, and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) is the most important soluble C60 derivative as an acceptor in BHJ solar cells. Power conversion efficiencies (PCE) around 4–5% have been reached based on the P3HT/PCBM system by device optimization.4,5 The PCE of the bulk heterojunction solar cells has been steadily increased by research progress on both the new photovoltaic materials and new device structures. Molecular design of the photovoltaic materials will play a key role in promoting the commercial application of the bulk heterojunction solar cells.6 In order to further improve the PCE of the polymer solar cells (PSCs), finding new conjugated polymer donor materials bearing broader absorption, lower bandgap, higher hole mobility, and suitable electronic energy levels have been the main goal in OPV field and some copolymers using push–pull property in the conjugated backbone showed higher photovoltaic efficiency than P3HT.6–12 Nevertheless, the research efforts toward new C60 derivative acceptor materials to replace PCBM have not been very successful until now with different polymer donors.13–15Among fullerene derivatives, PCBM offers the advantages of good solubility in organic solvents (chloroform, chlorobenzene, dichlorobenzene, etc.), higher electron mobility and higher electron affinity. However, weak absorption in the visible region and low lying LUMO level are the weak points. Weak absorption of PCBM limits the light harvesting in photovoltaic conversion and low LUMO level of the acceptor result in lower open circuit voltage (Voc) in PSCs, since Voc is strongly related to the difference between the LUMO level of acceptor and the HOMO of the donor material.16–18 Therefore, it is very important to design and synthesize new soluble fullerene derivatives with stronger visible absorption and higher LUMO energy levels than PCBM. Although many C60 derivatives and a new C84 derivative have been synthesized and utilized as acceptors in OPVs, only handful C60 derivatives achieved good solubility in organic solvents, desired electron mobility to have good charge transport, broad absorption to contribute to the photocurrent and suitable electronic levels to have efficient charge dissociation.19,20 The research effort devoted to new fullerene acceptor materials are increasing but the new fullerene derivative acceptors showing better photovoltaic performances than PCBM are very few. PCBM is still the most important acceptor material used in the organic solar cell studies. The miscibility and energy level match of fullerene acceptors with the low bandgap polymer donors should also be considered for achieving a high power conversion efficiency of organic solar cells.21–28
Among these electron acceptors based on fullerene, fullerene bis-adducts, which have two solubilizing groups, effectively increase Voc compared to P3HT:PCBM blended systems. The fullerene bis-adducts have higher LUMO energy levels because of the reduction of the molecule's electron affinity due to the presence of fewer unsaturated bonds as compared with fullerene monoadducts.29
Blom et al. reported bis-PCBM (a regioisomeric bis-adduct of PCBM) used as an electron acceptor and the Voc of the P3HT:bis-PCBM BHJ solar cells was 0.15 V higher than that of P3HT:PCBM due to the higher LUMO energy level of bis-PCBM as compared to PCBM.30 Afterwards, Laird and Li et al.26,29,31 reported a remarkable bis-adduct fullerene derivative, indene-C60 bis-adduct (ICBA). Through the Diels–Alder reaction of indene with the C60, ICBA achieves a LUMO energy level that is 0.17 eV higher than that of singly functionalized PCBM. A PSC consisting of P3HT and ICBA demonstrated a Voc of 0.84 V. However, due to byproducts, multistep purification procedures and the high cost of ICBA their future commercial application will be limited in PSCs.
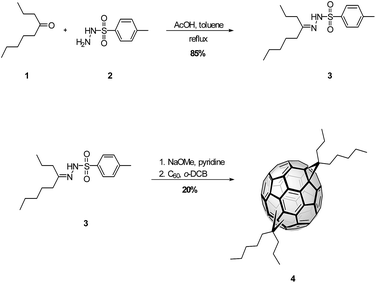
Herein, we introduce a new soluble fullerene based n-type semiconductor material, bis-4-propylpentyl-[6,6]-methanofullerene (NCBA), using 4-nonanone as a building block. Compared to indene bis-adducts which requires a large excess of indene to produce the final product (mole ratio of indene to C60 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), NCBA as shown in Scheme 1 is easily synthesized from of 4-nonyl-p-tosylhydrazone and C60 using 2
1), NCBA as shown in Scheme 1 is easily synthesized from of 4-nonyl-p-tosylhydrazone and C60 using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio. The synthesis of 4 was carried out by 1,3-dipolar cycloaddition reaction of diazo compound generated in situ by reaction between tosylhydrazone and sodium methoxide (Scheme 1). Condensation of 1 with p-tosylhydrazide in toluene affords tosylhydrazone 3, which after sequential treatment with sodium methoxide in pyridine and C60 in refluxing o-dichlorobenzene (o-DCB) affords bis-4-propylpentyl [6,6] methanofullerene (4) isomer mixture. Highly soluble bis-alkyl side chains raised the LUMO level 70 meV compared to PCBM because of bis-adduct of the methanofullerene derivative, resulting in an increase of Voc in devices with P3HT.
1 mole ratio. The synthesis of 4 was carried out by 1,3-dipolar cycloaddition reaction of diazo compound generated in situ by reaction between tosylhydrazone and sodium methoxide (Scheme 1). Condensation of 1 with p-tosylhydrazide in toluene affords tosylhydrazone 3, which after sequential treatment with sodium methoxide in pyridine and C60 in refluxing o-dichlorobenzene (o-DCB) affords bis-4-propylpentyl [6,6] methanofullerene (4) isomer mixture. Highly soluble bis-alkyl side chains raised the LUMO level 70 meV compared to PCBM because of bis-adduct of the methanofullerene derivative, resulting in an increase of Voc in devices with P3HT.
Experimental section
NMR spectroscopy was conducted using Bruker Avance 300 spectrometer. NMR-solvents were purchased from Deutero. The chemical shifts are reported in part per million (ppm) and referenced to the residual solvent. Spectral splitting patterns are designated as “s” (singlet), “d” (doublet), “m” (multiplet). The raw date was processed using MestReNova Lite.Mass spectrometry (MS) was done on a Shimadzu AXIMA Confidence MALDI-TOF MS-spectrometer (nitrogen UV-laser, 50 Hz, 337 nm). ESI mass spectrometry was carried out on a Bruker maxis 4 G UHR TOF MS/MS-Spectrometer.
1H NMR spectra were recorded on Bruker DPX 300 (300 MHz) spectrometer and chemical shifts are reported as δ values (ppm) and referenced to residual 1H signals in deuterated solvents.
Absorption profiles were recorded with a Perkin-Elmer Lambda-35 absorption spectrometer from 350 to 1100 nm. CV measurements were performed with an Metrohm μ Autolab III/FRA2 potentiostat/galvanostat. PL data were collected using a Perkin-Elmer LS55 Fluorescence Spectrometer. Unless otherwise stated, the PL excitation wavelength was set to 488 nm (approximately the absorption maximum for P3HT).
Bulk heterojunction devices were fabricated using polymers as electron donors and PC61BM (Solenne) as electron acceptor. The standard device structure was as follows: ITO/PEDOT:PSS (AL4083)/Polymer:PC61BM/Ca/Ag. ITO coated glass was used as transparent electrode. ITO was cleaned by ultrasonic treatment with acetone and isopropyl alcohol and dried under a flow of dry nitrogen. A PEDOT:PSS (AL4083, H.C. Stark) solution diluted in isopropanol was doctor-bladed onto clean substrates, resulting in a thickness of approximately 40 nm as determined with Dektak profilometer. PEDOT:PSS layer was annealed for 15 min at 140 °C in a nitrogen filled glovebox. The active layer consisting of P3HT (10 mg mL−1) and NCBA was stirred at 65 °C for 12 h before use. The active layers from chlorobenzene (CB) solution with different weight ratios were doctor-bladed onto PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS layer (∼100 nm). A Ca/Ag (∼15/85 nm) top electrode was evaporated via a mask in vacuum onto the active layers with an electrode area of 0.104 cm2. PCE was calculated from J–V characteristics recorded with Botest source measure unit by an Oriel Sol1A 94
PSS layer (∼100 nm). A Ca/Ag (∼15/85 nm) top electrode was evaporated via a mask in vacuum onto the active layers with an electrode area of 0.104 cm2. PCE was calculated from J–V characteristics recorded with Botest source measure unit by an Oriel Sol1A 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061 solar simulator with an intensity of 100 mW cm−2 where the light intensity was calibrated with a standard silicon photodiode. The EQE spectra were recorded with a Varian Cary 500 Scan spectrometer with tungsten light source using a lock-in amplifier. The electron and hole only devices with the same active layer thickness as actual devices were fabricated for space charge limited current (SCLC) measurements. A Rs of 4 Ω is considered for the voltage correction; V = Vappl−Vbi−Vrs, where V is the effective voltage, Vappl is the applied voltage, Vrs is the voltage drop and Vbi is the built-in voltage. The hole mobility was calculated by fitting dark J−V curves to SCLC model in which the current density is given by J = 9ε0εrμV2/8L3, where ε0 and εr represents the permittivity of the material, μ is the mobility, and L is the thickness of the active layer.
061 solar simulator with an intensity of 100 mW cm−2 where the light intensity was calibrated with a standard silicon photodiode. The EQE spectra were recorded with a Varian Cary 500 Scan spectrometer with tungsten light source using a lock-in amplifier. The electron and hole only devices with the same active layer thickness as actual devices were fabricated for space charge limited current (SCLC) measurements. A Rs of 4 Ω is considered for the voltage correction; V = Vappl−Vbi−Vrs, where V is the effective voltage, Vappl is the applied voltage, Vrs is the voltage drop and Vbi is the built-in voltage. The hole mobility was calculated by fitting dark J−V curves to SCLC model in which the current density is given by J = 9ε0εrμV2/8L3, where ε0 and εr represents the permittivity of the material, μ is the mobility, and L is the thickness of the active layer.
Morphology of the films were observed by Atomic Force Microscopy using Ambios AFM equipment.
Electrochemistry
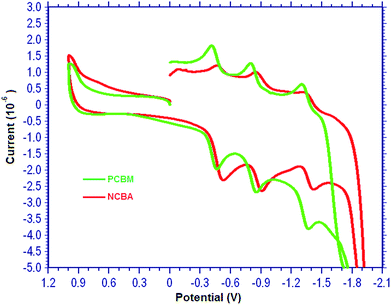
The electrochemical properties of compound NCBA have been studied at room temperature by means of cyclic voltammetry (CV). The cyclic voltammograms are collected using a CH-Instrument 660 B Model electrochemical analyzer. A three electrode cell set-up employed for the measurements consisted of glassy carbon working electrode, Pt wire counter electrode and Ag/AgCl reference electrode, all placed in a glass vessel. Tetrabutylammonium hexafluorophosphate (TBAPF6), 0.1 M, was used as supporting electrolyte. Ferrocene was used as internal reference electrode. The electrochemical properties of the NCBA are examined by cyclic voltammetry in o-DCB/MeCN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture with 100 mV s−1 scan rate. Compound NCBA display the typical redox behaviour of a [60]fullerene derivative, with quasi-reversible reduction waves at −0.49, −0.87 and −1.38 V (Fig. 2). Cyclic voltammogram was presented in Fig. 2 and all redox potentials of dimers are summarized in Table 1.
1) solvent mixture with 100 mV s−1 scan rate. Compound NCBA display the typical redox behaviour of a [60]fullerene derivative, with quasi-reversible reduction waves at −0.49, −0.87 and −1.38 V (Fig. 2). Cyclic voltammogram was presented in Fig. 2 and all redox potentials of dimers are summarized in Table 1.
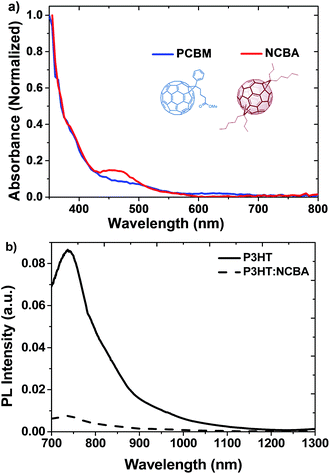 | ||
| Fig. 1 (a) UV-vis absorption spectra of NCBA and PCBM films and (b) Photoluminescence quenching of P3HT using 50% NCBA excited at 488 nm. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); scan rate 100 mV s−1
1); scan rate 100 mV s−1
| Ered1 (V) | Ered2 (V) | Ered3 (V) | Eferrocene (V) | |
|---|---|---|---|---|
| NCBA | −0.50 | −0.87 | −1.38 | 0.71 |
| PCBM | −0.43 | −0.82 | −1.33 | 0.71 |
Characterization
1H-NMR (CDCl3, 400 MHz) δ (ppm) = 7.85 (2H, d), 7.29 (2H, d), 2.42 (3H, s), 2.11 (4H, m), 1.2–1.8 (8H, m), 0.9 (6H, m).
1H-NMR (CDCl3, 400 MHz) δ (ppm) = 1.6–1.3 (24H, m), 0.84 (12H, t). Calcd: C 95.78, H 4.22; found C 95.75H 4.20. Mass (m/z): 972. Mass spectra supplied in ESI†.
Optical and electrochemical properties
Absorption in the visible region is a significant property for the acceptor material in organic photovoltaics (OPV)s. In literature, PC70BM and indene bis-adduct ICBA are superior to PCBM mainly due to their stronger absorption in the visible region.21 Fig. 1 represents the UV-vis absorption spectra of both PCBM and NCBA in thin film. The absorbance of NCBA is stronger in visible region between 400 nm and 500 nm than that of PCBM.The electrochemical properties of NCBA were studied by cyclic voltammetry (Fig. 2). The lowest unoccupied molecular orbital (LUMO) was calculated from their onset reduction (Eonsetred) obtained from the cyclic voltammogram. The LUMO energy level of NCBA is −3.59 eV, which is raised by 0.07 eV in comparison to that of PCBM (3.66 eV) in the same measurement system. The higher LUMO energy level of NCBA is desirable for its application in PSCs to get higher Voc. The shift in the LUMO level toward the vacuum level is due to the presence of the fewer unsaturated bonds as compared with fullerene monoadducts.
Photovoltaic properties
Photovoltaic performances of NCBA are investigated with P3HT as donor group in the normal structure BHJ solar cells using different donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor ratios. In BHJ devices, the weight ratio of donor/acceptor plays a significant role and has a large effect on PCE due to the balance between the absorption and the charge transport network of the active layer.33 Therefore, different weight ratios (2
acceptor ratios. In BHJ devices, the weight ratio of donor/acceptor plays a significant role and has a large effect on PCE due to the balance between the absorption and the charge transport network of the active layer.33 Therefore, different weight ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for P3HT
2) for P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA (10 mg mL−1) were prepared using chlorobenzene (CB) for device fabrication and 50% NCBA loading (1
NCBA (10 mg mL−1) were prepared using chlorobenzene (CB) for device fabrication and 50% NCBA loading (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be the optimized ratio. When reporting improvements in photovoltaic performance, it is critical to provide sufficient information in the form of statistics and experimental conditions to enable the work to be reproduced, and to allow the reader to evaluate the reliability of the data.34–36 Therefore, the J–V characteristics of P3HT:NCBA devices, under illumination, using different D
1) was found to be the optimized ratio. When reporting improvements in photovoltaic performance, it is critical to provide sufficient information in the form of statistics and experimental conditions to enable the work to be reproduced, and to allow the reader to evaluate the reliability of the data.34–36 Therefore, the J–V characteristics of P3HT:NCBA devices, under illumination, using different D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
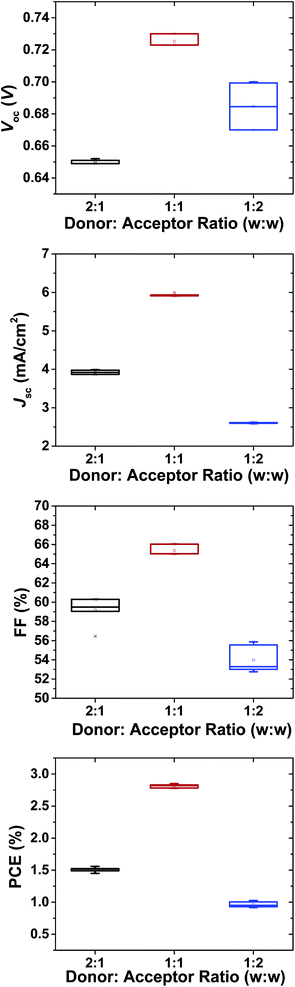
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios and the effect of annealing to the best ratio are shown in Fig. 3 and the key parameters from the average 6 devices are summarized in Fig. 4 and Table 2. As seen from Fig. 3, low amount of acceptor addition suffers from the low current in 2
A ratios and the effect of annealing to the best ratio are shown in Fig. 3 and the key parameters from the average 6 devices are summarized in Fig. 4 and Table 2. As seen from Fig. 3, low amount of acceptor addition suffers from the low current in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (3.9 mA cm−2) P3HT
1 ratio (3.9 mA cm−2) P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA cells. Thermal annealing the best ratio of P3HT
NCBA cells. Thermal annealing the best ratio of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA (1
NCBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 140 °C for 5 min. increased the fill factor remarkably from 53% to 65% and relatively high open circuit voltage (Voc) of 0.73 V was observed which is much higher than that of conventional P3HT:PCBM cells.37 Higher loading of NCBA to the blend system decreased the short circuit current (Jsc) and the open circuit voltage (Voc) which is due to transport properties in the blend. The preliminary performance results of best P3HT:NCBA solar cells are confirmed by external quantum efficiency (EQE) measurement. Fig. 5 exhibits the EQE of not annealed and annealed best ratio P3HT
1) at 140 °C for 5 min. increased the fill factor remarkably from 53% to 65% and relatively high open circuit voltage (Voc) of 0.73 V was observed which is much higher than that of conventional P3HT:PCBM cells.37 Higher loading of NCBA to the blend system decreased the short circuit current (Jsc) and the open circuit voltage (Voc) which is due to transport properties in the blend. The preliminary performance results of best P3HT:NCBA solar cells are confirmed by external quantum efficiency (EQE) measurement. Fig. 5 exhibits the EQE of not annealed and annealed best ratio P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA cells. From EQE measurements, the short-circuit current density (under air mass (AM) 1.5 conditions) was estimated to be 5.9 mA cm−2 for 1
NCBA cells. From EQE measurements, the short-circuit current density (under air mass (AM) 1.5 conditions) was estimated to be 5.9 mA cm−2 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 P3HT
1 P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
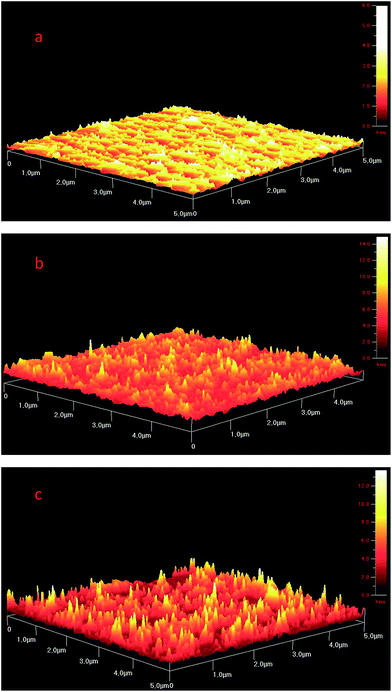
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA cell suggesting that the photon–electron conversion processes are rather efficient. Fig. 6 shows the atomic force microscopy (AFM) images of 1
NCBA cell suggesting that the photon–electron conversion processes are rather efficient. Fig. 6 shows the atomic force microscopy (AFM) images of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 P3HT
1 P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA solar cell before annealing (b) and after annealing (c) in noncontact mode. Annealing improved the surface morphology and increased the efficiency of P3HT
NCBA solar cell before annealing (b) and after annealing (c) in noncontact mode. Annealing improved the surface morphology and increased the efficiency of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA (1
NCBA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) solar cell.
1 ratio) solar cell.
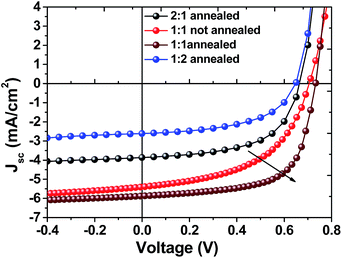 | ||
Fig. 3 J–V characteristics of P3HT:NCBA devices using different donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor ratios, under illumination at 100 mW cm−2. acceptor ratios, under illumination at 100 mW cm−2. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratios and comparison with P3HT:PCBM solar cell
A ratios and comparison with P3HT:PCBM solar cell
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA annealed NCBA annealed |
Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.65 | 3.9 | 59.7 | 1.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.73 | 5.9 | 65.1 | 2.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.67 | 2.6 | 53.5 | 0.93 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (1 PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.56 | 6.77 | 58.7 | 2.22 |
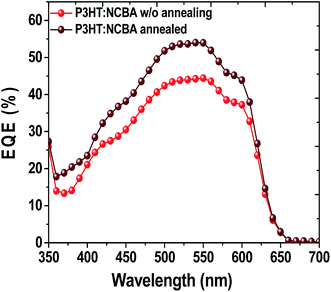 | ||
Fig. 5 External quantum efficiency (EQE) spectra of devices based on P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA with and without annealing. NCBA with and without annealing. | ||
Layers of NCBA and P3HT:NCBA were investigated to determine the charge transport properties. Charge carrier mobilities in NCBA and in the blend were measured by the space charge limited current (SCLC) method. The SCLC mobility measures the hole and electron mobility in the direction perpendicular to the electrodes; thus, it is the most representative measurement of charge-carrier mobility for solar cells.38,39 To measure the hole and electron mobilities of different fullerene derivative blends, the ITO/PEDOT:PSS/active layer (100 nm)/PEDOT:PSS/Ag (100 nm) and ITO/AZO/active layer/Ca (15 nm)/Ag (85 nm) devices were fabricated, respectively. Table 3 summarizes electron and hole mobilities for NCBA and P3HT:NCBA blends with and without thermal annealing (see Fig. SI-3†). Electron mobility of NCBA was found to be comparable to PCBM40 and was decreased one order of magnitude in the annealed blend. The parameter that represents the ratio of the hole-to-electron mobilities (μh/μe) in the D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A blends is crucial to understand the optoelectronic properties of BHJ, particularly with respect to FF. It is known that balanced charge-carrier transport is an important factor for increasing the fill factor (FF) in BHJ solar cells. Annealed 1
A blends is crucial to understand the optoelectronic properties of BHJ, particularly with respect to FF. It is known that balanced charge-carrier transport is an important factor for increasing the fill factor (FF) in BHJ solar cells. Annealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio P3HT
1 ratio P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA films showed a relatively balanced μh/μe value in the same order yield in high FF in the devices.
NCBA films showed a relatively balanced μh/μe value in the same order yield in high FF in the devices.
| Hole mobility (cm2 V−1 s−1) | Electron mobility (cm2 V−1 s−1) | |
|---|---|---|
| Pristine NCBA | 9.4 × 10−5 | 2.14 × 10−3 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA 1 NCBA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 not annealed 1 not annealed |
1.04 × 10−4 | 1.24 × 10−5 |
P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NCBA 1 NCBA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 annealed 1 annealed |
8.84 × 10−4 | 1.14 × 10−4 |
Conclusion
In summary, we have successfully synthesized a bis-adduct C60 derivative using a nonyl solubilizing group with simple synthetic route. NCBA offers a higher LUMO level compared to that of PCBM in order to minimize the energy losses in the electron transfer from the donor to the acceptor material. Besides a high electron mobility of 2 × 10−3 cm2 V−1 s−1, NCBA showed slightly higher absorption in 400–500 nm region compared to PCBM. Bulk-heterojunction photovoltaic devices are constructed using the fullerene derivative as acceptor with P3HT donor and efficiencies up to 2.8% have been achieved without further device optimization. As depicted, higher LUMO level resulted in significantly enhanced Voc of 0.73 V. Our successful preliminary results suggested that further optimization of this novel fullerene bis-adduct can yield higher efficiencies with chemical modifications to fine tuning the electronic properties and different conjugated polymers can be used to achieve higher power conversion efficiencies.Acknowledgements
Sule Erten-Ela acknowledges Alexander von Humboldt Foundation (AvH), Turkish Scientific and Technological Research Council (TUBITAK), UNESCO-LOREAL Foundation and Turkish Academy of Sciences (TUBA). We acknowledge Bavarian Research Foundation and the project Synthetic Carbon Allotropes (SFB953). The authors gratefully acknowledge the Cluster of Excellence “Engineering of Advanced Materials” at the University of Erlangen-Nuremberg, which is funded by the German Research Foundation (DFG) within the framework of “Excellence Initiative”.Notes and references
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Solar Cell Efficiency Tables (version 42), Prog. Photovoltaics, 2013, 21, 827–837 Search PubMed.
- G. Li, R. Zhu and Y. Yang, Polymer Solar Cells, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS PubMed.
- N. Li, D. Baran, K. Forberich, F. Machui, T. Ameri, M. Turbiez, M. Carrasco-Orozco, M. Drees, A. Facchetti, F. C. Krebs and C. J. Brabec, Towards 15% Energy Conversion Efficiency: A Systematic Study of the Solution-Processed Organic Tandem Solar Cells based on Commercially Available Materials, Energy Environ. Sci., 2013, 6, 3407–3413 CAS.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, High-Efficiency Solution Processable Polymer Photovoltaic Cells by Self-Organization of Polymer Blends, Nat. Mater., 2005, 4, 864–868 CrossRef CAS PubMed.
- Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, I. McCulloch, C.-S. Ha and M. Ree, A Strong Regioregularity Effect in Self-organizing Conjugated Polymer Films and High-efficiency Polythiophene:Fullerene Solar Cells, Nat. Mater., 2006, 5, 197–203 CrossRef CAS PubMed.
- Y. Li, Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- W.-Y. Wong, X.-Z. Wang, Z. He, A. B. Djurisic, C.-T. Yip, K.-Y. Cheung, H. Wang, C. S. K. Mak and W.-K. Chan, Metallated Conjugated Polymers as a New Avenue towards High-efficiency Polymer Solar Cells, Nat. Mater., 2007, 6, 521–527 CrossRef CAS PubMed.
- R. S. Ashraf, B. C. Schroeder, H. A. Bronstein, Z. Huang, S. Thomas, R. J. Kline, C. J. Brabec, P. Rannou, T. D. Anthopoulos, J. R. Durrant and I. McCulloch, The Influence of Polymer Purification on Photovoltaic Device Performance of a Series of Indacenodithiophene Donor Polymers, Adv. Mater., 2013, 25, 2029–2034 CrossRef CAS PubMed.
- L. A. Perez, K. W. Chou, J. A. Love, T. S. van der Poll, D.-M. Smilgies, T.-Q. Nguyen, E. J. Kramer, A. Amassian and G. C. Bazan, Solvent Additive Effects on Small Molecule Crystallization in Bulk Heterojunction Solar Cells Probed During Spin Casting, Adv. Mater., 2013, 25, 6380–6384 CrossRef CAS PubMed.
- T. Stubhan, M. Salinas, A. Ebel, F. C. Krebs, A. Hirsch, M. Halik and C. J. Brabec, Increasing the Fill Factor of Inverted P3HT:PCBM Solar Cells Through Surface Modification of Al-Doped ZnO via Phosphonic Acid-Anchored C60 SAMs, Adv. Energy Mater., 2012, 2, 532–535 CrossRef CAS PubMed.
- M. Halik and A. Hirsch, The Potential of Molecular Self-Assembled Monolayers in Organic Electronic Devices, Adv. Mater., 2011, 23, 2689–2695 CrossRef CAS PubMed.
- D. Baran, F. M. Pasker, S. L. Blanc, G. Schnakenburg, T. Ameri, S. Höger and C. J. Brabec, Introducing a New Triazoloquinoxaline-based Fluorene Copolymer for Organic Photovoltaics: Synthesis, Characterization, and Photovoltaic Properties, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 987–992 CrossRef CAS PubMed.
- L.-L. Deng, S.-L. Xie, C. Yuan, R.-F. Liu, J. Feng, L.-C. Sun, X. Lu, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, High LUMO Energy Level C60(OCH3)4 Derivatives: Electronic Acceptors for Photovoltaic Cells with Higher Open-Circuit Voltage, Sol. Energy Mater. Sol. Cells, 2013, 111, 193–199 CrossRef CAS PubMed.
- G. D. Han, W. R. Collins, T. L. Andrew, V. Bulović and T. M. Swager, Cyclobutadiene–C60 Adducts: N-Type Materials for Organic Photovoltaic Cells with High Voc, Adv. Funct. Mater., 2013, 23, 3061–3069 CrossRef CAS PubMed.
- C. Liu, S. Xiao, X. Shu, Y. Li, L. Xu, T. Liu, Y. Yu, L. Zhang, H. Liu and Y. Li, Synthesis and Photovoltaic Properties of Novel Monoadducts and Bis-adducts Based on Amide Methanofullerene, ACS Appl. Mater. Interfaces, 2012, 4, 1065–1071 CAS.
- S. Kolemen, Y. Cakmak, T. Ozdemir, S. Erten-Ela, M. Buyuktemiz, Y. Dede and E. U. Akkaya, Design and Characterization of Bodipy Derivatives for Bulk Heterojunction Solar Cells, Tetrahedron, 2014, 70, 6229–6234 CrossRef CAS PubMed.
- G. D. Sharma, M. Singh, R. Kurchania, E. N. Koukaras and J. A. Mikroyannidis, Synthesis and characterization of two carbazole-based alternating copolymers with 4-nitrophenylcyanovinylene pendant groups and their use as electron donors for bulk heterojunction solar cells, RSC Adv., 2013, 3, 18821–18834 RSC.
- Q. Tao, Y. Xia, X. Xu, S. Hedström, O. Backe, D. I. James, P. Persson, E. Olsson, O. Inganas, L. Hou, W. Zhu and E. Wang, D−A1−D−A2 Copolymers with Extended Donor Segments for Efficient Polymer Solar Cells, Macromolecules, 2015, 48, 1009–1016 CrossRef CAS.
- C. L. Chochos, N. Tagmatarchis and V. G. Gregoriou, Rational design on n-type organic materials for high performance organic photovoltaics, RSC Adv., 2013, 3, 7160–7181 RSC.
- M. A. Faist, S. Shoaee, S. Tuladhar, G. F. A. Dibb, S. Foster, W. Gong, T. Kirchartz, D. D. C. Bradley, J. R. Durrant and J. Nelson, Understanding the Reduced Effi ciencies of Organic Solar Cells Employing Fullerene Multiadducts as Acceptors, Adv. Energy Mater., 2013, 3, 744–752 CrossRef CAS PubMed.
- Y. He, H.-Y. Chen, J. Hou and Y. Li, Indene-C60 Bis-adduct: A New Acceptor for High-Performance Polymer Solar Cells, J. Am. Chem. Soc., 2010, 132, 1377–1382 CrossRef CAS PubMed.
- (a) Y. Li, Fullerene derivative acceptors for high performance polymer solar cells, Phys. Chem. Chem. Phys., 2011, 13, 1970–1983 RSC; (b) Y. He, B. Peng, G. Zhao, Y. Zou and Y. Li, Indene Addition of [6,6]-Phenyl-C61-butyric Acid Methyl Ester for High-Performance Acceptor in Polymer Solar Cells, J. Phys. Chem. C, 2011, 115, 4340–4344 CrossRef CAS; (c) Y. Li, Fullerene-bis-adduct Acceptors for Polymer Solar Cells, Chem.–Asian J., 2013, 8(10), 2316–2328 CrossRef CAS PubMed; (d) X. Guo, C. Cui, M. Zhang, L. Huo, Y. Huang, J. Hou and Y. Li, High efficiency polymer solar cells based on poly(3-hexylthiophene)/indene C70 bis-adduct with solvent additive, Energy Environ. Sci., 2012, 5, 7943–7949 RSC.
- Y.-J. Cheng, C.-H. Hsieh, Y. He, C.-S. Hsu and Y. Li, Combination of Indene-C60 Bis-Adduct and Cross-Linked Fullerene Interlayer Leading to Highly Efficient Inverted Polymer Solar Cells, J. Am. Chem. Soc., 2010, 132, 17381–17383 CrossRef CAS PubMed.
- R. B. Ross, C. M. Cardona, D. M. Guldi, S. G. Sankaranarayanan, M. O. Reese, N. Kopidakis, J. Peet, B. Walker, G. C. Bazan, E. Van Keuren, B. C. Holloway and M. Drees, Endohedral Fullerenes for Organic Photovoltaic Devices, Nat. Mater., 2009, 8, 208–212 CrossRef CAS PubMed.
- Y. Matsuo, J. Kawai, H. Inada, T. Nakagawa, H. Ota, S. Otsubo and E. Nakamura, Addition of Dihydromethano Group to Fullerenes to Improve the Performance of Bulk Heterojunction Organic Solar Cells, Adv. Mater., 2013, 25, 6266–6269 CrossRef CAS PubMed.
- E. Voroshazi, K. Vasseur, T. Aernouts, P. Heremans, A. Baumann, C. Deibel, X. Xue, A. J. Herring, A. J. Athans, T. A. Lada, H. Richter and B. P. Rand, Novel bis-C60 Derivative Compared to Other Fullerene Bis-adducts in High Efficiency Polymer Photovoltaic Cells, J. Mater. Chem., 2011, 21, 17345–17352 RSC.
- C.-Z. Li, H.-L. Yip and A. K. Y. Jen, Functional Fullerenes for Organic Photovoltaics, J. Mater. Chem., 2012, 22, 4161–4177 RSC.
- K.-H. Kim, H. Kang, S. Y. Nam, J. Jung, P. S. Kim, C.-H. Cho, C. Lee, S. C. Yoon and B. Kim, Facile Synthesis of o-Xylenyl Fullerene Multiadducts for High Open Circuit Voltage and Efficient Polymer Solar Cells, J. Chem. Eng. Mater. Sci., 2011, 23, 5090–5095 CAS.
- G. Zhao, Y. He and Y. Li, 6.5% Efficiency of Polymer Solar Cells Based on poly(3-hexylthiophene) and Indene-C60 Bis-adduct by Device Optimization, Adv. Mater., 2010, 22, 4355–4358 CrossRef CAS PubMed.
- M. Lenes, G.-J. A. H. Wetzelaer, F. B. Kooistra, S. C. Veenstra, J. C. Hummelen and P. W. M. Blom, Fullerene Bis-adducts for Enhanced Open-Circuit Voltages and Efficiencies in Polymer Solar Cells, Adv. Mater., 2008, 20, 2116–2119 CrossRef CAS PubMed.
- Y. He, G. Zhao, B. Peng and Y. Li, High-Yield Synthesis and Electrochemical and Photovoltaic Properties of Indene-C70 Bis-adduct, Adv. Funct. Mater., 2010, 20, 3383–3389 CrossRef CAS PubMed.
- R. Gomez, J. L. Segura and N. Martin, Highly Efficient Light-Harvesting Organofullerenes, Org. Lett., 2005, 7, 717–720 CrossRef CAS PubMed.
- D. Baran, N. Li, A.-C. Breton, A. Osvet, T. Ameri, M. Leclerc and C. J. Brabec, Qualitative Analysis of Bulk-Heterojunction Solar Cells without Device Fabrication: An Elegant and Contactless Method, J. Am. Chem. Soc., 2014, 136, 10949–10955 CrossRef CAS PubMed.
- O. G. Poluektov, J. Niklas, S. Beaupre, M. Leclerc, C. Villegas, S. Erten-Ela, J. L. Delgado, N. Martin, A. Sperlich and V. Dyakonov, Electronic Structure of Fullerene Heterodimer in Bulk Heterojunction Blends, Adv. Energy Mater., 2014, 7, 1–7 Search PubMed.
- S. Erten-Ela, C. Villegas, J. L. Delgado and N. Martin, Pyrrolidino [60] and [70] Fullerene Homo and Heterodimers as Electron Acceptors for OPV, New J. Chem., 2015, 39, 1477–1482 RSC.
- Y. Cakmak, S. Kolemen, M. Kucukyavuz, Y. Dede and S. Erten-Ela, Synthesis and Dye Sensitized Solar Cell Applications of Bodipy Derivatives with Bis-dimethylfluorenyl Amino Donor Groups, New J. Chem., 2015, 39, 4086–4092 RSC.
- T. Ameri, J. Min, N. Li, F. Machui, D. Baran, M. Forster, K. J. Schottler, D. Dolfen, U. Scherf and C. J. Brabec, Performance Enhancement of the P3HT/PCBM Solar Cells through NIR Sensitization Using a Small-Bandgap Polymer, Adv. Energy Mater., 2012, 2, 1198–1202 CrossRef CAS PubMed.
- J. Min, T. Ameri, R. Gresser, M. Lorenz-Rothe, D. Baran, A. Troeger, V. Sgobba, K. Leo, M. Riede, D. M. Guldi and C. J. Brabec, Two Similar Near-Infrared (IR) Absorbing Benzannulated Aza-BODIPY Dyes as Near-IR Sensitizers for Ternary Solar Cells, ACS Appl. Mater. Interfaces, 2013, 5, 5609–5616 CAS.
- H. Azimi, A. Senes, M. C. Scharber, K. Hingerl and C. J. Brabec, Charge Transport and Recombination in Low-Bandgap Bulk Heterojunction Solar Cell using Bis-adduct Fullerene, Adv. Energy Mater., 2011, 1, 1162–1168 CrossRef CAS PubMed.
- V. D. Mihailetchi, J. K. J. van Duren, P. W. M. Blom, J. C. Hummelen, R. A. J. Janssen, J. M. Kroon, M. T. Rispens, W. J. H. Verhees and M. M. Wienk, Electron Transport in a Methanofullerene, Adv. Funct. Mater., 2003, 13, 43–46 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10089e |
| This journal is © The Royal Society of Chemistry 2015 |