Open Access Article
Open Access ArticleAdvances in technical strategies for monitoring the reduction of platinum(IV) complexes
Shu
Chen
ab,
Qiyuan
Zhou
ab,
Ka-Yan
Ng
ab,
Zoufeng
Xu
ab,
Weikang
Xu
a and
Guangyu
Zhu
 *ab
*ab
aDepartment of Chemistry, City University of Hong Kong, Hong Kong SAR, P. R. China. E-mail: guangzhu@cityu.edu.hk
bCity University of Hong Kong Shenzhen Research Institute, Shenzhen 518057, P. R. China
First published on 16th April 2024
Abstract
Platinum(IV) prodrugs have emerged as highly promising candidates for next-generation anticancer drugs. The activation of these prodrugs heavily relies on the critical step of chemical reduction of platinum, which determines their ultimate efficacy as potent anticancer agents. Therefore, it is essential to employ effective strategies to monitor the reduction of Pt(IV) complexes and the generation of active Pt(II) counterparts. These strategies not only unravel the intracellular mechanisms but also facilitate the design of novel Pt(IV) prodrugs for cancer therapy and enable the prediction of their anticancer performance. In this review, we summarize recent advances in strategies used to monitor the reduction profiles of Pt(IV) complexes from an introductory yet comprehensive viewpoint. We first delve into the principles underlying the reduction of Pt(IV) prodrugs to Pt(II) species, with a focus on the detection foundations that rely on changes in molecular weight, electronic arrangement, and coordination patterns. We subsequently summarize the strategies employed to investigate the reduction progress of Pt(IV) complexes in both aqueous solutions and at the cellular level, while highlighting the scope of applications, advantages, and disadvantages of each method. Finally, we provide a concise summary and a critical assessment of the discussed approaches. We hope this account will empower researchers with a deeper understanding of the strategies for monitoring the activation of Pt(IV) prodrugs and shed light on the underlying mechanism of prodrug activation.
1. Introduction
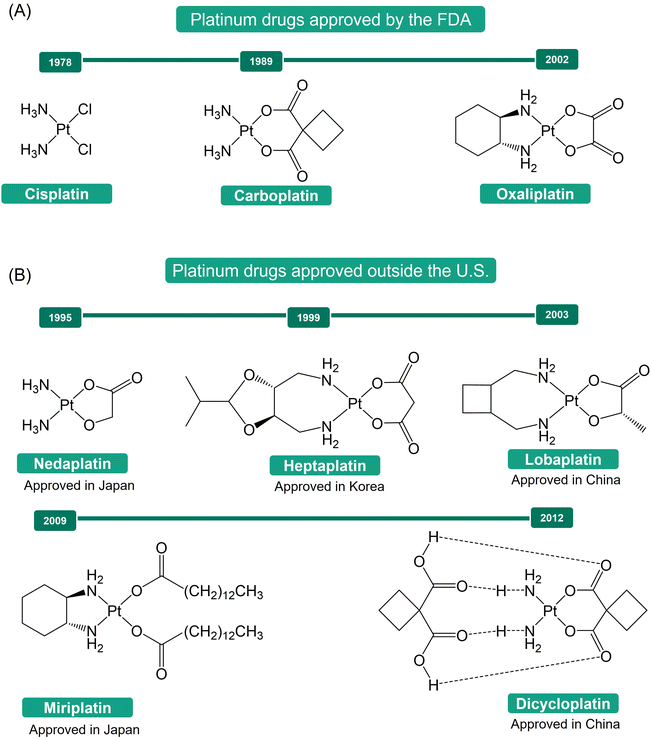
The discovery of cisplatin's antiproliferative properties by Rosenberg, through a serendipitous finding, marked a significant breakthrough in cancer treatment.1–4 Cisplatin's successful application in treating metastatic ovarian and testicular cancers has led to significant improvements in the survival rates of numerous cancer patients.5,6 This success spurred a further exploration into platinum-based anticancer agents, resulting in the development of carboplatin and oxaliplatin as second- and third-generation platinum-based drugs (Fig. 1A).7–10 These drugs have become indispensable tools in the global fight against cancer.11,12 Additionally, other Pt(II) drugs, such as nedaplatin, heptaplatin, lobaplatin, miriplatin, and dicycloplatin (Fig. 1B), have been approved for use in Japan (1995), Korea (1999), China (2003), Japan (2009), and China (2012), respectively, further expanding the armamentarium against cancer.13The mechanism of action of Pt(II) drugs has been extensively explored.5,11,14–16 Upon crossing the cell membrane, these drugs undergo aquation, a process that activates them. Subsequently, they bind to the N7 positions of guanine nucleobases in DNA, forming platinum-DNA adducts, which include both intra- and inter-strand crosslinks. These adducts distort the DNA structure, triggering a range of cellular responses that lead to cell cycle arrest and apoptosis, ultimately resulting in the death of cancer cells. While Pt(II) drugs have shown great promise in cancer treatment, their lack of inertness and selectivity has been associated with severe side effects during drug administration.1,17,18 Moreover, tumor cells can develop resistance to these drugs, either as intrinsic properties of certain types of tumor cells or as a consequence of prolonged drug exposure.1,19,20 These challenges present substantial obstacles to the effectiveness of Pt(II)-based antitumor therapy.
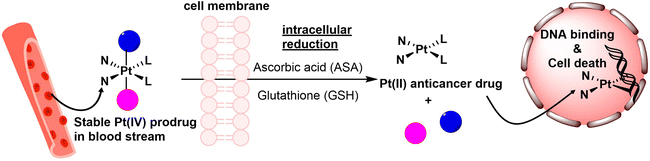
In recent years, significant progress has been made with the development of Pt(IV) prodrugs. These Pt(IV) complexes have emerged as alternative Pt-based drugs, aiming to minimize side effects, improve drug efficiency, and overcome drug resistance. Pt(IV) complexes possess a low-spin d6 electronic configuration and an octahedral geometry with six coordinating ligands. This configuration reduces the likelihood of ligand exchange reactions, thus minimizing side effects.21–23 Additionally, Pt(IV) prodrugs offer an advantage over Pt(II) counterparts by providing two additional axial ligands that can be tailored to improve drug efficiency and overcome drug resistance. It should be noted that in octahedral geometry, the terms “equatorial” and “axial” are not strictly defined. In order to differentiate, however, the intrinsic ligands of Pt(II) drugs are typically referred to as equatorial ligands, whereas the ligands introduced during the oxidation process are commonly called axial ligands. The incorporation of lipophilic moieties in Pt(IV) complexes promotes optimal cellular uptake,24–26 while the inclusion of tumor-targeting agents enhances specificity for cancer cells,27–30 thereby potentially amplifying the effectiveness of anticancer therapies. Moreover, upon reduction, the bioactive moieties initially integrated into Pt(IV) complexes can act synergically with released Pt(II) drugs, ultimately overcoming drug resistance.31–34 For example, mitaplatin is a dual-targeting prodrug that overcomes cisplatin resistance by selectively attacking nuclear DNA with cisplatin and targeting mitochondria with dichloroacetate (DCA) in cancer cells, resulting a reduced resistance factor (RF) of 3.0 compared to 10.7 for cisplatin.31
The activation of Pt(IV) prodrugs involves the reduction of the Pt(IV) center to Pt(II) by bio-reductants such as sodium ascorbate, glutathione (GSH), L-cysteine, and L-methionine. This reduction process can occur through either an inner-sphere or outer-sphere electron transfer mechanism.11,35,36 Upon reduction, the axial ligands that are in a trans position to the Pt(IV) center are typically detached (Scheme 1). Recent studies have shown that the structures of Pt(IV) complexes and the types of reducing agents employed can influence the structures of resulting Pt(II) products, and multiple Pt(II) species can be formed.22,35,37,38 To measure these reduction processes, both pseudo-first-order and second-order kinetic laws have been employed. Pseudo-first-order kinetics are commonly utilized for reduction measurements, where the reaction occurs with a significant excess of the reducing agent compared to the Pt(IV) complex.39–42 This approach is often used to simulate the intracellular environment, where reducing agents are typically much more abundant than Pt(IV) complexes. The application of the second-order kinetic law is typically employed when the signal from the Pt(IV) complex is affected by a high concentration of the reducing agent or when investigating the impact of varying concentrations of the reducing agent on the reduction rate.43,44
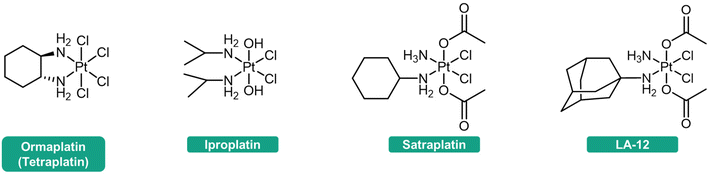
The reduction step is considered crucial for the activation of these complexes.45–47 The efficacy of Pt(IV) prodrugs as anticancer agents heavily relies on their reduction profile. If premature activation occurs, the advantages of Pt(IV) prodrugs in addressing the limitations of Pt(II) drugs may not be fully utilized. Conversely, if the reduction rate is significantly slow, the Pt(IV) prodrugs cannot be efficiently activated, and their latent cytotoxic activity remains unreleased. For example, ormaplatin (Fig. 2) has severe neurotoxicity as a result of rapid reduction to the active Pt(II) form, thereby rendering it unable to mitigate the typical side effects associated with Pt(II) drugs.48 In contrast, iproplatin (Fig. 2), which features two hydroxido axial ligands and a rather negative reduction potential, exhibits resistance to reducing agents, potentially explaining its lack of superior efficacy compared to cisplatin or carboplatin.36,49–51 Satraplatin and LA-12 (Fig. 2), however, have shown promising outcomes in preclinical studies. These compounds possess favorable properties that ensure stability in the bloodstream and efficient activation upon penetration into cancer cells.11,52–56
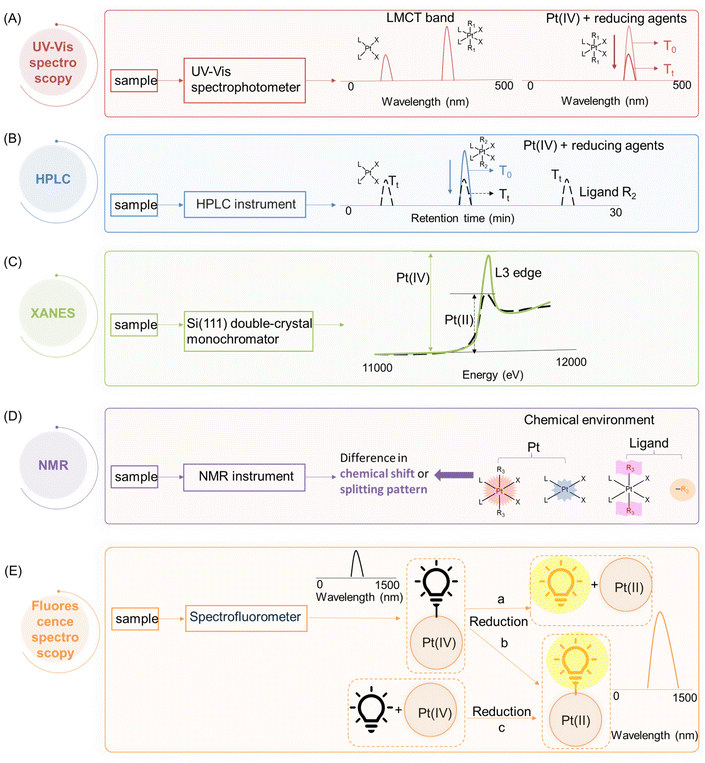
Therefore, a considerable amount of research has been dedicated to investigating the reduction properties of Pt(IV) prodrugs.22,32,35,57 A comprehensive understanding of the reduction scenarios of Pt(IV) complexes and the principles behind the reduction is essential for the rational design of innovative Pt(IV) prodrug candidates. To effectively track and analyze the reduction process, different analytical techniques, including ultraviolet–visible (UV-Vis) spectroscopy, high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR) spectroscopy, X-ray absorption near edge spectroscopy (XANES), and fluorescence spectroscopy, have been employed. These methods are favored for studying the fate of Pt(IV) complexes in buffer systems or at a cellular level, which provide valuable insights into predicting the cytotoxicity of Pt(IV) complexes, exploring the mechanisms of their cellular activation, and facilitating the development of new Pt(IV) prodrugs for cancer treatment.
In this review, we summarize recent advances in techniques used to monitor the reduction profiles of Pt(IV) complexes. Firstly, we delve into the fundamental principles underlying the techniques employed to track the reduction process of Pt(IV) prodrugs to their Pt(II) counterparts. Furthermore, we summarize and discuss the various techniques utilized to investigate the reduction progress of Pt(IV) complexes in aqueous solutions and at the cellular level. Specifically, we highlight the scope of applications, advantages, and disadvantages of each method. Finally, we offer a concise summary along with a critical evaluation. This review aims to equip researchers with a comprehensive understanding of strategies for monitoring the activation of Pt(IV) prodrugs and shed light on the underlying mechanisms of prodrug activation.
2. Overview of detection principles
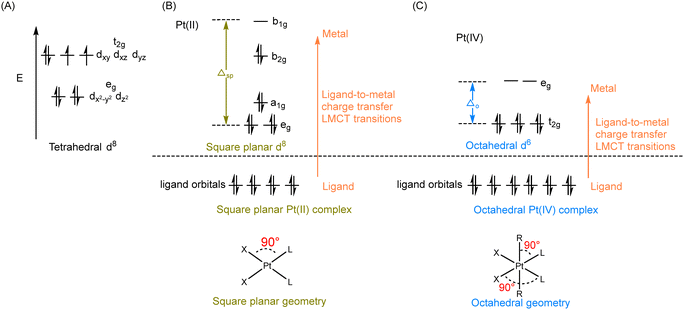
According to crystal field theory, the d orbitals of the Pt atom, whose electronic configuration is [Xe]4f145d96s1, initially feature degeneracy without splitting. Upon oxidation to Pt(II), they adopt a d8 configuration and undergo d orbital splitting when ligands approach.58,59 The d8 Pt(II) drugs prefer a square planar geometry as all eight d-electrons are paired in the lower-energy orbitals, rather than populating the higher-energy t2g set of tetrahedral orbitals (Fig. 3A and B). Additionally, square planar Pt(II) drugs, known for their high crystal field splitting energy surpassing the pairing energy, typically exhibit low-spin configurations. The Pt(IV) prodrug also adopts a low spin configuration due to the larger crystal field splitting energy Δo, leading to the pairing of the fourth to sixth electrons in the t2g orbitals; while the hybridization of the two vacant 5d orbitals with the vacant 6s and three of the 6p orbitals results in the formation of six d2sp3 hybrid orbitals, promoting the adoption of octahedral geometries in Pt(IV) prodrugs (Fig. 3C). During the reduction of Pt(IV) prodrugs to Pt(II) species, the geometry of the platinum center transforms from octahedral to square planar.35,60,61 This change in coordination geometry induces a reorganization of electrons around the platinum center, which can be applied as the basis to monitor the reduction of Pt(IV) complex.For instance, UV-Vis spectroscopy can be applied to monitor the reduction of Pt(IV) complexes. The reduction of simple Pt(IV) complexes containing axial ligands lacking UV absorbances can be monitored by observing the decrease in their ligand-to-metal charge transfer (LMCT) band. The electron transition of Pt(IV) complex from a ligand orbital to a metal d-orbital results in a more intense and red-shifted LMCT band compared with its Pt(II) counterpart, which can be attributed to several factors.11,62 The relative electron deficiency and presence of two vacant d orbitals in Pt(IV) prodrugs, compared to Pt(II) drugs, favor a higher likelihood of accepting electrons from the ligand, resulting in a more intense LMCT band in Pt(IV) prodrugs.62–65 The reduced energy gap between the ligand and metal orbitals in Pt(IV) prodrugs, compared to Pt(II) species, leads to the absorption of red-shifted UV light during the LMCT process (Fig. 3B and C). Accordingly, characteristic absorption bands of Pt(IV) complexes appear in the UV or visible spectrum. Monitoring the decrease in intensity of the LMCT band in Pt(IV) complexes before and after reduction enables rapid determination of the extent of reduction (Fig. 4A).
In cases where Pt(IV) complexes are modified with axial ligands possessing strong UV absorbance, their intrinsic UV absorbance is often overshadowed. Therefore, high-performance liquid chromatography (HPLC) can be used to monitor their reduction. HPLC separates Pt(II) and Pt(IV) complexes based on their differential hydrophobicity, resulting in distinct retention times in HPLC.66,67 By measuring the change in peak intensity of Pt(IV) complexes, the reduction process can be accurately monitored (Fig. 4B).68,69 The identification of reduction products, including the Pt(II) counterparts and the released axial ligands, can be achieved by comparing their respective retention times.
X-ray absorption near edge spectroscopy (XANES) enables the capture of X-ray absorption spectra of Pt(II) and Pt(IV) complexes at the Pt L3 edge, which is caused by the excitation of an electron from the occupied 2p orbital to the unfilled 5d orbital.70,71 The XANES spectra of Pt(IV) complexes show significantly higher edge heights compared to Pt(II) species (Fig. 4C). In Pt(II) drugs, the electronic configuration of Pt is 5d8, whereas Pt(IV) exhibits two additional vacancies in the d-shell, resulting in a configuration of 5d6 (Fig. 3B and C).35 Lytle et al. attributed the disparities in peak heights to the greater number of unoccupied d states in Pt(IV) complexes. The lower occupancy of the 5d orbitals increases the statistical probability of transitions to these states, thereby intensifying the L3 edge.70,72 Leveraging these differences in peak height, XANES proves to be a valuable tool for monitoring the reduction of Pt(IV) complexes and discerning the varying oxidation states of platinum.
The reduction of Pt(IV) complexes induces changes in the chemical environment surrounding the Pt nucleus, which consequently causes a variation in the chemical shift of the Pt atom in nuclear magnetic resonance (NMR) spectra.73–76 In addition, when Pt is conjugated to ligands, it influences their chemical environment, leading to differential chemical shifts compared to the free ligands.77,78 Moreover, this conjugation often results in the splitting of NMR peaks in ligands due to coupling interactions with platinum or other coordinated ligands, resulting in characteristic split patterns that distinguish them from free ligands.39,79 The chemical environment of non-leaving groups in Pt(IV) prodrugs also differs from that in Pt(II) drugs.77,80 NMR spectroscopy utilizes these variations to determine the reduction of Pt(IV) complexes and identify the reduction products (Fig. 4D).
In addition to the aforementioned techniques, fluorescence spectroscopy can also be employed to monitor the reduction of Pt(IV) complexes. The attachment of fluorophores to Pt(IV) complexes, whether in an axial or non-leaving position, usually results in a noticeable difference in fluorescence intensity compared to their unbound state. The decrease in fluorescence intensity can be attributed to the phenomena such as homo fluorescence resonance energy transfer (homo-FRET) and the heavy metal effect that occurs upon the conjugation with the platinum centers.81,82 Conversely, the reduction of Pt(IV) complexes is accompanied by an enhancement in fluorescence intensity, which can be attributed to either the generation of free fluorophores (Fig. 4E-a) or a reduced quenching effect exerted by the Pt(II) center on the fluorophore (Fig. 4E-b), thereby offering a direct visualization of the reduction process both extracellularly and intracellularly.83,84 Furthermore, an exogenous fluorescent probe can be specifically designed to interact exclusively with Pt(II) species, whereby the turn-on of its fluorescence is observed upon reaction with the Pt(II) center (Fig. 4E-c).85,86 Such fluorescent probes generally incorporate soft bases, such as sulfur, which prefer bonding to the Pt(II) center that is classified as soft acid, aligning with the principles of the Hard–Soft Acid–Base (HSAB) theory.1,87
3. Techniques used to monitor the reduction of Pt(IV) complexes
3.1 Ultraviolet–visible (UV-Vis) spectroscopy
The reduction kinetics of Pt(IV) complexes have often been investigated using UV spectroscopy.41,68,88–95 As mentioned above, the basis for using UV spectroscopy is that Pt(IV) prodrugs exhibit a more intense and red-shifted LMCT band compared with their Pt(II) counterparts.11,62 The wavelength of the LMCT band is influenced by the overall configuration (i.e., cis and trans geometry) of the Pt(IV) prodrugs62,64,65 and the inherent properties of coordinating ligands (e.g., electron-donating and steric characters).62,63 During the activation process, the intensity of the LMCT band in Pt(IV) complexes decreases due to the detachment of axial ligands. For example, the band of complex 1 centered at 304 nm exhibits a predominantly LMCT (py → Pt, N3) character (Fig. 5A and B). Following UVA exposure, this complex undergoes reduction.96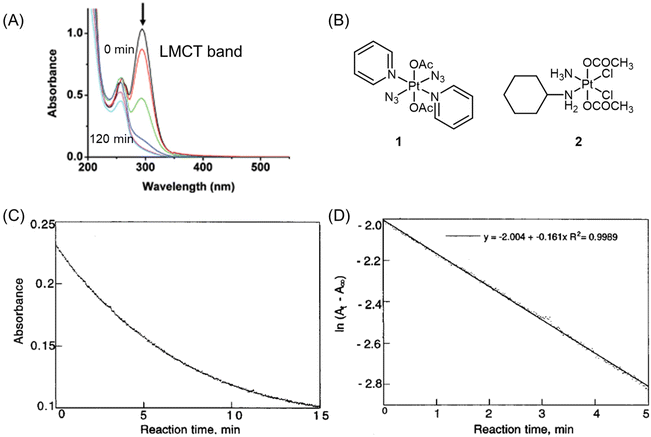 | ||
| Fig. 5 (A) UV-visible spectra of complex 1 after UVA irradiation for 0, 1, 5, 15, 30, 60, and 120 min. The arrow denotes a decrease in absorbance with increasing irradiation time. Adapted with permission from ref. 96. Copyright 2009, the Royal Society of Chemistry. (B) Chemical structures of complex 1 and 2. (C) The plot of absorbance at 330 nm versus reaction time for complex 2 (0.75 mM) and ascorbic acid (7.5 mM) at pH = 7.1. (D) A plot of ln(At − A∞) versus time for complex 2 (0.75 mM) and ascorbic acid (7.5 mM) at pH = 7.1. At = absorbance at 330 nm at time t. A∞ = absorbance at 330 nm after 30 min. Adapted with permission from ref. 41. Copyright 1998, American Chemical Society. | ||
To monitor the reduction of Pt(IV) complexes, the initial step involves determining the working wavelength for each tested Pt(IV) by recording their spectra across a wide range of wavelengths. Subsequently, the reduction reaction is initiated by mixing the Pt(IV) complexes and reducing agents in quartz cuvettes. For most kinetic measurements, pseudo-first-order conditions are employed, with a minimum of 10-fold excess of the reducing agent used. The reduction of Pt(IV) complexes is then studied spectrophotometrically by tracking the decrease in the LMCT band at a specific wavelength over time at a specific temperature. To obtain the rate constant, plots of ln(At − A∞) versus time are generated, where At represents the absorbances at time t, and A∞ represents the absorbance at infinity. These plots are constructed at wavelengths where the absorbance decreases maximally. By analyzing these plots, the reduction rate of Pt(IV) complexes can be determined.92
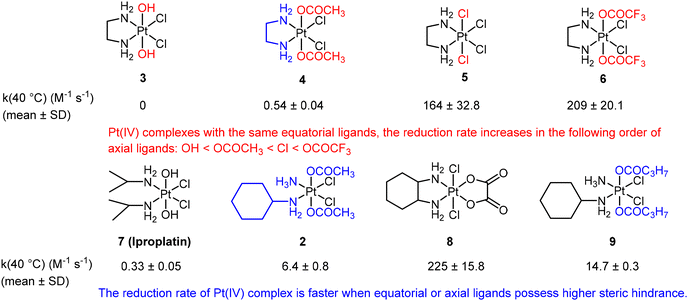
Choi et al. conducted a study utilizing UV spectroscopy to monitor the reduction of complex 2 (Fig. 5B) in the presence of a 10-fold excess of ascorbic acid.41 The reduction process was assessed by measuring the decrease in absorbance (At) of the Pt(IV) complex at 330 nm (Fig. 5C). The reduction of complex 2 by excess ascorbic acid followed a pseudo-first-order pattern, as evidenced by the linear plot of ln(At − A∞) versus time, which exhibited a high coefficient of determination (R2 = 0.998; Fig. 5D). The slope of this plot provided the pseudo-first-order rate constants. The authors employed this method to measure the reduction rate constants of seven other complexes (Fig. 6). Their findings revealed that the reduction rate of the Pt(IV) complexes was influenced by two factors: the electron-withdrawing power of axial ligands and the steric hindrance of both axial and equatorial ligands. Specifically, complexes with bulkier and more electron-withdrawing ligands exhibited faster reduction rates.
UV-Vis spectroscopy offers several advantages in studying the reduction process of Pt(IV) complexes. The non-destructive nature of this technique allows for the reuse of the Pt(IV) sample for further analysis after measurement. Additionally, UV-Vis spectroscopy provides rapid measurements that can capture swift changes in Pt(IV) complexes during reduction, with measurements typically taking only seconds. However, this approach does not provide any information about the identity of the reduction products. Besides, due to the presence of various biomolecules such as proteins, nucleic acids, and pigments in cells that have strong UV-Vis absorption, utilizing this technique to monitor the reduction process of Pt(IV) complexes in live cells is impractical.
3.2 High-performance liquid chromatography (HPLC)
Reversed-phase high-performance liquid chromatography (RP-HPLC) is a widely employed technique for monitoring the reduction of Pt(IV) complexes. This analytical approach provides researchers with the ability to determine whether a Pt(IV) complex will undergo reduction and release the reduction product. Additionally, it allows for the calculation of the rate at which the ligand dissociates from the complex, referred to as its reduction half-life. Therefore, HPLC is an indispensable analytical tool for analyzing Pt(IV) prodrugs before their evaluation in cellular systems.The concentrations of Pt(IV) complexes used for HPLC analysis usually range from 10 μM to 3 mM.24,38,97–99 The reduction half-lives (t1/2) of Pt(IV) complexes can be determined through several steps. Initially, the HPLC peaks corresponding to Pt(IV) complexes are integrated and analyzed at different time intervals. Subsequently, a linear regression of ln(At/A0) versus time (t) is plotted to calculate the rate constant (k), which is obtained using the pseudo-first-order equation ln(At/A0) = −kt, where A0 and At represent the integrals of the Pt(IV) complex peaks at the beginning and time t, respectively.100 The reduction half-life can then be calculated using the equation t1/2 = 0.693/k. The chromatographic peaks of Pt(IV) complexes in the HPLC can be processed using four methods to obtain peak areas that reflect the amount of Pt(IV) complexes reduced at different time points.
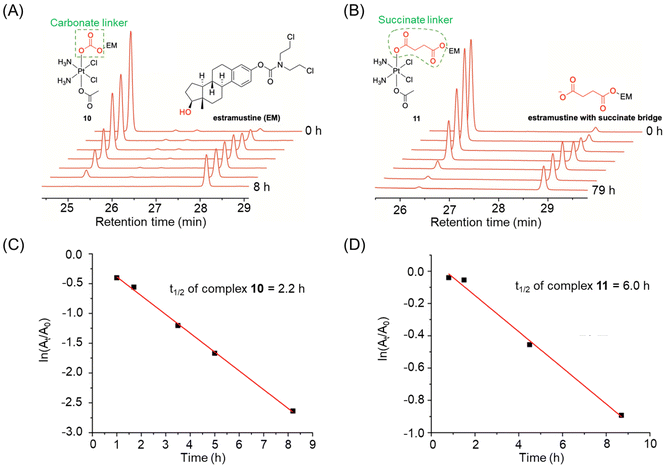 | ||
| Fig. 7 (A and B) The reduction of complexes 10 and 11, whose half-lives were calculated based on their absolute peak area at different time points. (C and D) Half-lives of complexes 10 and 11. Adapted with permission from ref. 39. Copyright 2019, Wiley Online Library. | ||
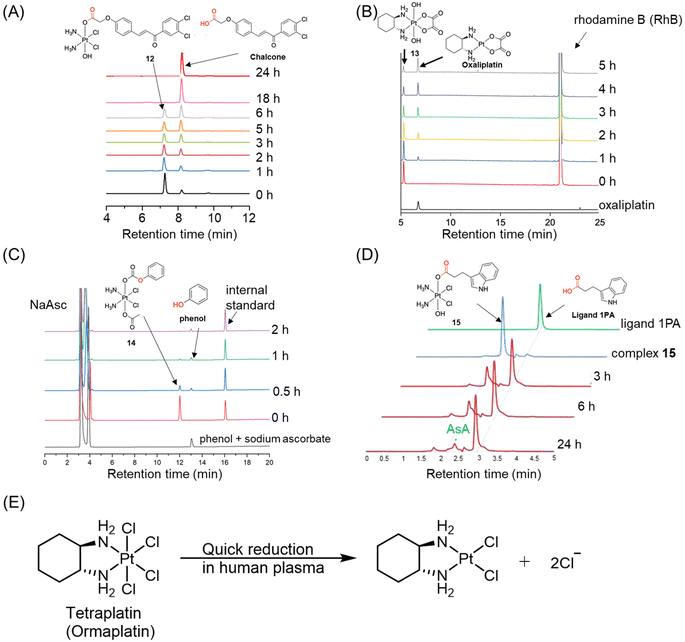 | ||
| Fig. 8 (A) The reduction of complex 12, whose half-life was calculated based on its area ratio at different time points. Adapted with permission from ref. 101. Copyright 2019, Wiley Online Library. (B) The reduction of complex 13, whose peak area was recorded at the wavelength of 303 nm. Adapted with permission from ref. 102. Copyright 2021, the Royal Society of Chemistry. (C) The reduction of complex 14, whose peak area was normalized with internal standard. (D) The peak overlapping of Pt(IV) complex 15 with reducing agent in HPLC chromatogram. Adapted with permission from ref. 103. Copyright 2016, Springer Nature. (E) The reduction pathway of ormapltin in plasma. | ||
Ormaplatin (Fig. 2) was among the first Pt(IV) complexes to undergo clinical trials. The reduction of ormaplatin was tracked using HPLC with the aid of an internal standard.107–109 The reduction half-lives of ormaplatin was determined to be 5–15 minutes in tissue culture medium and a mere 3 seconds in undiluted rat plasma (Fig. 8E).107,109 This rapid reduction of ormaplatin to its active Pt(II) form, attributed to the presence of axial chloride ligands, resulted in severe neurotoxicity.48 Consequently, ormaplatin did not progress beyond Phase I clinical trials.
It is worth noting that the reduction of Pt(IV) complexes can be investigated using various techniques. For example, the reduction of iproplatin (Fig. 2) has been extensively studied utilizing HPLC and UV spectroscopy.50,110,111 Iproplatin possesses hydroxo ligands in the axial positions, which impart resistance to reducing agents.36 As a result, significant amounts of iproplatin remain unaltered both in vitro and in vivo, contributing to its low toxicity. Despite undergoing extensive clinical trials ranging from Phase I to III, iproplatin was eventually discontinued due to its inability to demonstrate superior efficacy compared to cisplatin or carboplatin.50,51
Although HPLC-UV is an advanced tool for quantitatively detecting the reduction process of Pt(IV) complexes (Table 1), there are several limitations that hinder its broader applications. One limitation is that HPLC with a UV detector can only monitor the release of the reduction product throughout the reduction test if the released product has absorbance in the UV or visible region. However, some commonly used axial ligands in the investigation of Pt(IV) complexes, such as acetate and dichloroacetate, lack UV absorbance.112,113 Consequently, HPLC with a UV detector cannot be employed to confirm the release and identity of these reduction products. In such cases, alternative detection methods that do not rely on absorbance, such as electrospray ionization (ESI) mass spectrometry and liquid chromatography-mass spectrometry (LC-MS) techniques, can be employed to ascertain their identities.62,84,114–116 Besides, Pt(II) species in HPLC-UV analysis frequently display low absorbance, posing challenges to their accurate determination. Combining HPLC with ICP-MS could resolve this issue by enabling sensitive detection of Pt species. Moreover, HPLC-ICP-MS allows quantitative monitoring of Pt(II) growth and Pt(IV) reduction, providing precise insights into the conversion process.117,118 Another limitation arises from peak overlap in the HPLC chromatogram, which can affect data processing. In certain cases, the peaks of reduction products or reducing agents are too close to those of the Pt(IV) complexes.104 For instance, Erxleben et al. analyzed the reduction of Pt(IV) complex 15 using HPLC.103 The HPLC chromatogram revealed that a portion of the peak of complex 15 overlapped with that of sodium ascorbate (Fig. 8D), posing challenges in obtaining independent peak areas and affecting the accuracy of determining the reduction kinetics of Pt(IV) complexes. The overlap between complex 15 and ascorbate in the HPLC chromatogram can be addressed by using a column with a higher separation efficiency or by optimizing the gradient program to enhance peak separation.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Absolute peak area | A convenient method for calculating the reduction rate of Pt(IV) complexes | The results can be affected by inconsistent injection volume |
| The ratio of peak area | Enhancing the accuracy of calculating the reduction kinetics of Pt(IV) complexes by eliminating variation in sample injection | Peaks overlapping of Pt(IV) complexes with other analytes in the tested solution may affect the calculation of the reduction rate for Pt(IV) complexes |
| Selecting a wavelength where analytes share the same molar extinction coefficient | This method allows us to determine the percentage of remaining Pt(IV) complexes and that of reduction products simultaneously | This method is inapplicable when molar extinction coefficients of Pt(IV) complexes and reduction products differ |
| Including an internal standard | Improving the analytical quantification of the peak area of Pt(IV) complexes by eliminating the variation caused by sample injection | An appropriate internal standard should fulfill the following criteria: |
| (1) Having compatible absorption intensity with Pt(IV) complexes | ||
| (2) Stable during sample preparation and HPLC analysis | ||
| (3) Its peak should not overlap with the peaks of analytes present in the tested solution |
3.3 X-ray absorption near edge spectroscopy (XANES)
X-ray absorption near-edge structure, also known as near-edge X-ray absorption fine structure, is an absorption spectroscopy technique that provides valuable insights into the X-ray absorption spectra (XAS) of condensed matter. X-ray absorption near edge spectroscopy (XANES) can be used to identify the oxidation state of platinum complexes. The XANES spectra of Pt(II) and Pt(IV) species exhibit different edge heights, with Pt(IV) complexes displaying significantly higher edge heights. The peak height ratio, denoted as a/b, where a represents the maximum value of the edge and b corresponds to the local minimum of the edge, in the XANES spectra of platinum complexes, serves as a characteristic feature of the oxidation state (Fig. 9A).37,42,70,71 Mixtures containing different ratios of the two oxidation states produce a/b ratios that are intermediate between those of the individual oxidation states; these ratios demonstrate a linear relationship with the proportion of the two components in the mixture (Fig. 9B and C).70,71 Consequently, the peak height ratios can be used to monitor the reduction of Pt(IV) complexes in biological environments.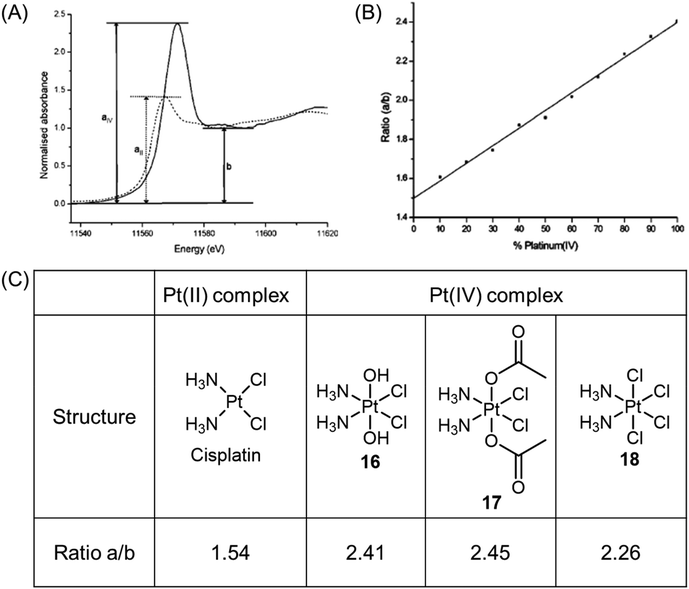 | ||
| Fig. 9 (A) XANES spectra of Pt(II) (dotted line) and Pt(IV) (solid line) complexes, showing the difference in peak heights for the two oxidation states, and the parameters a and b used in determining the ratio a/b. (B) Linear fit of peak height ratio (a/b) extracted from the Pt(II)/Pt(IV) standard mixtures. Determining the white line height ratio showed a good linear fit (R2 = 0.99788, P < 0.0001). Adapted with permission from ref. 71. Copyright 2003, American Chemical Society. (C) Experimentally determined peak height ratios (a/b) for XANES spectra of Pt(II) and Pt(IV) complexes. | ||
Hambley et al. conducted a study that demonstrated the applicability of XANES in monitoring the reduction of Pt(IV) prodrugs in cells.71 In their experiment, A2780 ovarian cancer cells were treated with a high concentration of complex 16, and the reduction process was analyzed using XANES. The reduction from Pt(IV) to Pt(II) and the accompanying change in the edge height were clearly observed. The Pt XANES spectrum of cells treated with complex 16 showed a significantly lower intensity of the edge height after 24 h compared to the spectrum taken at 2 h (Fig. 10A). Based on these observations, the authors calculated the percentage of complexes 16–18 present in the cells (Fig. 10B). The ease of reduction was found to depend on the nature of the axial ligands, denoted as X, in complexes of c,t,c-[PtCl2X2(NH3)2]. Specifically, the order of ease of reduction was found to be X = Cl > OAc > OH.119 This order was reflected in the extent of cellular reduction observed for the three Pt(IV) complexes after 2 h, where complex 18 was nearly fully reduced, while complexes 16 and 17 still had a substantial proportion remaining as Pt(IV). These results validate the importance of modifying axial ligands to fine-tune the reduction rate of Pt(IV) complexes, thereby offering valuable insights for the rational design of Pt(IV) complexes.
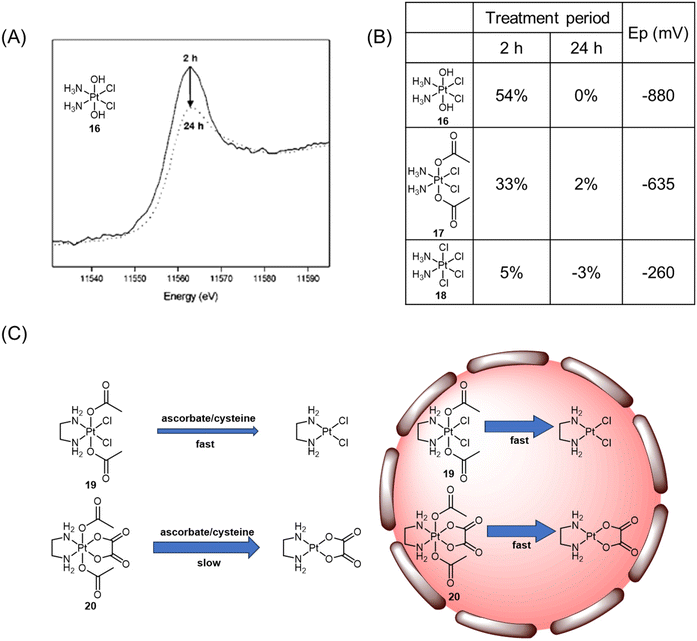 | ||
| Fig. 10 (A) Normalized XANES spectra of A2780 cells incubated with Pt(IV) complex 16 after 2 h (solid line) and 24 h (dotted line). Adapted with permission from ref. 71. Copyright 2003, American Chemical Society. (B) Proportion of Pt(IV) (±5%) remaining after incubation of complexes with A2780 cells and their reduction potentials (Ep). (C) The reduction of complexes 19 and 20 in various biological contexts. | ||
The analysis of XANES spectra also plays a crucial role in investigating how the Pt(IV) coordination sphere influences the ease of reduction of the platinum center in various biological contexts.120 In the presence of biological reductants such as ascorbate and cysteine, the Pt(IV) complex 19 with dichlorido equatorial ligands, having a higher reduction potential, exhibited a faster reduction rate (t1/2 = 30 min for ascorbate; t1/2 = 4.1 h for cysteine) compared with the analogous complex 20 with dicarboxylato equatorial ligands (t1/2 = 20 h for ascorbate; t1/2 = 27 days for cysteine; Fig. 10C). XANES spectroscopy analysis provided insights into the reduction rates of complexes 19 and 20 within DLD-1 cancer cells. It was observed that both complexes were reduced at similar rates, with 14% and 24% of complexes 19 and 20 remaining, respectively, after 6 h. Despite the unusual kinetic inertness of complex 20 with bidentate oxalato equatorial groups in simple single reductant model systems, its short half-live within cancer cells indicated that the intracellular environment could overcome the stabilizing effects of the tetracarboxylato coordination sphere. The significant variability in kinetic inertness exhibited by complex 20 in different biological contexts has important implications for the design of Pt(IV) prodrugs. While incorporating the tetracarboxylato coordination sphere may render Pt(IV) complexes less susceptible to reduction in the extracellular environment, this study demonstrated that such complexes are rapidly reduced upon entry into cancer cells. Delaying the reduction of the Pt(IV) complexes until they have penetrated the cancer cells may lead to more effective and less detrimental outcomes.
The direct measurement of the relative proportions of two different oxidation states of Pt(II) and Pt(IV) in biological environments using XANES is effective for observing the reduction of Pt(IV) complexes. XANES analysis demonstrates a detection limit of approximately 10 mg kg−1,121 influenced by factors such as the nature of the sample, experimental setup, and the specific element being analyzed. In the case of platinum complexes, clear XANES spectra are typically obtained by exposing cells in one 75 cm2 flask to the platinum complex at a concentration of 50 μM.120 However, this technique is time-consuming and requires sophisticated sample preparation, such as collecting and freezing cells, making it unsuitable for real-time monitoring of Pt(IV) prodrug reduction in live cells. Additionally, access to the instruments required for XANES analysis is limited, and conducting experiments using synchrotron radiation can be challenging due to the logistical and technical requirements.
3.4 Nuclear magnetic resonance (NMR) spectroscopy
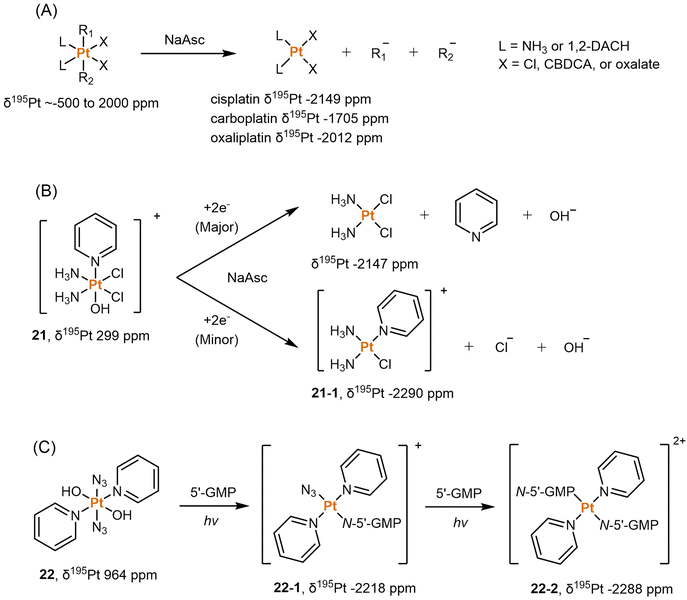
NMR spectroscopy is an informative technique for monitoring the reduction of Pt(IV) prodrugs. It offers several advantages, including the absence of damage to the analysts and the ability to perform time-dependent measurements. NMR can provide insights into the reduction process by analyzing NMR-active isotopes present in Pt(IV) prodrugs, such as 1H, 13C, 15N, 19F, and 195Pt. These isotopes can be scrutinized to track changes during reduction. The choice of NMR technique depends on the structure of Pt(IV) complexes and the interferences from the solution. In numerous studies conducted over the last few decades, deuterated water (D2O) has been a popular solvent for NMR detection. Unless otherwise specified, the chemical shifts discussed in subsequent sessions were determined using D2O as the solvent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm.75 This wide range allows for differentiation between different oxidation states of platinum (+2, +4), as the chemical shits can differ by thousands of ppm. Additionally, the substitution of ligands in the Pt complex can lead to chemical shift changes of around 100 ppm from the original values.74 Hence, 195Pt NMR spectroscopy is extensively used for structural determination and to compare the oxidation state and coordination environment of various Pt complexes. Sadler et al. compiled a comprehensive database of δ195Pt values for a broad range of Pt(II) complexes, including clinically approved anticancer drugs like cisplatin and carboplatin. The δ195Pt value for cisplatin was reported as −2149 ppm, while carboplatin had a value of −1705 ppm. Derivatives of these drugs, where the leaving group ligands (such as Cl and CBDCA) were substituted with other O-, N-, or S-donor ligands, displayed δ195Pt values ranging from −3685 to −1460 ppm.122 This study provides a valuable reference database of δ195Pt values for Pt(II) anticancer agents, and these values can be utilized when investigating the reduction processes from Pt(IV) prodrugs to Pt(II) drugs.
000 ppm.75 This wide range allows for differentiation between different oxidation states of platinum (+2, +4), as the chemical shits can differ by thousands of ppm. Additionally, the substitution of ligands in the Pt complex can lead to chemical shift changes of around 100 ppm from the original values.74 Hence, 195Pt NMR spectroscopy is extensively used for structural determination and to compare the oxidation state and coordination environment of various Pt complexes. Sadler et al. compiled a comprehensive database of δ195Pt values for a broad range of Pt(II) complexes, including clinically approved anticancer drugs like cisplatin and carboplatin. The δ195Pt value for cisplatin was reported as −2149 ppm, while carboplatin had a value of −1705 ppm. Derivatives of these drugs, where the leaving group ligands (such as Cl and CBDCA) were substituted with other O-, N-, or S-donor ligands, displayed δ195Pt values ranging from −3685 to −1460 ppm.122 This study provides a valuable reference database of δ195Pt values for Pt(II) anticancer agents, and these values can be utilized when investigating the reduction processes from Pt(IV) prodrugs to Pt(II) drugs.
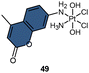
Dabrowiak et al. conducted a study utilizing 195Pt NMR spectroscopy to investigate the reduction of c,c,t-[Pt(NH3)2Cl2(OH)2] (oxoplatin, δ195Pt 853 ppm) in the presence of sodium ascorbate (NaAsc) (Scheme 2A).123 Cisplatin was confirmed as the major reduction product, as indicated by the 195Pt NMR peak at −2149 ppm, while the presence of other Pt(II) species was also observed, displaying δ195Pt values ranging from −1670 to −1830 ppm.123 This investigation confirmed that the Pt(IV) complex exhibits “prodrug” properties, as it only binds to DNA after being reduced to cisplatin. Following this discovery, numerous studies have endeavored to track the reduction process of Pt(IV) prodrugs to Pt(II) agents by utilizing 195Pt NMR spectroscopy. Some notable examples include cisplatin-, carboplatin-, and oxaliplatin-based Pt(IV) prodrugs containing axial carboxylate ligands; the corresponding Pt(II) drugs are usually observed in 195Pt NMR spectra (Scheme 2A).49,100,124,125 In our previous study on pyridinyl-Pt(IV) complex 21, a unique reduction pathway that involved the detachment of an axial hydroxido and an equatorial chlorine ligand was discovered. This led to the formation of pyriplatin 21-1, which was identified by 195Pt NMR (Scheme 2B).38 Besides, Sadler et al. reported a photoactivatable azido-Pt(IV) prodrug 22 that yielded Pt(II) active species (22-1 and 22-2) upon irradiation, as confirmed by 195Pt NMR spectroscopy (Scheme 2C).64
195Pt NMR spectroscopy possesses the ability to distinguish Pt in diverse chemical environments, detect Pt-containing substances in situ, and monitor chemical reactions in aqueous solutions. However, a relatively large amount of sample (approximately 10 mM for 600 MHz NMR) is usually required for 195Pt NMR measurement due to its low sensitivity.22,126 Moreover, the resolution of 195Pt NMR may not be sufficient to depict all spin-coupling patterns, particularly for Pt complexes coordinated with multiple N- or P-donor ligands.75 As a result, the Pt peaks in the spectrum can appear broadened, making their assignment challenging.
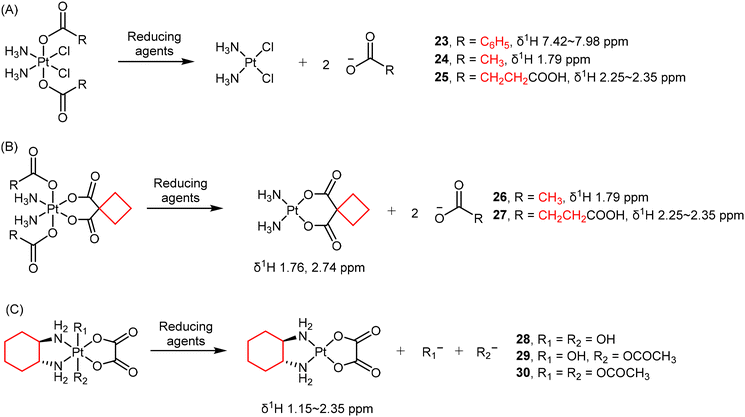
Due to different electron densities, the chemical shifts of protons in coordinated or free ligands can exhibit detectable differences. For example, the loss of axial carboxylate ligands such as benzoate (23), acetate (24), and succinate (25) in Pt(IV) complexes has been observed using 1H NMR spectroscopy (Scheme 3A).49,128,129 Salassa and co-authors reported that the reduction of complexes 26 and 27 (Scheme 3B) to carboplatin by NADH are photocatalyzed by flavins.1301H NMR signals from the axial acetato (26) and succinato (27) ligands, as well as the protons in the CBDCA ligand are quantified to determine the conversion percentage.
In some cases, 1H NMR is also helpful in characterizing the structure of resulting Pt(II) products, providing direct confirmation of the reduction process.131 For oxaliplatin-based Pt(IV) complexes, the hydrogen atoms in their DACH ligands are also usually monitored as indicators of reduction (Scheme 3C).77,124 For example, Gibson and colleagues investigated the reduction of three oxaliplatin-based Pt(IV) complexes 28–30 by monitoring the 1H NMR signals in the DACH ligand over time (Scheme 3C).77 Intriguingly, there is no direct correlation between the reduction potentials and the rates of reduction. Complex 30, with the least negative reduction potential (Ep = −0.48 V), exhibited only a 5% reduction after 12 hours. In contrast, complex 29 (Ep = −0.64 V) had a half-life of approximately 5.5 h, and complex 28, with the most negative reduction potential (Ep = −0.80 V), underwent the most rapid reduction and possessing a half-life of about 2.5 h. These findings contradict reports for Pt(IV) complexes with N2Cl2 coordination spheres, where a correlation between the reduction potential and the rates of reduction exists. Therefore, the electrochemically measured reduction potentials may not necessarily reflect the rates of reduction for Pt(IV) complexes, which hold significance in the design of novel Pt(IV) prodrugs based on oxaliplatin.
Although 1H NMR spectroscopy can offer high-resolution spectra when samples are measured in buffer solutions, it encounters challenges when detecting samples in biological environments such as cell culture medium or serum due to the high background present. Additionally, the analyte's active H atoms (OH, NH2, or COOH) may exchange with D in the deuterated solvent, leading to diminished or absent NMR signals.
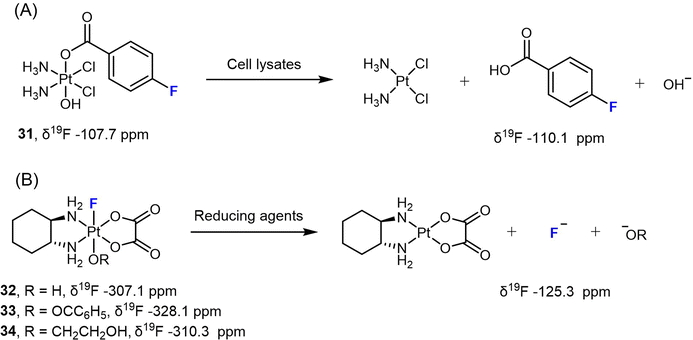
Liu et al. designed a Pt(IV) prodrug 31 bearing a 4-fluorobenzoate ligand (FBA) to monitor its reduction process under various biological conditions (Scheme 4A).78 Upon reduction, the NMR signal of 31 at −107.7 ppm vanished, while a signal corresponding to free FBA at −110.1 ppm emerged. By utilizing 19F NMR spectroscopy, the authors could accurately measure the consumption of the Pt(IV) complex and the release of the free ligand, and were able to determine the reduction rate. More importantly, this approach was also used to track the reduction process of the Pt(IV) prodrug in different types of cells, including A431, A549, and HeLa cancer cells, as well as red blood cells (RBCs) and Escherichia coli BL21 (DE3) bacteria cells. The authors detected the release of the FBA ligand from complex 31 over time using 19F NMR spectroscopy. In RBCs, however, a weak 19F signal was only observed after 2 h of incubation, indicating a slower reduction rate. This effect could be attributed to the lower uptake of the Pt(IV) complex in RBCs compared to cancer cells and bacteria.
When F coordinates directly to Pt in Pt(IV) prodrugs, the resulting 19F NMR spectrum typically shows distinct (s + d, JPt–F) peaks, which arise from the spin–spin coupling between 195Pt and 19F nuclei. Since 195Pt is present at a natural abundance of only 33.8%, approximately one-third of the 19F peak undergoes splitting due to spin–spin coupling, resulting in a doublet peak. The remaining two-thirds of the peak appears as a singlet. In our previous studies, we investigated the reduction of various F-coordinated oxaliplatin-based Pt(IV) complexes using 19F-NMR spectroscopy (Scheme 4B).24,79 We observed distinct 19F peaks in the spectra ranging from −328.1 to −307.1 ppm, which gradually diminished, while a single peak at −125.3 ppm (representing free F−) steadily increased. These results indicate that the axial ligands opposite to F significantly affect the reduction rate [carboxylato (33) > hydroxido (32) > alkoxido (34)]. For instance, complex 33 with a benzoato axial ligand is completely reduced to oxaliplatin within 1 hour when treated with 10 equivalents of NaAsc, whereas complex 34 with an axial 2-hydroxyethoxido group remains 95% intact after one-hour incubation with NaAsc, displaying a half-life over 8 h.
The 19F isotope is known for its high sensitivity in NMR spectroscopy and exhibits a wide range of chemical shifts. This sensitivity allows for efficient tracking of the F-containing molecules and assessing the dissociation of F− or F-containing ligands from Pt(IV) complexes, as it can detect molecular and conformational changes in labeled molecules. In biological settings, 19F NMR provides a non-destructive approach to monitor the reduction of Pt(IV) prodrug in live cells. The wide range of chemical shifts exhibited by 19F, however, poses limitations for its application in 2D NMR spectroscopy, primarily due to hardware requirements. For Pt(IV) complexes, the use of 19F NMR is focused on monitoring the axial F-containing ligands but not the equatorial ligands. As a result, 19F NMR spectroscopy itself cannot differentiate between hydrolysis and reduction, both of which result in the loss of an F-containing axial ligand.
Wong et al. employed 15N NMR to examine the reduction of complex c,c,t-[PtCl2(15NH3)2(COOCH3)2] (17 (15N)) by L-cysteine and L-methionine. The authors observed the formation of different products during the reduction process.128 Complex 17 (15N) was reduced to cisplatin (15N-labelled) by these reducing agents. Furthermore, L-methionine reacted with cisplatin, resulting in the formation of bidentate Pt(II) complexes in either cis- (17-1) or trans-conformation (17-2) (Scheme 5A). Osella et al. also investigated the reduction process of complex 17 (15N) using 15N NMR to analyze the reduction products resulting from sodium ascorbate.134 The reduction of the Pt(IV) prodrug 17 (15N) resulted in the clear observation of both 15N-cisplatin (−67.8 ppm) and the mono-aquated product (17-3, −65.8 ppm and −88.8 ppm; Scheme 5B). These examples highlight the sensitivity of 15N NMR spectroscopy in detecting and identifying the reduction products of Pt(IV) prodrugs, which can vary depending on the specific reducing agents used. Sadler et al. synthesized the 15N-labelled derivative of the photoactivable Pt(IV) prodrug t,t,t-[Pt(N3)2(OH)2(15NH3)2] (35) and tracked its photoreduction pathways using 15N NMR in an acidic solution or PBS buffer.135 The appearance of 15N peaks (35-1, −42.2, −41.3 ppm) in the Pt(IV) region indicated the hydrolysis of azido ligands upon irradiation, followed by reduction to Pt(II) complex 35-2, accompanied by the emergence of a new 15N peak (−69.6 ppm) (Scheme 5C).
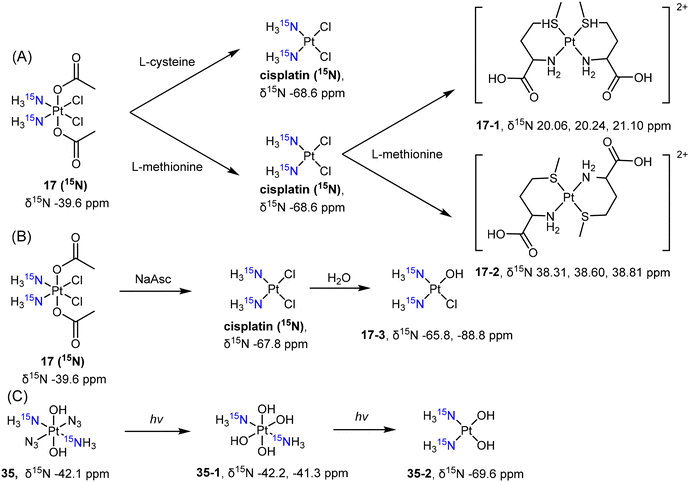 | ||
| Scheme 5 Reduction pathway of (A, B) 15N-labelled cisplatin-based Pt(IV) complex 17 and (C) diazido-Pt(IV) complex 35. | ||
The synthesis of 15N-labelled Pt(IV) complexes remains the main challenge in conducting 15N NMR detection and proves to be both a time-consuming and costly process, given the high expense of materials containing 15N. Consequently, the application of 15N NMR spectroscopy to analyze the reduction process of Pt(IV) prodrugs is limited.
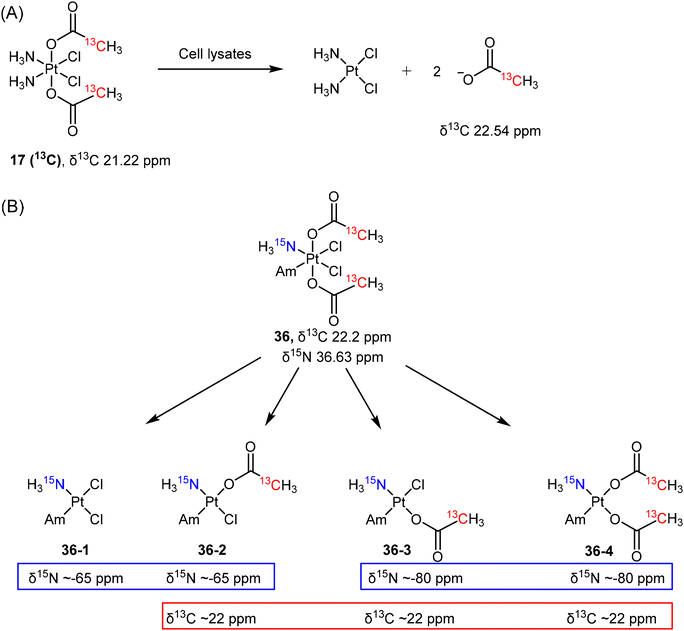
Reducing agents with low molecular mass, such as ascorbate or glutathione, are believed to be responsible for the reduction of Pt(IV) prodrugs in cellular environments. While most studies on the reduction of Pt(IV) complexes utilized ascorbate or GSH as reducing agents, it is crucial to recognize that various other reducing agents present in cells can also activate these complexes. As such, determining which specific cellular reducing agents are involved in the activation of Pt(IV) complexes is warranted. Gibson et al. utilized whole-cell extracts as a more realistic model for cellular conditions to study the reduction of Pt(IV) complexes.136 They synthesized a 13C-labelled Pt(IV) complex c,c,t-[PtCl2(NH3)2(COO13CH3)2] (17 (13C)) and utilized advanced 2D NMR spectroscopy ([1H,13C] HSQC) to monitor its reduction (Scheme 6A). The authors evaluated the reduction of prodrug 17 (13C) in extracts from A2780, A2780cisR, and HT29 cells by monitoring the change in 2D NMR peaks of complex 17 (13C) [δ(1H,13C) 2.13, 21.22 ppm] and the free acetate ligand [δ(1H,13C) 1.94, 22.54 ppm].136 The reduction curves were plotted, and the rates were calculated based on the quantification of 2D NMR peaks. The study revealed that the aqueous extracts from different cell lines exhibited varying rates of reduction for the same Pt(IV) complex, following the order A2780cisR (t1/2 = 36 min) > A2780 (t1/2 = 90 min) > HT-29 (t1/2 = 130 min). This result suggests that the rate of reduction depends on the reductive capacity, i.e., the contents, of each cancer cell line. Notably, the resistant A2780cisR cells, which have high levels of GSH, showed the most rapid reduction of 17 (13C). This observation supports the hypothesis that GSH is responsible for activating Pt(IV) prodrugs in cells. To test this hypothesis further, the authors divided the cell extracts into high and low molecular weight (MW) fractions and examined their ability to reduce the Pt(IV) complex. While the high MW fraction displayed a reduction rate (t1/2 = 35 min) similar to that of the whole extracts (t1/2 = 36 min), the low MW fraction, which contains both ascorbate and GSH, appeared to have limited efficiency in reducing the Pt complexes (with only 20% reduction after 500 min). The study suggests that the low MW antioxidants, previously considered responsible for reducing Pt(IV) complexes, exhibit lower reduction abilities compared to the whole cell extracts. Hence, it is reasonable to conclude that an aqueous solution containing a single reducing agent (such as GSH, cysteine, or ascorbic acid) may not adequately model the reduction of Pt(IV) complexes within cancer cells.
The reduction mechanisms proposed for Pt(IV) complexes generally suggest that the reduction process yields a single Pt(II) complex by eliminating two trans-oriented axial ligands from the octahedral Pt(IV) complex. The reduction of c,c,t-[PtCl2(15NH3)(Am)(COO13CH3)2] (36) with axial acetato ligands does not solely proceed by the loss of two axial ligands.37 When complex 36, labeled with both 13C- and 15N, was reduced by ascorbate, four distinct reduction products (36-1 to 36-4) were obtained and identified using [1H,13C] and [1H,15N] HSQC techniques (Scheme 6B).37 In the [1H,15N] spectrum, two peaks at δ15N −65 ppm and two peaks at δ15N −80 ppm were observed, which indicate that these 15NH3 groups were trans to either a chlorido or an acetato ligand. In addition, a group of peaks was observed at around δ13C 22 ppm (Scheme 6B) and differentiated from the peak of free acetate (δ13C 23.2). Collectively, the four possible reduction products were identified as c-[PtCl2(15NH3)(Am)] (36-1), c-[PtCl(13CH3COO)(Am)(15NH3)] (36-2 and 36-3, two isomers), and c-[Pt(13CH3COO)2(15NH3)(Am)] (36-4). This example indicates the possibility of losing one or two equatorial ligands during the reduction of a Pt(IV) complex and demonstrates that the use of 2D NMR is effective in identifying the mixtures of reduction products.
In the investigation of Pt(IV) prodrugs’ reduction, the [1H,15N] HSQC technique is more sensitive over [1H,13C] HSQC, and may show >10 ppm difference in chemical shifts. Arnesano et al. studied the reduction of complex c,c,t-[PtCl2(15NH3)2(COOCH3)2] 17 (15N) by NADH and cytochrome c (cyt c) (Scheme 7A).137 The reduction profile was obtained by quantifying the peaks in the [1H,15N] spectrum, and it was concluded that cyt c accelerated the reduction of the Pt(IV) prodrug in the presence of NADH. Another example reported by Osella involved the reduction of c,c,t-[PtCl2(15NH3)2(Gln)(COOCH3)] (37), a glutamine-conjugated Pt(IV) complex, by cytosol from A549 cells.138 The reduction process finished within 1 h and resulted in the formation of 15N-labelled cisplatin, which was observed through [1H,15N] HSQC at δ15N −66.8 ppm (Scheme 7B). Intriguingly, when the authors studied the reduction of amidato-Pt(IV) complex c,c,t-[PtCl2(15NH3)2(COOCH3)(NHCOCH3)] (38) in the presence of sodium ascorbate, a monofunctional Pt(II) complex c-[PtCl(NHCOCH3)(15NH3)2] (38-1, δ15N −69.0 ppm), as the minor reduction product, was also observed using [1H,15N] HSQC (Scheme 7C).80
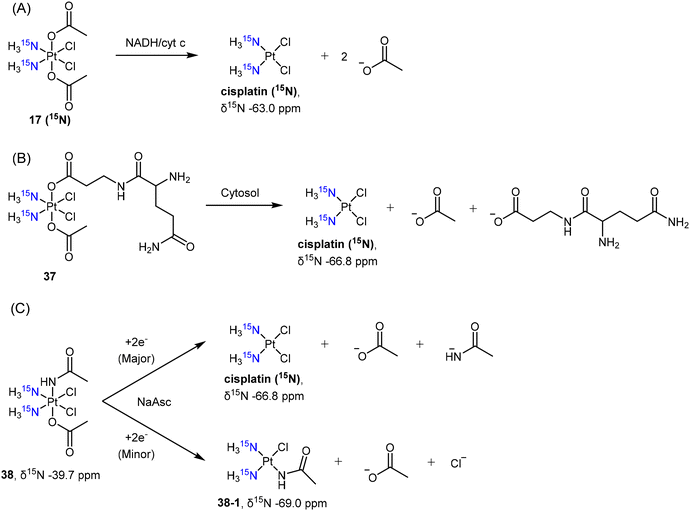 | ||
| Scheme 7 Reduction pathway of 15N-labelled cisplatin-based Pt(IV) complex 17 (15N) with (A) axial acetato ligand, (B) complex 37 with glutamine-mimic ligand, and (C) complex 38 with amidato ligand. | ||
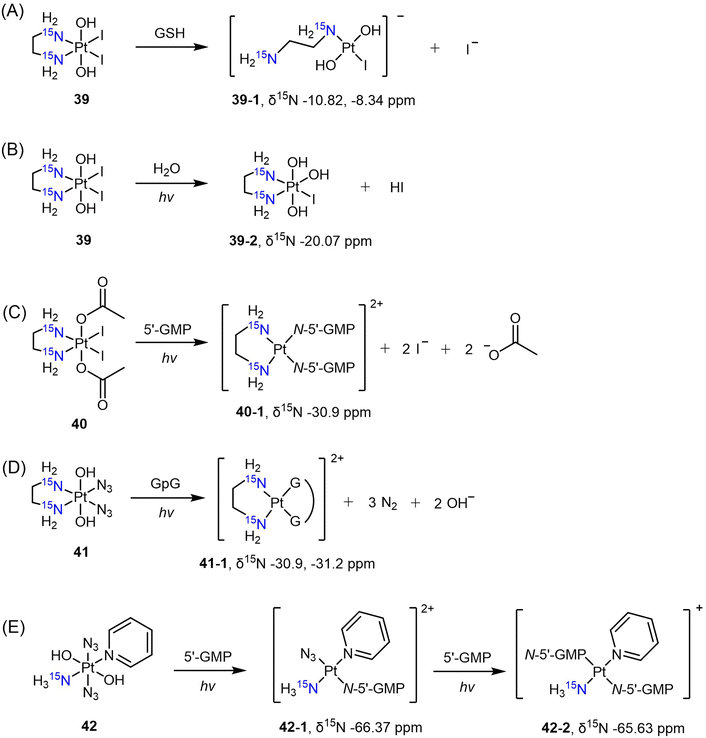
The diiodo-Pt(IV) complex t,c-[Pt(OH)2I2(en)] was initially developed as a photoactivatable Pt(IV) prodrug.139 Understanding the reduction process and mechanism of this photosensitive complex is essential, and this was achieved through the use of [1H,15N] HSQC with its 15N-analogue 39. Sadler et al. monitored the reduction of complex 39 in the dark in the presence of GSH.140 Based on the observation of 15N peaks at δ −10.82 and −8.34 ppm after reduction, the authors confirmed that the major reduction product at pH 7 is the ring-opened Pt(II) complex 39-1 (Scheme 8A). When the complex was irradiated in pure water, however, the I ligand was hydrolyzed to OH, leading to the formation of complex 39-2 instead of reduced Pt(II) species, as evidenced by the δ(1H,15N) peak observed at 6.33, −20.07 ppm (Scheme 8B).141 In contrast, the diacetato complex t,c-[Pt(CH3CO2)2I2(15N-en)] (40) was found to undergo photoreduction and bind to 5′-GMP, yielding the Pt-GMP adduct c-[Pt(5′-GMP)2(15N-en)] (40-1), as indicated by δ(1H,15N) peaks observed at 5.72, −30.9 ppm and 5.79, −30.9 ppm (Scheme 8C).
Other photoactivatable Pt(IV) prodrugs reported by Sadler and colleagues include c,t-[Pt(15N-en)(N3)2(OH)2] (41)142,143 and t,t,t-[Pt(N3)2(OH)2(15NH3)py] (42).144 The azido and pyridine ligands, due to the lack of hydrogen atoms attached to these N atoms, cannot be detected by [1H,15N] HSQC. Upon irradiation, complex 41 was reduced to the aquated Pt(II) species within 40 min and bound to a nucleotide d(GpG), resulting in the formation of the chelating Pt(II) adduct c-[Pt(15N-en)(GpG)] (41-1). The δ(1H,15N) peaks observed at 5.65, −30.9 ppm and 5.56, −31.2 ppm confirmed the presence of this adduct (Scheme 8D).143 In the presence of 5′-GMP, t,t,t-[Pt(N3)2(OH)2(15NH3)py] (42) was reduced, yielding the monofunctional Pt(II) adduct t,t-[Pt(N3)(5′-GMP)(15NH3)py] [42-1, δ(1H,15N) 4.15, −66.37 ppm] within 5 min (Scheme 8E).144 Subsequently, a second-step photolysis was initiated, which took one hour, eventually leading to the formation of the bis-5′-GMP adduct t-[Pt(5′-GMP)2(15NH3)py] [42-2, δ(1H,15N) 4.42, −65.63 ppm] (Scheme 8E).144
The [1H,15N] HSQC technique is highly effective in monitoring the reduction process of Pt(IV) prodrugs by various reducing agents. It also enables differentiation between hydrolysis and reduction during photoreactions. Furthermore, this technique demonstrates remarkable sensitivity and can be employed in biological fluids, with the potential to detect 15N-labeled compounds in live cells.
In analyzing the reduction profiles of Pt(IV) prodrugs, several types of NMR techniques can be used, as summarized in Table 2. The detection limit of NMR spectroscopy is affected by many parameters such as sample volume, sample type, measurement frequency, number of scans, NMR setup, and the specific isotopes being detected.145 For example, the detection limit of 1H NMR spectroscopy can be as low as 1 ppm when using a 600 MHz instrument and 1024 scans. With the same frequency and number of scans, the detection limit of other isotopes is generally scaled up relative to their sensitivity, as shown in Table 2. The most sensitive NMR techniques include 1H NMR and its 2D variants, such as [1H,13C] and [1H,15N] HSQC. These techniques are commonly utilized for quantification and time-dependent measurements. 195Pt NMR is particularly useful as it directly detects the Pt center, allowing the differentiation of Pt(II) and Pt(IV) complexes in various coordination environments. 15N NMR and [1H,15N] HSQC can help identify the ligands opposite to the 15N-labelled ligand, thereby facilitating the determination of the reduction process of Pt(IV) prodrugs. Furthermore, 19F NMR provides another sensitive method for monitoring the change of ligands in F-containing Pt(IV) complexes in buffered solutions or cells. The choice of NMR spectroscopy depends on specific conditions and research objectives. Overall, this technique is critical in comprehending the reduction of Pt(IV) complexes and is instrumental in the design of novel Pt(IV) anticancer agents.
| NMR types | Relative sensitivitya | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| a Relative sensitivity is compared to 1H NMR, which is defined as 100. | ||||
| 195Pt | 0.99 | Direct measurement of Pt; can distinguish Pt(II) and Pt(IV) | Less sensitive, not quantitative, requires a large amount of sample | Trace Pt(IV) to Pt(II); identify Pt(II) products from the library of 195Pt chemical shifts75 |
| 1H | 100 | Sensitive, quantitative, and informative | Interfered by proton exchange; high background in biological fluid | Quantitatively plot the reduction curve, calculate the reduction rate, and identify free ligand released |
| 19F | 83.3 | Sensitive, applicable in cell lysates and live cells | Require 19F-containing compound; cannot distinguish hydrolysis and reduction | Monitor the loss of 19F-containing ligands in buffered solutions, cell lysates, or live cells |
| 13C | 0.016 | High resolution and large range of chemical shift | Require 13C-labelled compound, low sensitivity | No published applications |
| 15N | 0.104 | Can distinguish Pt(II) and Pt(IV), sensitive to the change of trans ligand | Require 15N-labelled compound, low sensitivity | Trace Pt(IV) to Pt(II) and detect ligand exchange |
| [1H,13C] HSQC | 100 | Sensitive, quantitative, and present C–H bonding, high resolution, extensive range of chemical shift, and applicable in cell lysates | Require 13C-labelled compound, not applicable to 13C atom without H | Monitor the loss of 13C-labelled ligands and identify reduction products in buffered solutions or cell lysates |
| [1H,15N] HSQC | 100 | Sensitive, quantitative, and present N–H bonding. It can distinguish Pt(II) and Pt(IV), and it is sensitive to the change of trans ligand and is applicable in cell lysates | Require 15N-labelled compound, not applicable to 15N atom without H | Trace Pt(IV) to Pt(II) in buffered solutions or cell lysates, detect ligand exchange, and identify reduction products |
3.5 Fluorescence spectroscopy
Fluorescence spectroscopy is an advantageous analytical technique due to several key features, including its ability to detect low concentrations of fluorescent molecules with high sensitivity, excellent selectivity that is derived from the unique fluorescence emission spectra of different compounds, and non-invasive and non-destructive nature, which allows for the preservation of sample structure and integrity throughout the analysis process.81,146–151 These advantages make fluorescence spectroscopy an effective tool for monitoring dynamic processes of Pt(IV) prodrugs, both in test tubes and at the cellular level.83,85,86,114,152–154 Fluorophores have been conjugated to Pt(IV) complexes to track their reduction process in different biological contexts. It was anticipated that the coordination of fluorophores with Pt(IV) complexes would result in fluorescence quenching, which can occur through homo-fluorescence resonance energy transfer (homo-FRET) or the heavy metal effect.81,82 Reduction of Pt(IV) complex was accompanied by a concomitant enhancement in fluorescence intensity, which, in principle, provides a direct method for visualizing the reduction process, either extracellularly or intracellularly.83,84,114 Besides, by designing an external probe that exclusively interacts with Pt(II) species, fluorescence can be “turned on” specifically upon reduction of Pt(IV) prodrugs, providing further visualization capabilities.85,86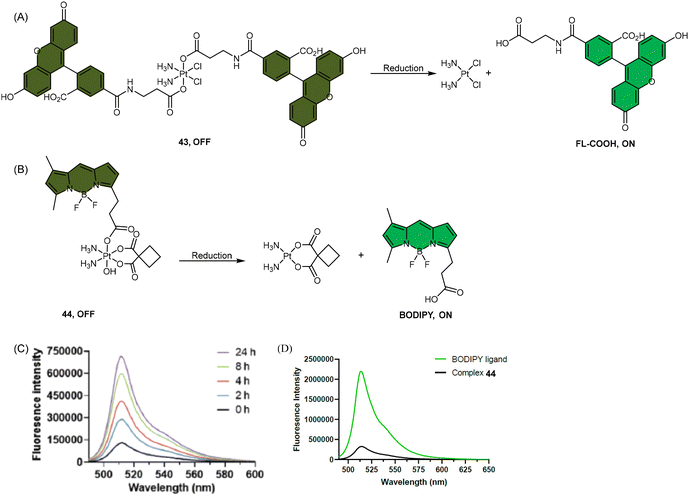 | ||
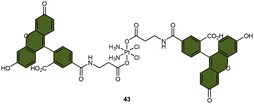
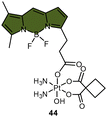
| Scheme 9 The Pt(IV) complexes with axial (A) fluorescein ligands (43) and (B) BODIPY ligands (44) exhibit fluorescence ‘turn-on’ following the reduction of Pt(IV) complexes and release of the axial fluorophore. (C) The time-dependent emission of 10 μM complex 44 in PBS buffer (pH 7.4) with 1 mM sodium ascorbate and 1% DMF at 37 °C. (D) Emission of 10 μM complex 44 and the BODIPY ligand in PBS buffer (pH 7.4) with 1% DMF. The excitation wavelength is 470 nm. Adapted with permission from ref. 83. Copyright 2022, the Royal Society of Chemistry. | ||
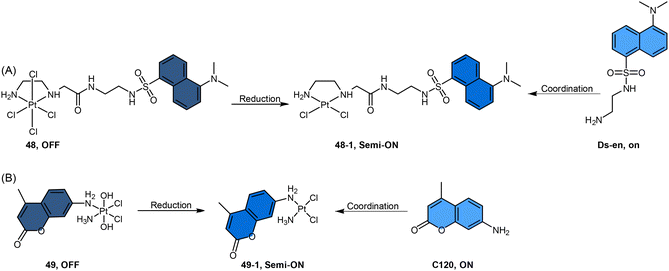
Recently, our group developed a fluorescent sensor 44 by attaching a BODIPY fluorophore to the axial position of a carboplatin Pt(IV) analogue. This sensor allows for real-time monitoring of the reduction of Pt(IV) complex (Scheme 9B).83 Upon incubation of the sensor with sodium ascorbate and cell extracts, a fluorescence turn-on effect was observed (Scheme 9C). This turn-on effect resulted from the reduction of 44 and thus, the liberation of the BODIPY ligand, whose fluorescence was previously suppressed when bound to platinum (Scheme 9D). Further investigation revealed that the fluorescence turn-on was predominantly caused by proteins with high molecular weights, especially those between 10 and 100 kDa. This finding confirms that high-molecular-weight proteins contributed significantly to the reduction of Pt(IV) complexes. Although the sensor is highly effective in monitoring the reduction of Pt(IV) prodrugs in a real-time mode, it could only be applied to cell lysates due to the rapid secretion of the complex from live cells.
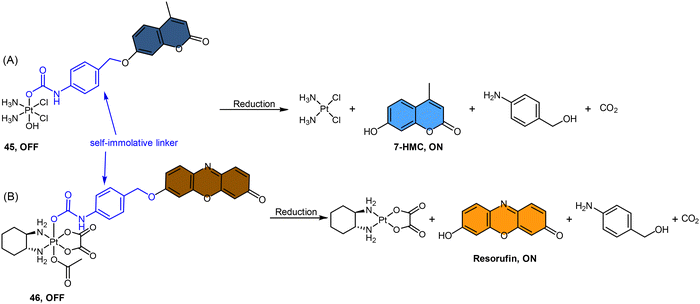
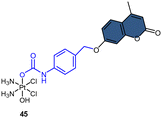
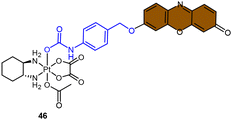
Fluorophores containing carboxylates have been commonly used in Pt(IV) sensors, likely due to the ease of carboxylation during the synthesis of Pt(IV) complexes and the relatively high stability of carboxylate ligands. Expanding the range of fluorophores beyond carboxylates has posed a challenge. Ang and coworkers developed an innovative method to obtain Pt(IV) prodrugs, in which they employed self-immolative 4-aminobenzyl linkers to conjugate a Pt(IV) complex with the OH of fluorophore 7-HMC, thus creating a stable carbamate bridge on the Pt(IV) scaffold 45 (Scheme 10A).84 Upon reduction of the self-immolative Pt(IV) prodrug 45, the carbamate ligand detached and underwent decarboxylation and 1,6-elimination processes, resulting in the generation of 4-aminobenzyl alcohol and carbon dioxide. This process also led to the release of 7-HMC and the concomitant restoration of fluorescence emission from the fluorophore. Upon the addition of sodium ascorbate to complex 45, a time-dependent enhancement in fluorescence emission at 450 nm was observed. Complex 45 underwent complete reduction within 18 hours, displaying a maximum fluorescence intensity at this time point comparable to the equimolar concentration of 7-HMC. Hence, the demonstration of complex 45 establishes the effectiveness of Pt(IV) prodrug and self-immolation strategies in achieving efficient co-delivery of Pt(II) and a bioactive ligand within cells through intracellular reduction. Sedgwick's group utilized a similar method to incorporate the fluorophore resorufin into a Pt(IV) complex, and a fluorescence probe 46 was obtained (Scheme 10B).156 This carbamate-functionalized probe displayed sodium ascorbate-dependent increases in fluorescence emission intensities. Complex 46 showed potential as a hypoxia-activated prodrug. Under conditions of <0.1% O2 (hypoxia), the probe displayed the highest fluorescence signal compared to the normoxic condition (21% O2), indicating that hypoxia was more efficient in inducing the bioreduction of Pt(IV) compounds to Pt(II) species compared to normoxia. Additionally, clonogenic cell survival assays revealed that the complex 46 was significantly more toxic in hypoxic conditions than in normoxic conditions. This hypoxia-activated prodrug contributes to elucidating the factors that affect intracellular reduction of Pt(IV) complexes.
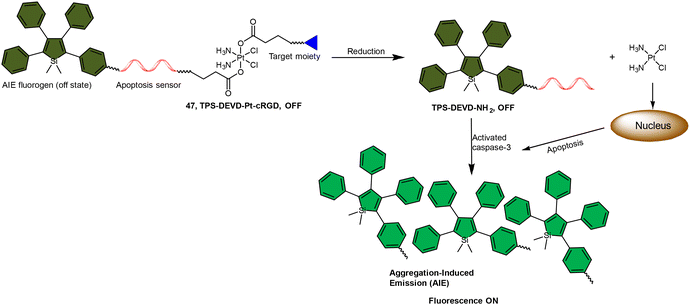
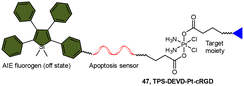
The fluorescence turn-on response resulting from the liberation of fluorophores has been utilized to monitor the reduction of Pt(IV) complexes, and its application can be expanded to indicate the activation of specific pathways in cells, such as apoptosis. Liu and coworkers reported a chemotherapeutic Pt(IV) prodrug 47 in which one axial position was functionalized with an apoptosis sensor composing a tetraphenylsilole (TPS) fluorophore with aggregation-induced emission (AIE) characteristics and a caspase-3-specific Asp-Glu-Val-Asp (DEVD) peptide (Scheme 11).157 This design allowed prodrug 47 to be reduced to an active Pt(II) drug within cells while simultaneously releasing the apoptosis sensor TPS-DEVD. The reduced Pt(II) drug induced apoptosis in cancer cells and activated caspase-3. The activated caspase-3 subsequently cleaved the DEVD sequence of the apoptosis sensor and triggered the AIE effect of TPS residue, ultimately leading to fluorescence enhancement. This research presents a fluorescent probe capable of tracking the reduction of Pt(IV) complexes and evaluating the therapeutic responses.
In another study by Hambley et al., coumarin dyes were incorporated into the non-leaving position of Pt(IV) complexes (Scheme 12B).152 Coordination of coumarin 120 (C120) at the non-leaving position of a Pt(II) drug afforded c-[PtCl2(C120)(NH3)] (complex 49-1), which resulted in partial quenching of fluorescence, with a 2.5-fold decrease observed. Oxidation of complex 49-1 to its Pt(IV) form (c,t,c-[PtCl2(OH)2(C120)(NH3)], complex 49) resulted in a further 7-fold decrease in fluorescence. When complex 49 was treated with the cellular reductant ascorbate for over 2 days, it underwent reduction to form complex 49-1. During this reduction process, a slight but steady increase in fluorescence was observed. The observed slow increment in fluorescence indicates a sluggish reduction process of the Pt(IV) complex 49, implying its resistance to reduction in the medium prior to cellular uptake.
These coordination-sensitive fluorescent probes can serve as valuable tools for studying the cellular metabolism of platinum complexes. These probes take advantage of the fact that free fluorophores show robust fluorescence, which can be partially quenched when conjugated in the non-leaving-group position of Pt(II) complexes and be further quenched upon oxidation to Pt(IV) complexes. In this way, the regeneration of fluorescence would reflect that the reduction of Pt(IV) complexes had occurred. The fluorophores exhibited remarkable stability when conjugated to the non-leaving positions of Pt drugs. Any potential loss of these fluorophores in the reduction environments was negligible in comparison to the reduction products. Hence, this strategy can be employed specifically to monitor the reduction of the Pt(IV) complex. The changes in fluorescence induced by changes in oxidation state can be utilized to obtain information about the oxidation or coordination state of platinum complexes.
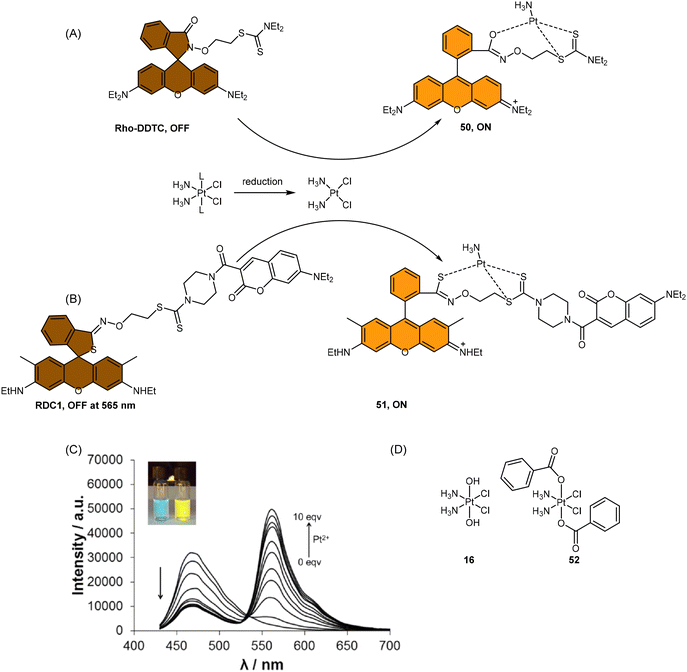 | ||
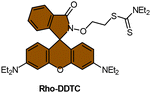
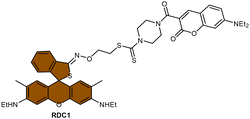
Scheme 13 (A) Proposed activation pathway of Rho-DDTC by Pt(II) complexes in the absence of aquation. (B) The FRET probe for the ratiometric sensing of cisplatin. (C) Fluorescence titration spectra of RDC1 (20 μM) in response to Pt2+ (0–10 eq.) in CH3CN/HEPES buffer (v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5 mM, pH 7.4) (λex = 400 nm). Inset: Change in fluorescence under irradiation by 365 nm UV lamp. Adapted with permission from ref. 85. Copyright 2018, Wiley Online Library. (D) The chemical structures of complexes 16 and 52. 3, 5 mM, pH 7.4) (λex = 400 nm). Inset: Change in fluorescence under irradiation by 365 nm UV lamp. Adapted with permission from ref. 85. Copyright 2018, Wiley Online Library. (D) The chemical structures of complexes 16 and 52. | ||
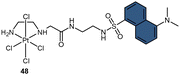
To address the aforementioned limitations and gain a deeper understanding of how Pt(IV) drugs are processed at the cellular level, Ang et al. developed a ratiometric probe RDC1 to detect cisplatin quantitively. This probe was utilized to investigate the fate of Pt(IV) prodrugs in a cellular environment (Scheme 13B).85 In the absence of Pt(II) compounds, the maximum emission of RDC1 was observed at a wavelength of 470 nm; upon interaction with Pt(II) species, the fluorescence intensity at 470 nm decreased while concomitant increased at 565 nm (Scheme 13C). The ratio of emission intensities (I565/I470) increased from 0.07 to 4.8 in the presence of Pt(II) compound, indicating a 68-fold enhancement. Hence, the ratio of fluorescence intensities (I565/I470), referred to as Rred (reduction ratio), is a reliable measurement for quantifying the reduced Pt(IV) complex. The ratiometric probe demonstrated a notably higher Rred value of 0.54 for complex 52, in contrast to 0.14 for complex 16 (Scheme 13D), showing that the ratiometric probe could quantitively determine the amount of Pt(IV) complex reduced. Furthermore, to gain deeper insights into the intracellular activation of Pt(IV) prodrugs, the authors conducted a study to examine the role of the cellular reductant GSH in the activation process utilizing the ratiometric probe. The results revealed that the reduction of complex 52 in GSH-depleted cells was similar to that in GSH-normal cells, suggesting that GSH may not be the dominant cellular reductant for Pt(IV) complexes. Although the ratiometric probe RDC1 has proven to be a reliable means for quantitatively detecting cisplatin and exploring the role of GSH in reducing Pt(IV) prodrugs in a cellular context, its usage necessitates the fixation of cells with paraformaldehyde, probably due to the probe's slow reaction kinetics, which prevents the real-time monitoring the activation of Pt(IV) prodrugs in live cells.
In general, fluorescence spectroscopy is a widely employed technique to monitor the activation of Pt(IV) complexes by observing the subsequent fluorescence turn-on during reduction reactions (Table 3). The high sensitivity of fluorescence spectroscopy enables researchers to track the reduction of Pt(IV) complexes, even at minimal concentrations. Furthermore, this technique provides valuable spatial information by enabling the observation of the uptake, distribution, and localization of Pt(IV) complexes, as well as their reduction process within cells or specific regions of interest. Fluorescent Pt(IV) probes that can enter cells have been particularly useful for real-time monitoring of their distribution and dynamic processes using fluorescence spectroscopy. However, accurately quantifying the amount of Pt(IV) complex reduced has proven challenging. While some probes can provide quantitative measurements of reduced Pt(IV) complexes, their efficacy is limited to fixed cells or cell lysate and not applicable to live cells. Therefore, there is a demand for fluorescent Pt(IV) probes that can enter live cells and allow investigation of their cellular processing. Furthermore, the challenges associated with fluorescence spectroscopy pose an additional obstacle to accurately quantifying the activation of Pt(IV) complexes within cells. The fluorophores used to monitor the activation of the Pt(IV) complex by fluorescence spectroscopy may undergo photobleaching, which leads to a decrease in fluorescence signal over time, limiting the duration of imaging experiments and affecting the accuracy of long-term observations.159 Moreover, noninvasive fluorescence imaging requires excitation light, and continuous exposure to such excitation light, especially at short wavelengths, may induce cellular damage and interfere with biological processes.
| Fluorescent probes | Advantages | Disadvantages | Applications |
|---|---|---|---|
|
Monitoring cellular uptake and conversion of Pt(IV) to Pt(II) | Not able to quantify the exact amount of Pt(IV) complexes reduced | Imaging localization and reduction of Pt(IV) complex 43 in HeLa cells114 |
|
Monitoring the reduction of Pt(IV) prodrugs in a real-time mode | Only works well in buffer solution and cell lysate | Proteins with high molecular weights contribute more to the reduction of the Pt(IV) complex in cell lysates83 |
|
Propose a novel method to tether fluorophore to Pt(IV) complexes | Only investigated in a buffer solution | Tether fluorophore without carboxylate to Pt(IV) complexes84 |
|
Monitoring the reduction of Pt(IV) complexes in hypoxia conditions | Fixed cell | Be utilized as a potential hypoxia-activated prodrug156 |
|
Reflecting both the reduction of Pt(IV) complexes and the activated pathway | The fluorescence enhancement could not reflect the amount of Pt(IV) complexes reduced | Used as an indicator for early evaluation of the therapeutic responses157 |
|
Monitoring the oxidation of the platinum center | Only investigated in a buffer solution | Providing new directions for imaging the reduction of Pt(IV) complexes158 |
|
Monitoring the reduction | The distinction between the Pt(II) and Pt(IV) states is unclear | The probe could give information about coordination or oxidation state152 |
|
Be able to distinguish Pt(II) and Pt(IV) complexes | (1) The fluorescence of the probe is affected by various factors | Imaging of cisplatin and several Pt(IV) prodrugs in HeLa cells86 |
| (2) Fixed cell only | |||
|
Be able to distinguish cisplatin from its Pt(IV) counterparts | Fixed cell only | The probe could figure out that GSH is not the dominant cellular reductant of Pt(IV) complexes85 |
4. Conclusions and perspectives
The reduction of Pt(IV) complexes could be monitored by various techniques (Table 4), each technique with its own advantages and limitations. UV spectroscopy is a convenient and easily accessible technique for monitoring Pt(IV) reduction processes and is comparatively more straightforward than other analytical techniques. However, it is not capable of identifying the specific reduction products and is not suitable for monitoring Pt(IV) reduction in live cells where there is a high background. HPLC is a reliable technique for analyzing and quantifying the reduction of Pt(IV) complexes, as well as the generation of Pt(II) species and the released ligands. The identity of these reduction products can be determined by their characteristic retention time; their identity can also be verified through electrospray ionization (ESI) mass and liquid chromatography-mass spectrometry (LC-MS) techniques.62,84,114–116 HPLC is also valuable for time-resolved analysis, allowing for investigating reaction kinetics and tracking the reduction progress over time. However, HPLC does not provide real-time monitoring and requires batch-wise analysis, which may not capture rapid changes or transient species during the reduction process. XANES is a powerful technique for detecting variations in the oxidation state of the platinum center, and it can provide quantitative information about the relative concentrations of different Pt states present in the cells. However, sample preparation for XANES analysis is complicated, time-consuming, and technically demanding. It is not suitable for real-time or live-cell monitoring of Pt(IV) prodrug reduction. Moreover, access to synchrotron radiation facilities is required for high-energy X-ray measurements using XANES, which may pose logistic challenges. NMR spectroscopy is an indispensable analytical tool in examining the activation of Pt(IV) complexes, providing detailed information about the oxidation state and coordination environment of Pt, and facilitating the identification and characterization of reduction products. Furthermore, it is a non-destructive technique, allowing for the recovery of samples for further analysis. However, NMR spectroscopy may face challenges related to low sensitivity, especially with low concentrations of Pt species or rapid reaction kinetics. In such cases, longer acquisition times or higher concentrations of the Pt(IV) complex may be required, respectively, to achieve adequate sensitivity and high signal-to-noise ratios. Fluorescence spectroscopy provides high resolution to identify the uptake, colocalization, and reduction of Pt(IV) complex within cells. Nevertheless, the use of fluorophores in fluorescence spectroscopy for monitoring the activation of the Pt(IV) complex can be limited by photobleaching, which may compromise the accuracy of long-term observations. Furthermore, noninvasive fluorescence imaging necessitates excitation light, and prolonged exposure to high-intensity light carries the inherent risk of inducing phototoxicity in cellular systems.159–161| Detection techniques | Advantages | Disadvantages | Applications |
|---|---|---|---|
| UV-Vis spectroscopy | The method is sensitive and easy to perform | This approach does not provide any information on the identity of the reduction products | It can be applied to monitor the reduction of Pt(IV) without other chromophores that absorb in the same region |
| Due to the high background in cells, it cannot be used to monitor the reduction of the Pt(IV) complexes in live cells | |||
| HPLC | This method could monitor the reduction of the Pt(IV) complex and the generation of Pt(II) species and the released ligands, whose identity could also be identified by retention time | HPLC requires solution-based samples, making it unsuitable for monitoring the reduction of Pt(IV) complex in live cells | HPLC analysis is the current gold standard for evaluating Pt(IV) reduction |
| XANES | The method could monitor the oxidation change of the platinum center | Requiring sophisticated sample preparation, including collecting and freezing cells, this technique fails to monitor the reduction of Pt(IV) prodrugs neither in live cells nor in a real-time mode | The reduction of Pt(IV) complexes in the biological environment could be observed by measuring the relative proportions of two different oxidation states of both Pt(II) and Pt(IV) |
| NMR spectroscopy | Providing sufficient information about the oxidation state and coordination environment of platinum, as well as reduction products | High concentration demanded; low sensitivity | Different types of NMR were used for the situations and research purposes to efficiently monitor the reduction of the Pt(IV) complex |
| Fluorescence spectroscopy | Providing good resolution to identify the uptake, colocalization, and reduction of Pt(IV) complex | Noninvasive fluorescence imaging needs excitation light, which can lead to tissue auto-fluorescence and cell damage | Fluorescence spectroscopy has been widely used to study the reduction of Pt(IV) complexes in cells |
| The fluorescence can be vigorously quenched by tissue components and influenced by various factors, leading to a high background |
Generally, the strategies applied in buffer systems, such as UV, HPLC, and NMR, provide sufficient information about the time scale of the reduction event and the identities of the reduction products. To evaluate the reduction of a novel Pt(IV) complex, HPLC analysis usually serves as the gold standard if the complex can be detected by the HPLC instrument and exhibits a distinguishable retention time on the column. The highly sensitive nature of HPLC allows for the measurement of the reduction rate of and identification of reduction products using only trace amounts of the complex (in μM). In cases where the Pt(IV) complex's retention time overlaps with the dead volume on the HPLC column, but distinct absorption bands are still observed in the UV-Vis spectrum, UV-Vis spectroscopy can be employed to monitor the reduction process. If a more in-depth analysis of the reduction of Pt(IV) complexes at the cellular level is required, researchers can utilize techniques such as XANES, 19F NMR, and fluorescence spectroscopy. These methods provide valuable insights into the role of biological reductants and ligands located at axial or equatorial positions in the reduction process.
In conclusion, Pt(IV) prodrugs have emerged as highly promising candidates as next-generation anticancer drugs. The effectiveness of these prodrugs in combating cancers heavily depends on their reduction profile, both in buffer solutions and, more importantly, within cells. Tracking the reduction process of various Pt(IV) complexes using different techniques can help anticipate and even improve the anticancer efficiency of these complexes. This review serves as a comprehensive resource for researchers aiming to stay updated on the latest advancements in strategies used to monitor the reduction, learn from exemplary research, and contribute to the mechanistic understanding of metal-based drugs in cellular and even in vivo contexts. Since current techniques have some limitations, it is necessary to develop novel strategies capable of real-time monitoring of Pt(IV) reduction in live cells and uncover the mechanisms and timing of the reduction process, including the exact roles of reducing agents. These advancements hold great potential in elucidating the intrinsic mechanisms involved in Pt(IV) prodrug activation in live cells and in vivo, thus providing invaluable insights for the rational design of novel Pt(IV) prodrugs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (Grant No. 22077108 and 22277103), the Hong Kong Research Grants Council (Grant No. CityU 11303320, 11302221, 11313222, and 11304923), the Science Technology and Innovation Committee of Shenzhen Municipality (JCYJ20210324120004011), and the City University of Hong Kong (Grant No. 7020014) for funding support.References
- R. A. Alderden, M. D. Hall and T. W. Hambley, The discovery and development of cisplatin, J. Chem. Educ., 2006, 83, 728 CrossRef CAS.
- S. Ghosh, Cisplatin: the first metal based anticancer drug, Bioorg. Chem., 2019, 88, 102925 CrossRef CAS PubMed.
- J. J. Wilson and S. J. Lippard, Synthetic methods for the preparation of platinum anticancer complexes, Chem. Rev., 2014, 114, 4470–4495 CrossRef CAS PubMed.
- B. Rosenberg, L. Van Camp and T. Krigas, Inhibition of cell division in Escherichia coli, by electrolysis products from a platinum electrode, Nature, 1965, 205, 698–699 CrossRef CAS PubMed.
- S. Dasari and P. B. Tchounwou, Cisplatin in cancer therapy: molecular mechanisms of action, Eur. J. Pharmacol., 2014, 740, 364–378 CrossRef CAS PubMed.
- I. Kostova, Platinum complexes as anticancer agents, Recent Pat. Anticancer Drug Discovery, 2006, 1, 1–22 CrossRef CAS PubMed.
- W. J. van der Vijgh, Clinical pharmacokinetics of carboplatin, Clin. Pharmacokinet., 1991, 21, 242–261 CrossRef CAS PubMed.
- H. Calvert, The clinical development of carboplatin – A personal perspective, Inorg. Chim. Acta, 2019, 498, 118987 CrossRef CAS.
- A. M. Di Francesco, A. Ruggiero and R. Riccardi, Cellular and molecular aspects of drugs of the future: oxaliplatin, Cell. Mol. Life Sci., 2002, 59, 1914–1927 CrossRef CAS PubMed.
- E. Raymond, S. G. Chaney, A. Taamma and E. Cvitkovic, Oxaliplatin: a review of preclinical and clinical studies, Ann. Oncol., 1998, 9, 1053–1071 CrossRef CAS PubMed.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- L. Kelland, The resurgence of platinum-based cancer chemotherapy, Nat. Rev. Cancer, 2007, 7, 573–584 CrossRef CAS PubMed.
- N. J. Wheate, S. Walker, G. E. Craig and R. Oun, The status of platinum anticancer drugs in the clinic and in clinical trials, Dalton Trans., 2010, 39, 8113–8127 RSC.
- E. R. Jamieson and S. J. Lippard, Structure, recognition, and processing of cisplatin–DNA adducts, Chem. Rev., 1999, 99, 2467–2498 CrossRef CAS PubMed.
- J. Reedijk, Why does cisplatin reach guanine-N7 with competing S-donor ligands available in the cell?, Chem. Rev., 1999, 99, 2499–2510 CrossRef CAS PubMed.
- B. W. Harper, A. M. Krause-Heuer, M. P. Grant, M. Manohar, K. B. Garbutcheon-Singh and J. R. Aldrich-Wright, Advances in platinum chemotherapeutics, Chem. – Eur. J., 2010, 16, 7064–7077 CrossRef CAS PubMed.
- R. Oun, Y. E. Moussa and N. J. Wheate, The side effects of platinum-based chemotherapy drugs: a review for chemists, Dalton Trans., 2018, 47, 6645–6653 RSC.
- J. T. Hartmann and H.-P. Lipp, Toxicity of platinum compounds, Expert Opin. Pharmacother., 2003, 4, 889–901 CrossRef CAS PubMed.
- Z. H. Siddik, Cisplatin: mode of cytotoxic action and molecular basis of resistance, Oncogene, 2003, 22, 7265–7279 CrossRef CAS PubMed.
- A.-M. Florea and D. Büsselberg, Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects, Cancers, 2011, 3, 1351–1371 CrossRef CAS PubMed.
- J. J. Wilson and S. J. Lippard, Synthesis, characterization, and cytotoxicity of platinum(IV) carbamate complexes, Inorg. Chem., 2011, 50, 3103–3115 CrossRef CAS PubMed.
- E. Wexselblatt and D. Gibson, What do we know about the reduction of Pt(IV) pro-drugs?, J. Inorg. Biochem., 2012, 117, 220–229 CrossRef CAS PubMed.
- C. K. J. Chen and T. W. Hambley, The impact of highly electron withdrawing carboxylato ligands on the stability and activity of platinum(IV) pro-drugs, Inorg. Chim. Acta, 2019, 494, 84–90 CrossRef CAS.
- S. Chen, H. Yao, Q. Zhou, M.-K. Tse, Y. F. Gunawan and G. Zhu, Stability, reduction, and cytotoxicity of platinum(IV) anticancer prodrugs bearing carbamate axial ligands: comparison with their carboxylate analogues, Inorg. Chem., 2020, 59, 11676–11687 CrossRef CAS PubMed.
- Z. Li, X.-J. Ding, X. Qiao, X.-M. Liu, X. Qiao, C.-Z. Xie, R.-P. Liu and J.-Y. Xu, Thalidomide-based Pt(IV) prodrugs designed to exert synergistic effect of immunomodulation and chemotherapy, J. Inorg. Biochem., 2022, 232, 111842 CrossRef CAS PubMed.
- X.-M. Liu, Z. Li, X.-R. He, R.-P. Liu, Z.-Y. Ma, X. Qiao, S.-Q. Wang and J.-Y. Xu, Dual-targeting of the aromatase binding domain of heme and androstenedione by Pt(IV) prodrugs: a new treatment for postmenopausal breast cancer, Inorg. Chem. Front., 2022, 9, 3470–3483 RSC.
- S. Dhar, Z. Liu, J. Thomale, H. Dai and S. J. Lippard, Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device, J. Am. Chem. Soc., 2008, 130, 11467–11476 CrossRef CAS PubMed.
- L. Ma, R. Ma, Y. Wang, X. Zhu, J. Zhang, H. C. Chan, X. Chen, W. Zhang, S.-K. Chiu and G. Zhu, Chalcoplatin, a dual-targeting and p53 activator-containing anticancer platinum(IV) prodrug with unique mode of action, Chem. Commun., 2015, 51, 6301–6304 RSC.
- N. Muhammad, N. Sadia, C. Zhu, C. Luo, Z. Guo and X. Wang, Biotin-tagged platinum(IV) complexes as targeted cytostatic agents against breast cancer cells, Chem. Commun., 2017, 53, 9971–9974 RSC.
- M. Srinivasarao and P. S. Low, Ligand-targeted drug delivery, Chem. Rev., 2017, 117, 12133–12164 CrossRef CAS PubMed.
- S. Dhar and S. J. Lippard, Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 22199–22204 CrossRef CAS PubMed.
- D. Gibson, Platinum(IV) anticancer agents; are we en route to the holy grail or to a dead end?, J. Inorg. Biochem., 2021, 217, 111353 CrossRef CAS PubMed.
- W. H. Ang, I. Khalaila, C. S. Allardyce, L. Juillerat-Jeanneret and P. J. Dyson, Rational design of platinum(IV) compounds to overcome glutathione-S-transferase mediated drug resistance, J. Am. Chem. Soc., 2005, 127, 1382–1383 CrossRef CAS PubMed.
- Q. Chen, Y. Yang, X. Lin, W. Ma, G. Chen, W. Li, X. Wang and Z. Yu, Platinum(IV) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance, Chem. Commun., 2018, 54, 5369–5372 RSC.
- Z. Xu, Z. Wang, Z. Deng and G. Zhu, Recent advances in the synthesis, stability, and activation of platinum(IV) anticancer prodrugs, Coord. Chem. Rev., 2021, 442, 213991 CrossRef CAS.
- M. D. Hall and T. W. Hambley, Platinum(IV) antitumour compounds: their bioinorganic chemistry, Coord. Chem. Rev., 2002, 232, 49–67 CrossRef CAS.
- A. Nemirovski, I. Vinograd, K. Takrouri, A. Mijovilovich, A. Rompel and D. Gibson, New reduction pathways for ctc-[PtCl2(CH3CO2)2(NH3)(Am)] anticancer prodrugs, Chem. Commun., 2010, 46, 1842–1844 RSC.
- Q. Zhou, S. Chen, Z. Xu, G. Liu, S. Zhang, Z. Wang, M. K. Tse, S. M. Yiu and G. Zhu, Multitargeted platinum(IV) anticancer complexes bearing pyridinyl ligands as axial leaving groups, Angew. Chem., Int. Ed., 2023, 62, e202302156 CrossRef CAS PubMed.
- T. Yempala, T. Babu, S. Karmakar, A. Nemirovski, M. Ishan, V. Gandin and D. Gibson, Expanding the arsenal of PtIV anticancer agents: multi–action PtIV anticancer agents with bioactive ligands possessing a hydroxy functional group, Angew. Chem., Int. Ed., 2019, 58, 18218–18223 CrossRef CAS PubMed.
- S. Chen, K.-Y. Ng, Q. Zhou, H. Yao, Z. Deng, M.-K. Tse and G. Zhu, The influence of different carbonate ligands on the hydrolytic stability and reduction of platinum(IV) prodrugs, Dalton Trans., 2022, 51, 885–897 RSC.
- S. Choi, C. Filotto, M. Bisanzo, S. Delaney, D. Lagasee, J. L. Whitworth, A. Jusko, C. Li, N. A. Wood, J. Willingham, A. Schwenker and K. Spaulding, Reduction and anticancer activity of platinum(IV) complexes, Inorg. Chem., 1998, 37, 2500–2504 CrossRef CAS.
- C. K. J. Chen, P. Kappen and T. W. Hambley, The reduction of cis-platinum(IV) complexes by ascorbate and in whole human blood models using 1H NMR and XANES spectroscopy, Metallomics, 2019, 11, 686–695 CrossRef CAS PubMed.
- Y. Liu, H. Tian, L. Xu, L. Zhou, J. Wang, B. Xu, C. Liu, L. I. Elding and T. Shi, Investigations of the kinetics and mechanism of reduction of a carboplatin Pt(IV) prodrug by the major small-molecule reductants in human plasma, Int. J. Mol. Sci., 2019, 20, 5660 CrossRef CAS PubMed.
- J. Dong, Y. Ren, S. Huo, S. Shen, J. Xu, H. Tian and T. Shi, Reduction of ormaplatin and cis-diamminetetrachloroplatinum(IV) by ascorbic acid and dominant thiols in human plasma: kinetic and mechanistic analyses, Dalton Trans., 2016, 45, 11326–11337 RSC.
- K. Lemma, J. Berglund, N. Farrell and L. I. Elding, Kinetics and mechanism for reduction of anticancer-active tetrachloroam(m)ine platinum(IV) compounds by glutathione, J. Biol. Inorg. Chem., 2000, 5, 300–306 CrossRef CAS PubMed.
- V. Pichler, P. Heffeter, S. M. Valiahdi, C. R. Kowol, A. Egger, W. Berger, M. A. Jakupec, M. S. Galanski and B. K. Keppler, Unsymmetric mono- and dinuclear platinum(IV) complexes featuring an ethylene glycol moiety: synthesis, characterization, and biological activity, J. Med. Chem., 2012, 55, 11052–11061 CrossRef CAS PubMed.
- V. Pichler, S. Göschl, E. Schreiber-Brynzak, M. A. Jakupec, M. S. Galanski and B. K. Keppler, Influence of reducing agents on the cytotoxic activity of platinum(IV) complexes: induction of G2/M arrest, apoptosis and oxidative stress in A2780 and cisplatin resistant A2780cis cell lines, Metallomics, 2015, 7, 1078–1090 CrossRef CAS PubMed.
- T. J. O'Rourke, G. R. Weiss, P. New, H. A. Burris III, G. Rodriguez, J. Eckhardt, J. Hardy, J. G. Kuhn, S. Fields, G. M. Clark and D. D. Von Hoff, Phase I clinical trial of ormaplatin (tetraplatin, NSC 363812), Anti-Cancer Drugs, 1994, 5, 520–526 CrossRef PubMed.
- Y. Shi, S.-A. Liu, D. J. Kerwood, J. Goodisman and J. C. Dabrowiak, Pt(IV) complexes as prodrugs for cisplatin, J. Inorg. Biochem., 2012, 107, 6–14 CrossRef CAS PubMed.
- L. Pendyala, J. W. Cowens, G. B. Chheda, S. P. Dutta and P. J. Creaven, Identification of cis-dichloro-bis-isopropylamine platinum(II) as a major metabolite of iproplatin in humans, Cancer Res., 1988, 48, 3533–3536 CAS.
- V. H. Bramwell, D. Crowther, S. O'Malley, R. Swindell, R. Johnson, E. H. Cooper, N. Thatcher and A. Howell, Activity of JM9 in advanced ovarian cancer: a phase I-II trial, Cancer Treat. Rep., 1985, 69, 409–416 CAS.
- L. R. Kelland, An update on satraplatin: the first orally available platinum anticancer drug, Expert Opin. Invest. Drugs, 2000, 9, 1373–1382 CrossRef CAS PubMed.
- N. Graf and S. J. Lippard, Redox activation of metal-based prodrugs as a strategy for drug delivery, Adv. Drug Delivery Rev., 2012, 64, 993–1004 CrossRef CAS PubMed.
- M. D. Hall, H. R. Mellor, R. Callaghan and T. W. Hambley, Basis for design and development of platinum(IV) anticancer complexes, J. Med. Chem., 2007, 50, 3403–3411 CrossRef CAS PubMed.
- P. Sova, A. Mistr, A. Kroutil, F. Zak, P. Pouckova and M. Zadinova, Preclinical anti-tumor activity of a new oral platinum(IV) drug LA-12, Anti-Cancer Drugs, 2005, 16, 653–657 CrossRef CAS PubMed.
- V. Kvardova, R. Hrstka, D. Walerych, P. Muller, E. Matoulkova, V. Hruskova, D. Stelclova, P. Sova and B. Vojtesek, The new platinum(IV) derivative LA-12 shows stronger inhibitory effect on Hsp90 function compared to cisplatin, Mol. Cancer, 2010, 9, 1–9 CrossRef PubMed.
- D. Gibson, Platinum(IV) anticancer prodrugs – hypotheses and facts, Dalton Trans., 2016, 45, 12983–12991 RSC.
- J. R. Gispert, Coordination chemistry, Wiley-VCH Weinheim, 2008 Search PubMed.
- G. L. Miessler, Inorganic chemistry, Pearson Education India, 2008 Search PubMed.
- T. C. Johnstone, S. M. Alexander, J. J. Wilson and S. J. Lippard, Oxidative halogenation of cisplatin and carboplatin: synthesis, spectroscopy, and crystal and molecular structures of Pt(IV) prodrugs, Dalton Trans., 2015, 44, 119–129 RSC.
- X. Hu, F. Li, N. Noor and D. Ling, Platinum drugs: from Pt(II) compounds, Pt(IV) prodrugs, to Pt nanocrystals/nanoclusters, Sci. Bull., 2017, 62, 589–596 CrossRef CAS PubMed.
- H. Shi, C. Imberti and P. J. Sadler, Diazido platinum(IV) complexes for photoactivated anticancer chemotherapy, Inorg. Chem. Front., 2019, 6, 1623–1638 RSC.
- F. S. Mackay, J. A. Woods, H. Moseley, J. Ferguson, A. Dawson, S. Parsons and P. J. Sadler, A photoactivated trans–diammine platinum complex as cytotoxic as cisplatin, Chem. – Eur. J., 2006, 12, 3155–3161 CrossRef CAS PubMed.
- N. J. Farrer, J. A. Woods, L. Salassa, Y. Zhao, K. S. Robinson, G. J. Clarkson, F. S. Mackay and P. J. Sadler, A potent trans-diimine platinum anticancer complex photoactivated by visible light, Angew. Chem., Int. Ed., 2010, 49, 8905–8908 CrossRef CAS PubMed.
- H.-C. Tai, Y. Zhao, N. J. Farrer, A. E. Anastasi, G. Clarkson, P. J. Sadler and R. J. Deeth, A computational approach to tuning the photochemistry of platinum(IV) anticancer agents, Chem. – Eur. J., 2012, 18, 10630–10642 CrossRef CAS PubMed.
- J. A. Platts, S. P. Oldfield, M. M. Reif, A. Palmucci, E. Gabano and D. Osella, The RP-HPLC measurement and QSPR analysis of logPo/w values of several Pt(II) complexes, J. Inorg. Biochem., 2006, 100, 1199–1207 CrossRef CAS PubMed.
- S. D. Kumar and D. R. H. Kumar, Importance of RP-HPLC in analytical method development: a review, Int. J. Pharma Sci. Res., 2012, 3, 4626–4633 Search PubMed.
- S. Jovanović, B. Petrović and Ž. D. Bugarčić, UV-Vis, HPLC, and 1H-NMR studies of the substitution reactions of some Pt(IV) complexes with 5′-GMP and L-histidine, J. Coord. Chem., 2010, 63, 2419–2430 CrossRef.
- T. Niu, W. Wan, X. Li, D. Su, S. Huo and S. Shen, Reduction of platinum(IV) prodrug model complex trans-[PtCl2(CN)4]2− by a peptide containing cysteine and methionine groups: HPLC and MS studies, J. Mol. Liq., 2018, 252, 24–29 CrossRef CAS.
- M. D. Hall, H. L. Daly, J. Z. Zhang, M. Zhang, R. A. Alderden, D. Pursche, G. J. Foran and T. W. Hambley, Quantitative measurement of the reduction of platinum(IV) complexes using X-ray absorption near-edge spectroscopy (XANES), Metallomics, 2012, 4, 568–575 CrossRef CAS PubMed.
- M. D. Hall, G. J. Foran, M. Zhang, P. J. Beale and T. W. Hambley, XANES determination of the platinum oxidation state distribution in cancer cells treated with platinum(IV) anticancer agents, J. Am. Chem. Soc., 2003, 125, 7524–7525 CrossRef CAS PubMed.
- F. W. Lytle, Determination of d-band occupancy in pure metals and supported catalysts by measurement of the LIII X-ray absorption threshold, J. Catal., 1976, 43, 376–379 CrossRef CAS.
- B. Shelimov, J.-F. Lambert, M. Che and B. Didillon, Application of NMR to interfacial coordination chemistry: a 195Pt NMR study of the interaction of hexachloroplatinic acid aqueous solutions with alumina, J. Am. Chem. Soc., 1999, 121, 545–556 CrossRef CAS.
- P. S. Pregosin, M. Kretschmer, W. Preetz and G. Rimkus, 195Pt NMR studies on stereoisomeric chloro bromo platinates(IV), Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1982, 37, 1422–1424 CrossRef.
- B. M. Still, P. G. A. Kumar, J. R. Aldrich-Wright and W. S. Price, 195Pt NMR—Theory and application, Chem. Soc. Rev., 2007, 36, 665–686 RSC.
- J. R. L. Priqueler, I. S. Butler and F. D. Rochon, An overview of 195Pt nuclear magnetic resonance spectroscopy, Appl. Spectrosc. Rev., 2006, 41, 185–226 CrossRef CAS.
- J. Z. Zhang, E. Wexselblatt, T. W. Hambley and D. Gibson, Pt(IV) analogs of oxaliplatin that do not follow the expected correlation between electrochemical reduction potential and rate of reduction by ascorbate, Chem. Commun., 2012, 48, 847–849 RSC.
- S. Yuan, Y. Zhu, Y. Dai, Y. Wang, D. Jin, M. Liu, L. Tang, F. Arnesano, G. Natile and Y. Liu, 19F NMR allows the investigation of the fate of platinum(IV) prodrugs in physiological conditions, Angew. Chem., Int. Ed., 2022, 61, e202114250 CrossRef CAS PubMed.
- Z. Xu, H. M. Chan, C. Li, Z. Wang, M.-K. Tse, Z. Tong and G. Zhu, Synthesis, structure, and cytotoxicity of oxaliplatin-based platinum(IV) anticancer prodrugs bearing one axial fluoride, Inorg. Chem., 2018, 57, 8227–8235 CrossRef CAS PubMed.
- M. Ravera, E. Gabano, I. Zanellato, F. Fregonese, G. Pelosi, J. A. Platts and D. Osella, Antiproliferative activity of a series of cisplatin-based Pt(IV)-acetylamido/carboxylato prodrugs, Dalton Trans., 2016, 45, 5300–5309 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer US, Boston, MA, 3rd edn, 2006 Search PubMed.
- J. Hernando, M. van der Schaaf, E. M. H. P. van Dijk, M. Sauer, M. F. García-Parajó and N. F. van Hulst, Excitonic behavior of rhodamine dimers: a single-molecule study, J. Phys. Chem. A, 2003, 107, 43–52 CrossRef CAS.
- H. Yao and G. Zhu, A platinum-based fluorescent “turn on” sensor to decipher the reduction of platinum(IV) prodrugs, Dalton Trans., 2022, 51, 5394–5398 RSC.
- V. E. Y. Lee, Z. C. Lim, S. L. Chew and W. H. Ang, Strategy for traceless codrug delivery with platinum(IV) prodrug complexes using self-immolative linkers, Inorg. Chem., 2021, 60, 1823–1831 CrossRef CAS PubMed.
- J. X. Ong, C. S. Q. Lim, H. V. Le and W. H. Ang, A ratiometric fluorescent probe for cisplatin: investigating the intracellular reduction of platinum(IV) prodrug complexes, Angew. Chem., Int. Ed., 2019, 58, 164–167 CrossRef CAS PubMed.
- D. Montagner, S. Q. Yap and W. H. Ang, A fluorescent probe for investigating the activation of anticancer platinum(IV) prodrugs based on the cisplatin scaffold, Angew. Chem., Int. Ed., 2013, 52, 11785–11789 CrossRef CAS PubMed.
- R. G. Pearson, Chemical hardness and bond dissociation energies, J. Am. Chem. Soc., 1988, 110, 7684–7690 CrossRef CAS.
- M. Arsenijević, M. Milovanović, V. Volarević, D. Čanović, N. Arsenijević, T. Soldatović, S. Jovanović and Ž. D. Bugarčić, Cytotoxic properties of platinum(IV) and dinuclear platinum(II) complexes and their ligand substitution reactions with guanosine-5′-monophosphate, Transition Met. Chem., 2012, 37, 481–488 CrossRef.
- M. Crespo, M. Font-Bardia, P. Hamidizadeh, M. Martínez and S. M. Nabavizadeh, Kinetico-mechanistic study on the reduction/complexation sequence of PtIV/PtII organometallic complexes by thiol-containing biological molecules, Inorg. Chim. Acta, 2019, 486, 8–16 CrossRef CAS.
- I. Zanellato, I. Bonarrigo, D. Colangelo, E. Gabano, M. Ravera, M. Alessio and D. Osella, Biological activity of a series of cisplatin-based aliphatic bis(carboxylato) Pt(IV) prodrugs: how long the organic chain should be?, J. Inorg. Biochem., 2014, 140, 219–227 CrossRef CAS PubMed.
- N. A. Kratochwil and P. J. Bednarski, Relationships between reduction properties and cancer cell growth inhibitory activities of cis–dichloro– and cis–diiodo–Pt(IV)–ethylenediamines, Arch. Pharm. Pharm. Med. Chem., 1999, 332, 279–285 CrossRef CAS PubMed.
- K. Lemma, T. Shi and L. I. Elding, Kinetics and mechanism for reduction of the anticancer prodrug trans,trans,trans-[PtCl2(OH)2(c,-C6H11NH2)(NH3)] (JM335) by thiols, Inorg. Chem., 2000, 39, 1728–1734 CrossRef CAS PubMed.
- N. G. Blanco, C. R. Maldonado and J. C. Mareque-Rivas, Effective photoreduction of a Pt(IV) complex with quantum dots: a feasible new light-induced method of releasing anticancer Pt(II) drugs, Chem. Commun., 2009, 5257–5259 RSC.
- S. Choi, S. Mahalingaiah, S. Delaney, N. R. Neale and S. Masood, Substitution and reduction of platinum(IV) complexes by a nucleotide, guanosine 5′-monophosphate, Inorg. Chem., 1999, 38, 1800–1805 CrossRef CAS PubMed.
- K. Lemma, A. M. Sargeson and L. I. Elding, Kinetics and mechanism for reduction of oral anticancer platinum(IV) dicarboxylate compounds by L-ascorbate ions, Dalton Trans., 2000, 1167–1172 RSC.
- F. S. Mackay, N. J. Farrer, L. Salassa, H.-C. Tai, R. J. Deeth, S. A. Moggach, P. A. Wood, S. Parsons and P. J. Sadler, Synthesis, characterisation and photochemistry of PtIV pyridyl azido acetato complexes, Dalton Trans., 2009, 2315–2325 RSC.
- Z. Wang, N. Wang, S.-C. Cheng, K. Xu, Z. Deng, S. Chen, Z. Xu, K. Xie, M.-K. Tse, P. Shi, H. Hirao, C.-C. Ko and G. Zhu, Phorbiplatin, a highly potent Pt(IV) antitumor prodrug that can be controllably activated by red light, Chem, 2019, 5, 3151–3165 CAS.
- Y. Kido, A. R. Khokhar and Z. H. Siddik, Glutathione-mediated modulation of tetraplatin activity against sensitive and resistant tumor cells, Biochem. Pharmacol., 1994, 47, 1635–1642 CrossRef CAS PubMed.
- E. L. Weaver and R. N. Bose, Platinum(II) catalysis and radical intervention in reductions of platinum(IV) antitumor drugs by ascorbic acid, J. Inorg. Biochem., 2003, 95, 231–239 CrossRef CAS PubMed.
- S. Karmakar, I. Poetsch, C. R. Kowol, P. Heffeter and D. Gibson, Synthesis and cytotoxicity of water-soluble dual- and triple-action satraplatin derivatives: replacement of equatorial chlorides of satraplatin by acetates, Inorg. Chem., 2019, 58, 16676–16688 CrossRef CAS PubMed.
- L. Ma, N. Wang, R. Ma, C. Li, Z. Xu, M. K. Tse and G. Zhu, Monochalcoplatin: an actively transported, quickly reducible, and highly potent Pt(IV) anticancer prodrug, Angew. Chem., Int. Ed., 2018, 57, 9098–9102 CrossRef CAS PubMed.
- Z. Deng, C. Li, S. Chen, Q. Zhou, Z. Xu, Z. Wang, H. Yao, H. Hirao and G. Zhu, An intramolecular photoswitch can significantly promote photoactivation of Pt(IV) prodrugs, Chem. Sci., 2021, 12, 6536–6542 RSC.
- D. Tolan, V. Gandin, L. Morrison, A. El-Nahas, C. Marzano, D. Montagner and A. Erxleben, Oxidative stress induced by Pt(IV) pro-drugs based on the cisplatin scaffold and indole carboxylic acids in axial position, Sci. Rep., 2016, 6, 29367 CrossRef PubMed.
- H. Yao, Z. Xu, C. Li, M. K. Tse, Z. Tong and G. Zhu, Synthesis and cytotoxic study of a platinum(IV) anticancer prodrug with selectivity toward luteinizing hormone-releasing hormone (LHRH) receptor-positive cancer cells, Inorg. Chem., 2019, 58, 11076–11084 CrossRef CAS PubMed.
- H. Yao, Y. F. Gunawan, G. Liu, M.-K. Tse and G. Zhu, Optimization of axial ligands to promote the photoactivation of BODIPY-conjugated platinum(IV) anticancer prodrugs, Dalton Trans., 2021, 50, 13737–13747 RSC.
- Z. Deng, N. Wang, Y. Liu, Z. Xu, Z. Wang, T.-C. Lau and G. Zhu, A photocaged, water-oxidizing, and nucleolus-targeted Pt(IV) complex with a distinct anticancer mechanism, J. Am. Chem. Soc., 2020, 142, 7803–7812 CrossRef CAS PubMed.
- G. R. Gibbons, S. Wyrick and S. G. Chaney, Rapid reduction of tetrachloro(D,L-trans)1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in RPMI 1640 tissue culture medium, Cancer Res., 1989, 49, 1402–1407 CAS.
- W. K. Anderson, D. A. Quagliato, R. D. Haugwitz, V. L. Narayanan and M. K. Wolpert-DeFilippes, Synthesis, physical properties, and antitumor activity of tetraplatin and related tetrachloroplatinum(IV) stereoisomers of 1,2-diaminocyclohexane, Cancer Treat. Rep., 1986, 70, 997–1002 CAS.
- S. G. Chaney, S. Wyrick and G. K. Till, In vitro biotransformations of tetrachloro(d,l-trans,)-1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in rat plasma, Cancer Res., 1990, 50, 4539–4545 CAS.
- L. Pendyala, J. W. Cowens and P. J. Creaven, Studies on the pharmacokinetics and metabolism of cis-dichloro-trans-dihydroxy-bis-isopropylamine platinum(IV) in the dog, Cancer Treat. Rep., 1982, 66, 509–516 CAS.
- D. J. Evans and M. Green, The rate of reduction of cis-, cis-, trans-[PtIV(NH2Pri)2Cl2(OH)2], CHIP, the anti-cancer drug by ascorbic acid, Inorg. Chim. Acta, 1987, 130, 183–184 CrossRef CAS.
- T. Babu, A. Sarkar, S. Karmakar, C. Schmidt and D. Gibson, Multiaction Pt(IV) carbamate complexes can codeliver Pt(II) drugs and amine containing bioactive molecules, Inorg. Chem., 2020, 59, 5182–5193 CrossRef CAS PubMed.
- S. Karmakar, H. Kostrhunova, T. Ctvrtlikova, V. Novohradsky, D. Gibson and V. Brabec, Platinum(IV)-estramustine multiaction prodrugs are effective antiproliferative agents against prostate cancer cells, J. Med. Chem., 2020, 63, 13861–13877 CrossRef CAS PubMed.
- Y. Song, K. Suntharalingam, J. S. Yeung, M. Royzen and S. J. Lippard, Synthesis and characterization of Pt(IV) fluorescein conjugates to investigate Pt(IV) intracellular transformations, Bioconjugate Chem., 2013, 24, 1733–1740 CrossRef CAS PubMed.
- H. Shi, Q. Wang, V. Venkatesh, G. Feng, L. S. Young, I. Romero-Canelón, M. Zeng and P. J. Sadler, Photoactive platinum(IV) complex conjugated to a cancer-cell-targeting cyclic peptide, Dalton Trans., 2019, 48, 8560–8564 RSC.
- G. Liu, Y. Zhang, H. Yao, Z. Deng, S. Chen, Y. Wang, W. Peng, G. Sun, M.-K. Tse, X. Chen, J. Yue, Y. Peng, L. Wang and G. Zhu, An ultrasound-activatable platinum prodrug for sono-sensitized chemotherapy, Sci. Adv., 2023, 9, eadg5964 CrossRef CAS PubMed.
- H. Xiao, R. Qi, S. Liu, X. Hu, T. Duan, Y. Zheng, Y. Huang and X. Jing, Biodegradable polymer – cisplatin(IV) conjugate as a pro-drug of cisplatin(II), Biomaterials, 2011, 32, 7732–7739 CrossRef CAS PubMed.
- S. Theiner, M. Grabarics, L. Galvez, H. P. Varbanov, N. S. Sommerfeld, M. S. Galanski, B. K. Keppler and G. Koellensperger, The impact of whole human blood on the kinetic inertness of platinum(IV) prodrugs – an HPLC-ICP-MS study, Dalton Trans., 2018, 47, 5252–5258 RSC.
- L. T. Ellis, H. M. Er and T. W. Hambley, The influence of the axial ligands of a series of platinum(IV) anti-cancer complexes on their reduction to platinum(II) and reaction with DNA, Aust. J. Chem., 1995, 48, 793–806 CrossRef CAS.
- C. K. J. Chen, J. Z. Zhang, J. B. Aitken and T. W. Hambley, Influence of equatorial and axial carboxylato ligands on the kinetic inertness of platinum(IV) complexes in the presence of ascorbate and cysteine and within DLD-1 cancer cells, J. Med. Chem., 2013, 56, 8757–8764 CrossRef CAS PubMed.
- O. Proux, E. Lahera, W. Del Net, I. Kieffer, M. Rovezzi, D. Testemale, M. Irar, S. Thomas, A. Aguilar-Tapia and E. F. Bazarkina, High-energy resolution fluorescence detected X–ray absorption spectroscopy: a powerful new structural tool in environmental biogeochemistry sciences, J. Environ. Qual., 2017, 46, 1146–1157 CrossRef CAS PubMed.
- S. J. Berners-Price, L. Ronconi and P. J. Sadler, Insights into the mechanism of action of platinum anticancer drugs from multinuclear NMR spectroscopy, Prog. Nucl. Magn. Reson. Spectrosc., 2006, 1, 65–98 CrossRef.
- E. E. Blatter, J. F. Vollano, B. S. Krishnan and J. C. Dabrowiak, Interaction of the antitumor agents cis,cis,trans-Pt(IV)(NH3)2Cl2(OH)2 and cis,cis,trans-Pt(IV)[(CH3)2CHNH2]2Cl2(OH)2 and their reduction products with PM2 DNA, Biochemistry, 1984, 23, 4817–4820 CrossRef CAS PubMed.
- H. Yao, S. Chen, Z. Deng, M.-K. Tse, Y. Matsuda and G. Zhu, BODI-Pt, a green-light-activatable and carboplatin-based platinum(IV) anticancer prodrug with enhanced activation and cytotoxicity, Inorg. Chem., 2020, 59, 11823–11833 CrossRef CAS PubMed.
- E. Wexselblatt, R. Raveendran, S. Salameh, A. Friedman-Ezra, E. Yavin and D. Gibson, On the stability of PtIV pro–drugs with haloacetato ligands in the axial positions, Chem. – Eur. J., 2015, 21, 3108–3114 CrossRef CAS PubMed.
- S. J. Berners-Price, L. Ronconi and P. J. Sadler, Insights into the mechanism of action of platinum anticancer drugs from multinuclear NMR spectroscopy, Prog. Nucl. Magn. Reson. Spectrosc., 2006, 49, 65–98 CrossRef CAS.
- C. K. J. Chen, P. Kappen, D. Gibson and T. W. Hambley, trans-Platinum(IV) pro-drugs that exhibit unusual resistance to reduction by endogenous reductants and blood serum but are rapidly activated inside cells: 1H NMR and XANES spectroscopy study, Dalton Trans., 2020, 49, 7722–7736 RSC.
- L. Chen, P. F. Lee, J. D. Ranford, J. J. Vittal and S. Y. Wong, Reduction of the anti-cancer drug analogue cis,trans,cis-[PtCl2(OCOCH3)2(NH3)2] by L-cysteine and L-methionine and its crystal structure, Dalton Trans., 1999, 1209–1212 RSC.
- V. E. Y. Lee, C. F. Chin and W. H. Ang, Design and investigation of photoactivatable platinum(IV) prodrug complexes of cisplatin, Dalton Trans., 2019, 48, 7388–7393 RSC.
- J. Gurruchaga-Pereda, V. Martínez-Martínez, E. Rezabal, X. Lopez, C. Garino, F. Mancin, A. L. Cortajarena and L. Salassa, Flavin bioorthogonal photocatalysis toward platinum substrates, ACS Catal., 2020, 10, 187–196 CrossRef CAS.
- M. Sinisi, F. P. Intini and G. Natile, Dependence of the reduction products of platinum(IV) prodrugs upon the configuration of the substrate, bulk of the carrier ligands, and nature of the reducing agent, Inorg. Chem., 2012, 51, 9694–9704 CrossRef CAS PubMed.
- T. G. Appleton, R. D. Berry, C. A. Davis, J. R. Hall and H. A. Kimlin, Reactions of platinum(II) aqua complexes. 1. Multinuclear (platinum-195, nitrogen-15, and phosphorus-31) NMR study of reactions between the cis-diamminediaquaplatinum(II) cation and the oxygen-donor ligands hydroxide, perchlorate, nitrate, sulfate, phosphate, and acetate, Inorg. Chem., 1984, 23, 3514–3521 CrossRef CAS.
- T. G. Appleton, J. R. Hall and S. F. Ralph, Reactions of platinum(II) aqua complexes. 3. Multinuclear (nitrogen-15, platinum-195, carbon-13, and proton) NMR study of reactions of aqua and hydroxo complexes with glycine and (methylimino)diacetic acid, Inorg. Chem., 1985, 24, 673–677 CrossRef CAS.
- D. Corinti, M. E. Crestoni, S. Fornarini, E. Dabbish, E. Sicilia, E. Gabano, E. Perin and D. Osella, A multi-methodological inquiry of the behavior of cisplatin-based Pt(IV) derivatives in the presence of bioreductants with a focus on the isolated encounter complexes, J. Biol. Inorg. Chem., 2020, 25, 655–670 CrossRef CAS PubMed.
- L. Ronconi and P. J. Sadler, Photoreaction pathways for the anticancer complex trans,trans,trans-[Pt(N3)2(OH)2(NH3)2], Dalton Trans., 2011, 40, 262–268 RSC.
- A. Nemirovski, Y. Kasherman, Y. Tzaraf and D. Gibson, Reduction of cis,trans,cis-[PtCl2(OCOCH3)2(NH3)2] by aqueous extracts of cancer cells, J. Med. Chem., 2007, 50, 5554–5556 CrossRef CAS PubMed.
- A. Lasorsa, O. Stuchlíková, V. Brabec, G. Natile and F. Arnesano, Activation of platinum(IV) prodrugs by cytochrome c and characterization of the protein binding sites, Mol. Pharmaceutics, 2016, 13, 3216–3223 CrossRef CAS PubMed.
- M. Ravera, E. Gabano, S. Tinello, I. Zanellato and D. Osella, May glutamine addiction drive the delivery of antitumor cisplatin-based Pt(IV) prodrugs?, J. Inorg. Biochem., 2017, 167, 27–35 CrossRef CAS PubMed.
- N. Kratochwil, P. J. Bednarski, H. Mrozek, A. Vogler and J. K. Nagle, Photolysis of an iodoplatinum(IV) diamine complex to cytotoxic species by visible light, Anti-Cancer Drug Des., 1996, 11, 155–171 CAS.
- N. A. Kratochwil, Z. Guo, P. S. Murdoch, J. A. Parkinson, P. J. Bednarski and P. J. Sadler, Electron-transfer-driven trans-ligand labilization: a novel activation mechanism for Pt(IV) anticancer complexes, J. Am. Chem. Soc., 1998, 120, 8253–8254 CrossRef CAS.
- N. A. Kratochwil, J. A. Parkinson, P. J. Bednarski and P. J. Sadler, Nucleotide platination induced by visible light, Angew. Chem., Int. Ed., 1999, 38, 1460–1463 CrossRef CAS PubMed.
- P. Müller, B. Schröder, J. A. Parkinson, N. A. Kratochwil, R. A. Coxall, A. Parkin, S. Parsons and P. J. Sadler, Nucleotide cross–linking induced by photoreactions of platinum(IV)–azide complexes, Angew. Chem., Int. Ed., 2003, 42, 335–339 CrossRef PubMed.
- P. J. Bednarski, R. Grünert, M. Zielzki, A. Wellner, F. S. Mackay and P. J. Sadler, Light-activated destruction of cancer cell nuclei by platinum diazide complexes, Chem. Biol., 2006, 13, 61–67 CrossRef CAS PubMed.
- F. S. Mackay, J. A. Woods, P. Heringová, J. Kašpárková, A. M. Pizarro, S. A. Moggach, S. Parsons, V. Brabec and P. J. Sadler, A potent cytotoxic photoactivated platinum complex, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20743–20748 CrossRef CAS PubMed.
- P. Lepucki, A. P. Dioguardi, D. Karnaushenko, O. G. Schmidt and H.-J. Grafe, The normalized limit of detection in NMR spectroscopy, J. Magn. Reson., 2021, 332, 107077 CrossRef CAS PubMed.
- E. P. Diamandis, Immunoassays with time-resolved fluorescence spectroscopy: principles and applications, Clin. Biochem., 1988, 21, 139–150 CrossRef CAS PubMed.
- J. Bridgeman, M. Bieroza and A. Baker, The application of fluorescence spectroscopy to organic matter characterisation in drinking water treatment, Rev. Environ. Sci. Biotechnol., 2011, 10, 277–290 CrossRef CAS.
- O. S. Wolfbeis, Fluorescence spectroscopy: new methods and applications, Springer Science & Business Media, 2012 Search PubMed.
- X. Jiang, Y. Yu, J. Chen, M. Zhao, H. Chen, X. Song, A. J. Matzuk, S. L. Carroll, X. Tan, A. Sizovs, N. Cheng, M. C. Wang and J. Wang, Quantitative imaging of glutathione in live cells using a reversible reaction-based ratiometric fluorescent probe, ACS Chem. Biol., 2015, 10, 864–874 CrossRef CAS PubMed.
- K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics, Nat. Chem., 2017, 9, 279–286 CrossRef CAS PubMed.
- H. Liu, W. Song, S. Zhang, K. S. Chan, Z. Guo and Z. Shen, A ratiometric fluorescent probe for real-time monitoring of intracellular glutathione fluctuations in response to cisplatin, Chem. Sci., 2020, 11, 8495–8501 RSC.
- E. J. New, R. Duan, J. Z. Zhang and T. W. Hambley, Investigations using fluorescent ligands to monitor platinum(IV) reduction and platinum(II) reactions in cancer cells, Dalton Trans., 2009, 3092–3101 RSC.
- Y. Yuan, Y. Chen, B. Z. Tang and B. Liu, A targeted theranostic platinum(IV) prodrug containing a luminogen with aggregation-induced emission (AIE) characteristics for in situ monitoring of drug activation, Chem. Commun., 2014, 50, 3868–3870 RSC.
- C. Shen, B. D. W. Harris, L. J. Dawson, K. A. Charles, T. W. Hambley and E. J. New, Fluorescent sensing of monofunctional platinum species, Chem. Commun., 2015, 51, 6312–6314 RSC.
- C. S. Wijesooriya, J. A. Peterson, P. Shrestha, E. J. Gehrmann, A. H. Winter and E. A. Smith, A photoactivatable BODIPY probe for localization–based super–resolution cellular imaging, Angew. Chem., Int. Ed., 2018, 57, 12685–12689 CrossRef CAS PubMed.
- M. H. C. Boulet, H. R. Bolland, E. M. Hammond and A. C. Sedgwick, Oxali(IV)Fluors: fluorescence responsive oxaliplatin(IV) complexes identify a hypoxia-dependent reduction in cancer cells, J. Am. Chem. Soc., 2023, 145, 12998–13002 CrossRef CAS PubMed.
- Y. Yuan, R. T. K. Kwok, B. Z. Tang and B. Liu, Targeted theranostic platinum(IV) prodrug with a built-in aggregation-induced emission light-up apoptosis sensor for noninvasive early evaluation of its therapeutic responses in situ, J. Am. Chem. Soc., 2014, 136, 2546–2554 CrossRef CAS PubMed.
- J. J. Wilson and S. J. Lippard, Modulation of ligand fluorescence by the Pt(II)/Pt(IV) redox couple, Inorg. Chim. Acta, 2012, 389, 77–84 CrossRef CAS PubMed.
- C. Boudreau, T.-L. Wee, Y.-R. Duh, M. P. Couto, K. H. Ardakani and C. M. Brown, Excitation light dose engineering to reduce photo-bleaching and photo-toxicity, Sci. Rep., 2016, 6, 30892 CrossRef CAS PubMed.
- P. M. Carlton, J. Boulanger, C. Kervrann, J.-B. Sibarita, J. Salamero, S. Gordon-Messer, D. Bressan, J. E. Haber, S. Haase, L. Shao, L. Winoto, A. Matsuda, P. Kner, S. Uzawa, M. Gustafsson, Z. Kam, D. A. Agard and J. W. Sedat, Fast live simultaneous multiwavelength four-dimensional optical microscopy, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16016–16022 CrossRef CAS PubMed.
- S. Wäldchen, J. Lehmann, T. Klein, S. Van De Linde and M. Sauer, Light-induced cell damage in live-cell super-resolution microscopy, Sci. Rep., 2015, 5, 15348 CrossRef PubMed.
| This journal is © the Partner Organisations 2024 |