Selective CO2 adsorption in a porphyrin polymer with benzimidazole linkages†
Venkata S. Pavan K. Netia,
Jun Wangb,
Shuguang Dengb and
Luis Echegoyen*a
aDepartment of Chemistry, University of Texas at El Paso, El Paso, TX 79968, USA. E-mail: echegoyen@utep.edu; Fax: +1-915-747-8807; Tel: +1-915-747-7573
bDepartment of Chemical Engineering, New Mexico State University, Las Cruces, NM 88003, USA
First published on 8th January 2015
Abstract
A new nanoporous porphyrin-based benzimidazole linked polymer, PBILP, was synthesized. The use of porphyrin monomers as molecular building units led to the formation of a rigid amorphous network that has a moderate surface area (SBET = 557 m2 g−1). The CO2 adsorption ability of PBILP is 12.1 wt% (2.76 mmol g−1) and it has a CO2/CH4 selectivity of 7.2 at 273 K/1 bar and a CO2/N2 selectivity of 72 at 273 K/1 bar.
The synthesis of nitrogen rich microporous materials has gained significant attention due to their potential as solid adsorbents for CO2 capture. These microporous materials include, but are not limited to, metal–organic frameworks (MOFs),1 zeolitic imidazolate frameworks (ZIFs),2 and hypercross-linked microporous polymers (BILPs, POPs, etc.).3–6 The key strategy to develop new and efficient POPs mainly relies on the design of nitrogen rich building blocks that possess high surface areas. In fact, many examples of new ligands and linkages have expanded the versatility of the resulting functional microporous materials. These materials have found a wide variety of applications in gas storage and separation,3–6 heterogeneous catalysis,6j etc. POPs and their membranes are best suited for selective gas adsorption and gas separation applications due to their physical, chemical, high temperature and pressure stabilities, and their resistance towards moisture, and basic and acidic conditions.4a,5a Recently, Hupp and Nguyen et al. reported an Al-porphyrin based POP for supercritical CO2 processing and for the degradation of nerve agents.6d El-Kaderi et al. developed benzimidazole-linked polymers (BILPs),5a azo-linked polymers (ALPs),5e and Zhang et al. reported imine linked polymers (ILPs),4b and Uyama et al. reported N-doped activated carbon monoliths for selective CO2 capture. Some advantages of porous polymers over activated carbons are higher CO2/N2 selectivity and efficient and reversible capture of CO2. On the other hand, advantages of the micro- and mesoporous activated carbons over the porous polymers are the low cost of the raw material and the high CO2 uptake. The selective CO2 adsorption in these frameworks over CH4 or N2 is believed to arise as a consequence of CO2-framework interactions through R–N(δ−)–C(δ+)O2.
In order to expand the BILP chemistry to porphyrins, we focused on carboxaldehyde based porphyrin, specifically meso-tetra-(4-phenylformyl) porphyrin (TCPP, 1), and benzene-1,2,4,5-tetramine (BTA, 2). We have prepared a porphyrin benzimidazole linked polymer (PBILP) containing poly-benzimidazole linkages as shown in Scheme 1. Part of the motivation behind this work is to demonstrate the capture of high amounts of CO2 in a metalloporphyrin porous polymer. In the future we want to convert the captured CO2 to polycarbonates or other polymers, similar to a report using cobalt-porphyrins to effect this catalytic transformation.7a High nitrogen and cobalt content of PBILP can be used for selective CO2 adsorption and also for catalytic transformations. In this report, we describe the synthesis and characterization of PBILP and the selective CO2 adsorption properties. The synthesis of PBILP was accomplished by the condensation reaction between 1 and 2, which is similar to a BILP synthesis reported by El-Kaderi et al. with a slight modification (see ESI†).5a Compound 1 was synthesized following a similar literature procedure.7b A homogeneous solution of 1 was added drop-wise to the suspension of 2 in N,N′-dimethylformamide (DMF) over 4 h while stirring at the −30 °C, followed by stirring at room temperature for 12 h. The reaction yielded a purple suspension which was bubbled with O2, and then heated at 130 °C for 36 h. The slow addition of 1 to 2 yielded the PBILP in a 60% yield as a purple solid. The resulting purple polymeric solid was unambiguously characterized by spectral and analytical methods. The PBILP was designed to possess a 2D network structure arising from the four benzimidazole-linkages (Scheme 1). It is well known that during the course of polymerization, planar porphyrins have a tendency to stack via π–π interactions. Consequently, the majority of porphyrin porous polymers are interpenetrating networks with relatively low total pore volumes.6 Thus we chose one large porphyrin building unit and one small building unit to reduce interpenetration. In fact, a small degree of interpenetration is observed in the polymeric skeleton (Fig. 2a).
The PBILP is stable and insoluble in common organic solvents, water. PBILP is also stable in a 2 M solution of HCl or NaOH and its elemental analysis did not indicate any decomposition, moreover the color of the material was unchanged. Due to the insoluble nature of the PBILP in organic solvents, FT-IR and solid-state 13C cross polarization, magic angle spinning (CP-MAS) NMR characterization was performed. A peak at about 1000 cm−1 in the FT-IR spectra of TCPP and PBILP is assigned to the Co–N stretching frequency, indicating the presence of Co–porphyrin units in the PBILP polymer. A new stretch appeared at 1482 cm−1, which is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching of the benzimidazole ring.5a The broad band at around 1630 cm−1 is presumably due to the overlap of C
N stretching of the benzimidazole ring.5a The broad band at around 1630 cm−1 is presumably due to the overlap of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bands, which is in good agreement with previous reports.5a In addition, the absence of a C
N stretching bands, which is in good agreement with previous reports.5a In addition, the absence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at ∼1700 cm−1 in PBILP indicates the full consumption of compound 1 (Fig. S1, ESI†). The 13C CP-MAS NMR spectrum exhibited a signal at δ = 153 ppm which corresponds to benzimidazole linkages and this is in good agreement with other reported benzimidazole frameworks.5a Other peaks at δ = 131.3 and 93.7 ppm were assigned to the aromatic carbon atoms of the PBILP (Fig. S2, ESI†).
O stretching band at ∼1700 cm−1 in PBILP indicates the full consumption of compound 1 (Fig. S1, ESI†). The 13C CP-MAS NMR spectrum exhibited a signal at δ = 153 ppm which corresponds to benzimidazole linkages and this is in good agreement with other reported benzimidazole frameworks.5a Other peaks at δ = 131.3 and 93.7 ppm were assigned to the aromatic carbon atoms of the PBILP (Fig. S2, ESI†).
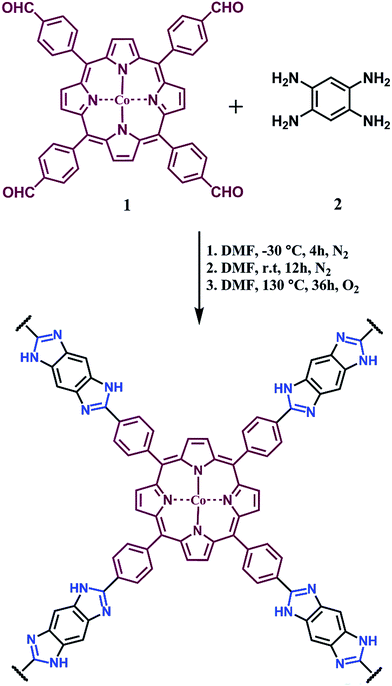
A scanning electron microscopy (SEM) image of PBILP (Fig. S3, ESI†) shows spherical-shaped irregular submicrometer particles with sizes between 50 and 100 nm. To evaluate the porosity of PBILP, N2 adsorption–desorption isotherms were measured at 77 K (Fig. 1). The N2-adsorption isotherms indicated Brunauer–Emmett–Teller (BET) and Langmuir surface areas of 557 and 1077 m2 g−1, respectively. The BET surface area of PBILP is lower than for other porphyrin polymers obtained by triazine/carbazole linkages (Table 2).6f,i The increase in the N2 adsorption at P/P0 = 0.9 may arise in part from interparticulate porosity associated with the intertwined nano- and microporous nature of PBILP. The pore size distribution analyzed by using non-local density functional theory (NLDFT) further confirmed the nanoroporosity nature of the material. The dominant pore size of PBILP is around 6 Å (Fig. 2a). Conjugated polymers with nanopores interact effectively with small gas molecules through improved molecular interactions.
 | ||
| Fig. 1 Adsorption (filled circles) and desorption (empty circles) for N2 at 77 K (black), 273 K (blue), and 298 K (red). | ||
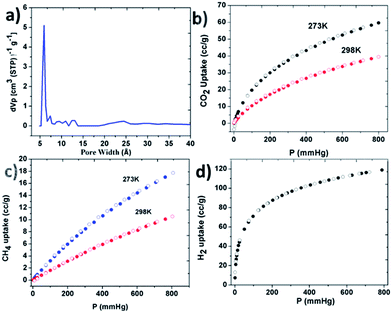 | ||
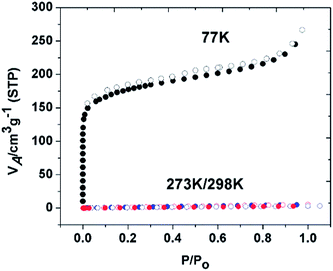
| Fig. 2 (a) NLDFT pore width analysis (b) CO2 at 273 K and 298 K (c) CH4 at 273 K and 298 K (d) H2 at 77 K. Adsorption (filled symbols) and desorption (empty symbols). | ||
The reasonably high surface area and nitrogen rich functional groups of PBILP are conducive to CO2 adsorption. The CO2, CH4, N2 and H2 adsorption isotherms of PBILP were measured at 77, 273, and 298 K, 1 atm and the data are summarized in Table 1. The isotherms show slight hystereses on desorption (Fig. 2). The PBILP shows a CO2 uptake of 2.76 mmol g−1 (12.1 wt%) at 273 K and 1.8 mmol g−1 (7.9 wt%) at 298 K, 1 atm (Fig. 2b). The isosteric heat (Qst) of the CO2 adsorption at low coverage is 25.7 kJ mol−1, calculated using the Clausius–Clapeyron equation (Fig. S6, ESI†) fitting the parameters obtained from the adsorption data measured at 273, and 298 K.4f The data suggest that strong physisorption (<40 kJ mol−1) rather than chemisorption of CO2 is in effect. According to previous reports, BILPs could be fully reactivated under vacuum at room temperature after CO2 adsorption and then readsorb the same amount of gas.5a The low-coverage Qst values of PBILP are comparable with those for MCTFs [24.5 kJ mol−1] and BILPs (26.5 kJ mol−1),6f,i and are lower than those previously reported for imine, benzothiazole and triazole containing porous polymers, and COFs.4,6 Although the BET surface area of PBILP is moderate, the CO2 adsorption capacity of PBILP is comparable to those for previously reported porphyrin based CPOPs and triazine porphyrin POPs, which had much higher surface areas (Table 2),6f,i and lower than activated carbons.6j
| Polymer | Selectivity | CO2 (wt%) | CH4 (wt%) |
|---|---|---|---|
| PBILP | 72 (CO2/N2) | 12.1 (273 K) | 1.3 (273 K) |
| 7.2 (CO2/CH4) | 7.9 (298 K) | 0.8 (298 K) |
| Polymer | CO2 | CH4 | SBET (m2 g−1) |
|---|---|---|---|
| PBILP | 12.1 wt% | 1.3 wt% | 557 |
| MCTF | 13.9 wt% | — | 1520 |
| CPOP | 13.8 wt% | 4.7 wt% | 1320 |
| BILP-1 | 18.8 wt% | 2.3 wt% | 1172 |
| Fe-POP-1 | 19 wt% | — | 875 |
Based on the measured physisorption isotherms with a pressure of up to 1 bar (Fig. 2), we found that PBILP showed a moderate uptake capacity for H2 (1 wt%) and for CH4 (1.3 wt%), which are similar to those for some reported porous polyporphyrins and COFs measured under similar conditions.4,6 At zero coverage, the Qst for CH4 is 18.3 kJ mol−1. A higher Qst value for CO2 compared to that for CH4 is likely due to R–N(δ−)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(δ+)O2 interactions. Furthermore, the selectivity of PBILP towards CO2 over N2 and CH4 was investigated (Fig. S5, ESI†). On the basis of Langmuir model fits and Henry's constant values in the pressure range between 0 and 1 bar, the estimated adsorption selectivity for CO2/CH4 is 7.2 and 72 for CO2/N2 at 273 K/298 K/1 bar. The thermal stability of the PBILP was examined by thermogravimetric analysis (TGA). The porphyrin monomer appears to have more than a 50% mass loss at around 600 °C, while the cross-linked polymer yields high residual masses of 70% at the same temperature (Fig. S7, ESI†). Comparing the thermogravimetric results of the polymer-network and the corresponding monomer, it is evident that Co–porphyrin oxidative coupling polymerization occurs. The powder X-ray diffraction pattern of the synthesized PBILP revealed no diffraction peaks, indicating that it is amorphous.
C(δ+)O2 interactions. Furthermore, the selectivity of PBILP towards CO2 over N2 and CH4 was investigated (Fig. S5, ESI†). On the basis of Langmuir model fits and Henry's constant values in the pressure range between 0 and 1 bar, the estimated adsorption selectivity for CO2/CH4 is 7.2 and 72 for CO2/N2 at 273 K/298 K/1 bar. The thermal stability of the PBILP was examined by thermogravimetric analysis (TGA). The porphyrin monomer appears to have more than a 50% mass loss at around 600 °C, while the cross-linked polymer yields high residual masses of 70% at the same temperature (Fig. S7, ESI†). Comparing the thermogravimetric results of the polymer-network and the corresponding monomer, it is evident that Co–porphyrin oxidative coupling polymerization occurs. The powder X-ray diffraction pattern of the synthesized PBILP revealed no diffraction peaks, indicating that it is amorphous.
Conclusions
In conclusion, we have synthesized, characterized, and described the use of a benzimidazole-linked porphyrin-based porous polymer, PBILP, for CO2 capture. Similar to Wang's cobalt porphyrin catalyst, the BILP could be used as a heterogeneous catalyst to convert the captured CO2 to poly (propylene carbonate) and other carbonate based polymers. In addition to the promising selective CO2 adsorption, PBILP also showed some storage capacity for H2 at 77 K and for CH4 at 273 K. The PBILP possesses high thermal and chemical stability, relatively high surface area, and its porosity can be tuned by changing the reaction conditions.Acknowledgements
This work was generously supported by NSF grant DMR-1205302 (PREM program), and the Robert A. Welch Foundation, grant # AH-0033.Notes and references
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- (a) B. Wang, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Nature, 2008, 453, 207 CrossRef CAS PubMed; (b) R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939 CrossRef CAS PubMed.
- (a) O. K. Farha, A. M. Spokoyny, B. G. Hauser, Y.-S. Bae, S. E. Brown, R. Q. Snurr, C. A. Mirkin and J. T. Hupp, Chem. Mater., 2009, 21, 3033 CrossRef CAS; (b) P. Pandey, A. P. Katsoulidis, I. Eryazici, Y. Wu, M. G. Kanatzidis and S. T. Nguyen, Chem. Mater., 2010, 22, 4974 CrossRef CAS; (c) N. B. McKeown, J. Mater. Chem., 2010, 20, 10588 RSC; (d) P. Pandey, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, J. Mater. Chem., 2011, 21, 1700 RSC.
- (a) M. Hashem, C. G. Bezzu, B. M. Kariuki and N. B. McKeown, Polym. Chem., 2011, 2, 2190 RSC; (b) Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630 CrossRef CAS; (c) H. A. Patel, S. H. Je, J. Park, D. P. Chen, Y. Jung, C. T. Yavuz and A. Coskun, Nat. Commun., 2013, 4, 1357 CrossRef PubMed; (d) P. Peng, F.-F. Li, F. Bowles, V. S. P. K. Neti, A. J. M. Magaña, M. Olmstead, A. Balch and L. Echegoyen, Chem. Commun., 2013, 49, 3209 RSC; (e) V. S. P. K. Neti, X. Wu, M. Hosseini, R. Bernal, S. Deng and L. Echegoyen, CrystEngComm., 2013, 15, 7157 RSC; (f) V. S. P. K. Neti, X. Wu, S. Deng and L. Echegoyen, RSC Adv., 2014, 4, 9669 RSC; (g) P. Peng, F.-F. Li, V. S. P. K. Neti, A. J. M. Magaña and L. Echegoyen, Angew. Chem., Int. Ed., 2014, 53, 160 CrossRef CAS PubMed.
- (a) M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2011, 23, 1650 CrossRef CAS; (b) M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2012, 24, 1511 CrossRef CAS; (c) M. G. Rabbani, T. E. Reich, R. M. Kassab, K. T. Jackson and H. M. El-Kaderi, Chem. Commun., 2012, 48, 1141 RSC; (d) T. E. Reich, S. Behera, K. T. Jackson, P. Jena and H. M. El-Kaderi, J. Mater. Chem., 2012, 22, 13524 RSC; (e) P. Arab, M. G. Rabbani, A. K. Sekizkardes, T. Islamoglu and H. M. El-Kaderi, Chem. Mater., 2014, 26, 1385 CrossRef CAS.
- (a) Z. Wang, S. Yuan, A. Mason, B. Reprogle, D.-J. Liu and L. Yu, Macromolecules, 2012, 45, 7413 CrossRef CAS; (b) A. Modak, M. Nandi, J. Mondal and A. Bhaumik, Chem. Commun., 2012, 48, 248 RSC; (c) M. Nandi, K. Okada, A. Dutta, A. Bhaumik, J. Maruyama, D. Derks and H. Uyama, Chem. Commun., 2012, 48, 10283 RSC; (d) R. K. Totten, Y.-S. Kim, M. H. Weston, O. K. Farha, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2013, 135, 11720 CrossRef CAS PubMed; (e) V. S. P. K. Neti, X. Wu, S. Deng and L. Echegoyen, Polym. Chem., 2013, 4, 4566 RSC; (f) X. Liu, H. Li, Y. Zhang, B. Xu, A. Sigen, H. Xia and Y. Mu, Polym. Chem., 2013, 4, 2445 RSC; (g) V. S. P. K. Neti, X. Wu, S. Deng and L. Echegoyen, CrystEngComm., 2013, 15, 6892 RSC; (h) V. S. P. K. Neti, A. J. M. Magaña and L. Echegoyen, J. Coord. Chem., 2013, 66, 3193 CrossRef CAS; (i) L.-J. Feng, Q. Chen, J.-H. Zhu, D.-P. Liu, Y.-C. Zhao and B.-H. Han, Polym. Chem., 2014, 5, 3081 RSC; (j) W. Zhang, P. Jiang, Y. Wang, J. Zhang and P. Zhang, Catal. Sci. Technol., 2015, 5, 101 RSC.
- (a) Y. Qin, X. Wang, S. Zhang, X. Zhao and F. Wang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5959 CrossRef CAS; (b) R. Akbarzadeh and H. Dehghani, Chin. J. Polym. Sci., 2013, 31, 139 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Fig. S1–S7. See DOI: 10.1039/c4ra15086d |
| This journal is © The Royal Society of Chemistry 2015 |