Stapled ligand for synthesis of highly emissive and stable CsPbBr3 perovskite nanocrystals in polar organic solvent†
Tianju
Chen
a,
Qi
Yang
a,
Peng
Zhang
 a,
Ruihao
Chen
a,
Ruihao
Chen
 *b,
Yuke
Lin
a,
Weifang
Zhou
a,
Laizhi
Sui
*c,
Xuan
Zheng
*a,
Guoliang
Chen
a and
Feiming
Li
*b,
Yuke
Lin
a,
Weifang
Zhou
a,
Laizhi
Sui
*c,
Xuan
Zheng
*a,
Guoliang
Chen
a and
Feiming
Li
 *a
*a
aCollege of Chemistry, Chemical Engineering and Environment, Minnan Normal University, Zhangzhou, 363000, P.R. China. E-mail: lfm1914@mnnu.edu.cn; zx1974@mnnu.edu.cn
bState Key Laboratory of Solidification Processing, Center for Nano Energy Materials, School of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, P. R. China. E-mail: rhchen@nwpu.edu.cn
cState Key Laboratory of Molecular Reaction Dynamics and Dalian Coherent Light Source, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023 China. E-mail: lzsui@dicp.ac.cn
First published on 28th July 2023
Abstract
Colloidal cesium lead halide perovskite nanocrystals (CsPbX3 PNCs) are prone to instability due to low lattice energy and dynamic ionic bonding between the PNC core and the inorganic–organic interface. To address this issue, we propose a stapled ligand, perfluoroglutaric acid (PFGA), that can effectively passivate the surface of CsPbBr3 PNCs through atomic-scale double chelating coordination with Pb2+ between neighboring [PbBr6]4− unit cells. Theoretical calculations show that this precise double chelating on the surface of CsPbBr3 PNCs can achieve a much stronger bonding energy (−5.19 eV) compared to single-chelated perfluoroadipic acid (−1.42 eV) or other similar ligands with trivial changes in chain length. The PFGA enables CsPbBr3 PNCs to achieve a high photoluminescence quantum yield of over 85% with a single acid as a capping agent, as well as outstanding dispersion in a variety of polar solvents. Furthermore, the PFGA-capped CsPbBr3 PNCs exhibit excellent purification stability after five rounds of vigorous washing and long-term stability after more than six months of storage within ethanol, which has been successfully applied for chloride sensing in different circumstances. Our findings provide insight into the design of surface engineering strategies to produce highly emissive and stable PNCs and broaden the sensing field of PNCs in polar media.
Introduction
Colloidal cesium lead halide perovskite nanocrystals (CsPbX3 PNCs) are efficient semiconductors that exhibit impressive optical properties, such as near-unity photoluminescence quantum yield (PLQY), wide colour gamut, high light-absorption coefficients, and highly tunable band-edge emission.1,2 These advantages make PNCs the system of choice for classical light emission devices, including light-emitting diodes,3,4 lasers,5,6 optical communication instruments,7,8 and scintillators,9,10 as well as promising light harvesters, such as solar cells11,12 and photodetectors.13,14 However, the structural instability caused by the intrinsic ionic crystal characteristics of CsPbX3 PNCs has hindered their current applications. In particular, the highly dynamic interface between the PNC core and surface ligands, which typically involves proton exchange and association–dissociation processes, has been the primary challenge in obtaining sufficiently robust PNCs.1On the one hand, it has been observed that protonated oleic acid (OA) and deprotonated oleyl amine (OAm) alone do not interact effectively with the surface of the PNCs. However, when the protonated N+H group interacts with lead species (Pb-oleate, PbX2) through hydrogen bridging bonds, it reduces their Coulomb force, ultimately resulting in the desorption of the lead oleate Z-type ligands during colloidal purification or storage.15 These processes lead to rapid PL quenching, as well as morphological degradation and coalescence of the colloids. Therefore, various amine-free or ammonium-free methods have been developed, involving use of glycyrrhizic acid,15 quaternary ammonium salts,16,17 trioctylphosphine oxide,18,19 alkylphosphonic acids,20,21 trioctylphosphine,22 and dodecylbenzene sulfonic acid,23 to eliminate internal proton exchange caused by Brønsted acid–base equilibria, resulting in significant improvements in colloidal purification and stability. Additionally, use of ligands that comprise more than one functional group have been demonstrated to be an effective strategy for improving the stability of PNCs by enhancing their adsorption capacity. Bakr et al. utilized a bidentate ligand (2,2′-iminodibenzoic acid (IDA)) to simultaneously passivate two surface-exposed Pb atoms, resulting in a larger binding energy of 1.4 eV compared to that of the single carboxylic OA ligand with 1.14 eV. This allowed the IDA-treated CsPbI3 PNCs to exhibit a high PLQY of 95% and improved stability.24 Kovalenko et al. used a zwitterionic soy lecithin as capping ligands to maintain the structural integrity of CsPbBr3 PNCs over a wide range of concentrations.25 More recently, Snaith et al. employed multidentate ligands, namely ethylenediaminetetraacetic acid (EDTA) and reduced L-glutathione, to “clean” the lead atoms of mixed-halide PNC surfaces, which suppressed the formation of iodine Frenkel defects and inhibited halide segregation.26 These results demonstrate the excellent potential of polydentate ligands for improving the stability and optical properties of PNCs. Interestingly, multidentate ligands with functional groups spaced more than eight atoms apart have seldom been reported for passivation of PNCs due to incomplete passivation resulting from inappropriate bonding sites. Spatial matching between the ligand functional group sites and PNC surfaces is also a key parameter for the design of ligands, although it has not been taken seriously so far, as more desirable binding energy can be achieved by eliminating unnecessary energy loss from molecular geometrical distortion.
In this study, we propose a novel approach to passivate the surface of CsPbBr3 PNCs using a stapled ligand, perfluoroglutaric acid (PFGA). The bidentate positions of PFGA are highly matched to the cubic CsPbBr3 lattice parameter at an atomic level, where Pb2+–Pb2+ interaction between neighboring [PbBr6]4− unit cells is stapled via a bidentate coordination approach (see Scheme 1), allowing for precise passivation of the PNC surface. Our first-principles density functional theory (DFT) calculations confirm that the surface of CsPbBr3 PNCs precisely double-chelated with PFGA possesses much stronger bonding than that with single-chelated ligands or similar double-chelated ligands, even with a difference of only one atom. Moreover, CsPbBr3 PNCs passivated with PFGA using only a single ligand display a high PLQY of over 85% with outstanding dispersion and stability in a variety of polar organic solvents. The strong bonding energy and exposed hydrophilic carboxyl interface of PFGA effectively passivate the CsPbBr3 PNC surface, making this an effective and promising approach for improving the stability and optical properties of PNCs. Our results suggest that the spatial matching between ligand functional group sites and PNC surface is a critical parameter for designing ligand molecules and should be taken into account to achieve desirable binding energy while minimizing unnecessary energy loss from molecular geometrical distortion.
Results and discussion
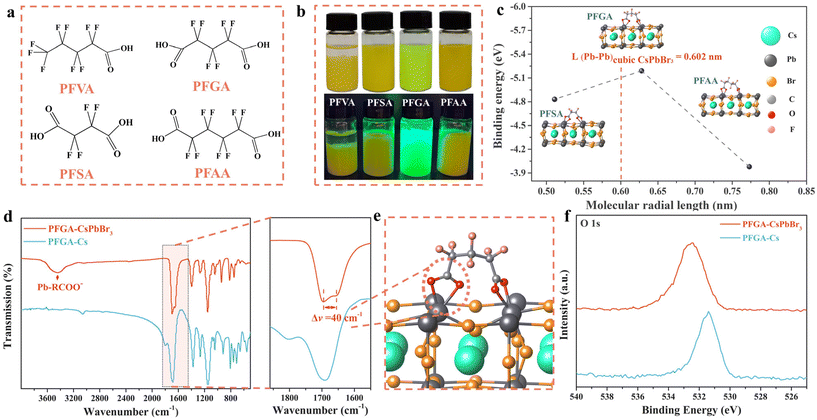
In this study, we investigated the effectiveness of a proposed stapled ligand for the synthesis of highly emissive CsPbBr3 PNCs. We synthesized CsPbBr3 PNCs using a series of ligands as capping agents, namely perfluoroglutaric acid (PFGA), perfluorovaleric acid (PFVA), perfluorosuccinic acid (PFSA), and perfluoroadipic acid (PFAA), as shown in Fig. 1a. We found that only PFGA was an appropriate ligand for the synthesis of CsPbBr3 PNCs, despite the similarity in molecular structures of these ligands. We ruled out the possibility of partial PFGA binding on the CsPbBr3 surface through single chelating coordination by comparing the PFGA and PFVA cases. To gain further insights into the underlying mechanism, we calculated the binding energy of each adsorbate by DFT methods (see ESI† for computational details). During the calculation process, we also considered PFSA and PFAA as having double chelating coordination since their single chelating coordination is similar to that of PFVA. We found that PFGA was anchored at two surface-adjacent Pb atoms on the optimized CsPbBr3 (001) surface with a larger binding energy (−5.19 eV) than those of the PFSA/PFAA/PFVA cases (−4.83 eV/−3.98 eV/−1.42 eV, respectively), indicating that the strongest stability of the chelate bonds was that between Pb2+ and carboxyl (COO−) of PFGA. This strong stability may be due to the molecular size of PFGA being the most suitable, where the optimized molecular radial length of PFGA (6.33 Å) is well matched with the edge length of the CsPbBr3 cell (i.e., Pb–Br–Pb, 6.02 Å). In contrast, the radial length of PFSA or PFAA (5.17 Å or 7.75 Å) appears to be too short or too long, respectively (see Fig. 1c).To gain a deeper understanding of how PFGA affects the NC surface, Fourier-transform infrared (FTIR) spectra and X-ray photoelectron spectroscopy (XPS) spectra were acquired for Cs2PFGA and PFGA-CsPbBr3 PNCs. As shown in Fig. 1d, the absorption peaks of PFGA-CsPbBr3 PNCs were similar to those of Cs2PFGA, except for a strong band at the 3200–3600 cm−1 region corresponding to the coordinate bond of COO− and Pb2+. This confirms the existence of chelating agents on the PNC surface. These results were consistent with those of the full-scan XPS spectrum, in which all the related ligand elements can be observed above the detection limits (Fig. S1†). Compared to Cs2PFGA, the antisymmetric stretching vibrations vas(COO−) and symmetric stretching vibrations vs(COO−) of the PFGA-CsPbBr3 PNCs were shifted from 1803 and 1691 cm−1 to 1696 and 1656 cm−1, respectively (Fig. 1d). The frequency difference (Δv = vas − vs) of PNCs reducing by 40 cm−1 revealed the chelating geometry of the dicarboxylic acid moiety under their variation of bond order (Fig. 1e).27 Furthermore, the high-resolution XPS spectrum showed that the O 1s signal of PFGA-CsPbBr3 PNCs shifted from 532.5 eV to 531.4 eV (Fig. 1f). The large low-field offset indicates the stronger interaction between PFGA and CsPbBr3 surface, as well as the electron-donating coordination from carboxyl.
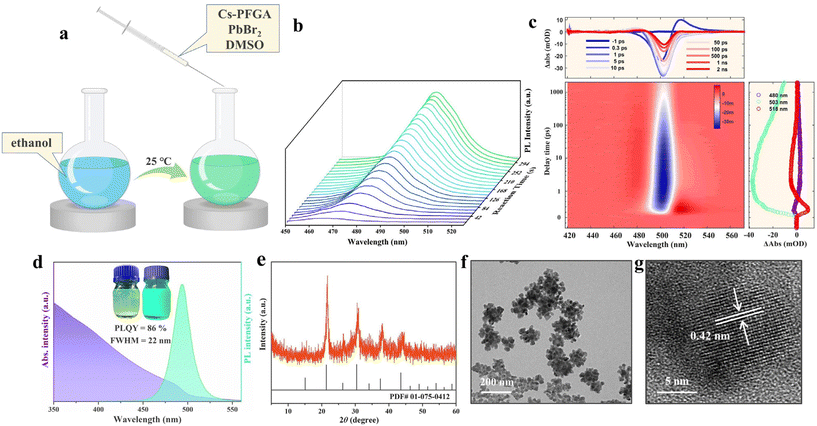
The PFGA-CsPbBr3 PNCs were synthesized using a modified ligand-assisted precipitation method (see detailed methods in the ESI† and related Fig. 2a).28 In this method, equimolar amounts of cesium perfluoroglutarate (Cs2PFGA) and PbBr2 were dissolved in DMSO to prepare the precursor, which was then injected into ethanol under magnetic stirring using a water bath at 25 °C. Despite the shortage of bromine ions and the use of a single acid as ligand, the PFGA-CsPbBr3 PNCs exhibited a relatively high QY of 86% (Fig. 2d). Previous approaches that used only acid molecules as additives for surface ligands resulted in enormous negatively charged defects due to charge competition,16 leading to a low QY generated from the negative exciton trapping effect of the halogen vacancy.29
We believe that the relatively high QY of the resulting PNCs can be attributed mainly to the precise passivation and little steric repulsion in stapled PFGA, which can effectively remove most of the lead atoms on the PNC surface. Furthermore, the in situ PL spectrum shown in Fig. 2b demonstrates that the PFGA-assisted precipitation process involves a rapid nucleation and several minutes of slow growth due to the weak interfacial tension between polar anti-solvent and ionic crystals, which reduces the reactivity of crystal growth. In contrast, classic precipitation typically completes nucleation and growth within several seconds.28 It is worth noting that the slow growth process seems to promote the crystalline structural integrity and optical performance of the PNCs, as a growth rate that is too fast may not be able to compensate for all the missing bromide ions in the [PbBr6]4− octahedra.29 Therefore, the different precipitation process may also be an important factor influencing the optical performance of the PFGA-CsPbBr3 PNCs. We measured the ultrafast dynamics of PFGA-CsPbBr3 PNCs dispersed in ethanol using femtosecond transient absorption (TA). Within the first 1 ps, the photon-induced absorption (PIA) at 518 nm decayed immediately, and the ground state bleaching (GSB) at 505 nm began to increase, which was caused by the relaxation of hot carriers. The absence of an obvious GSB signal in the low-energy region indicates that the trap states are effectively passivated.30
Transmission electron microscopy (TEM) images revealed that PFGA-CsPbBr3 PNCs tend to form loose and irregular clusters (Fig. 2f) due to the lack of stereoscopic barrier on PFGA ligands and the low surface energy of –CF3 and –CF2– groups (16 and 18 mN M−1, respectively).31 The practical particle size distribution of the PNCs was around 120 nm, as measured by dynamic light scattering (DLS) (Fig. S2†), which is consistent with the TEM image. The PFGA-CsPbBr3 PNCs exhibited a green PL peak at 500 nm with a full width at half-maximum of 22 nm (Fig. 2d). The obvious quantum confinement effect in the PL peak suggests that the PNCs are individual aggregates rather than being fused together. X-ray diffraction (XRD) patterns of PFGA-CsPbBr3 PNCs showed that their diffraction peaks match well with the cubic phase of CsPbBr3 (standard card, ICSD#01-075-0412) (Fig. 2e). Moreover, the lattice spacing of 4.2 Å observed in the high-resolution TEM (HR-TEM) image, corresponding to the (110) crystal plane of the cubic CsPbBr3, further confirms the crystalline phase of the CsPbBr3 PNCs (Fig. 2g).
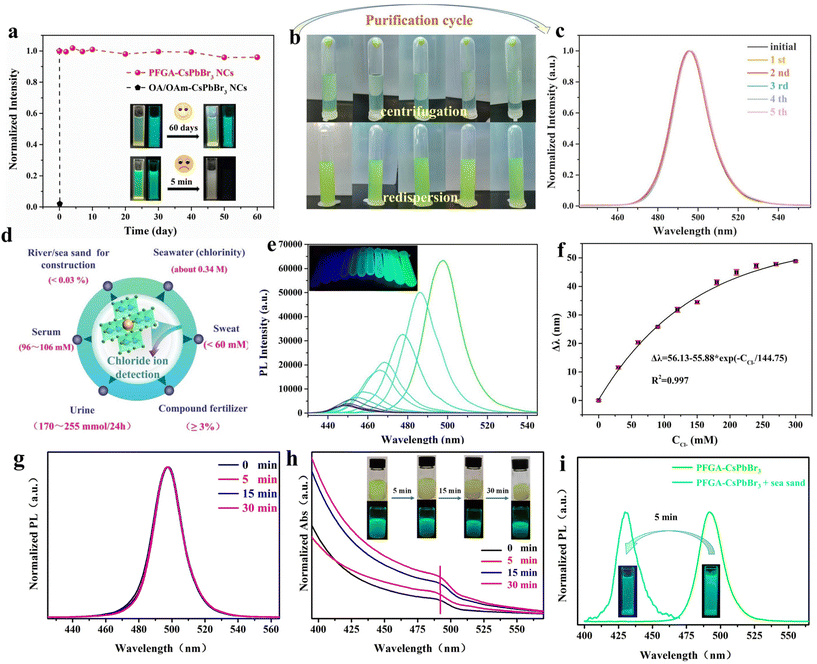
Theoretical calculations suggest that the ligands bind tightly to the CsPbBr3 PNCs, resulting in excellent stability and dispersion in various polar solvents. The orientation forces between the exposed hydrophilic carboxyl group and solvent molecules contribute to the dispersity in polar solvents. However, this dispersity comes at the cost of poor deposition in non-polar solvents, as illustrated in Fig. S3.† To demonstrate the stability of PFGA-CsPbBr3 PNCs, we compared their photoluminescence (PL) emission with that of OA/OAm-capped CsPbBr3 PNCs in ethanol over a period of two months. While the PL intensity of OA/OAm-CsPbBr3 PNCs rapidly quenched within 5 min, PFGA-CsPbBr3 PNCs showed no significant difference in PL intensity even after 60 days (Fig. 3a). This indicates a strong interaction between PFGA and the PNC surface. Another important issue for practical applications is the purification problem caused by the highly dynamic OA and OAm, which easily escape from the surface of NCs. During purification of OA/OAm-CsPbBr3 PNCs in toluene, imperfect passivation and particle aggregation caused the PL spectra to gradually redshift and impure peaks to appear (Fig. S4†). In contrast, after violent washing of PFGA-CsPbBr3 PNCs with ethanol five times, there was no significant difference in product color, colloidal dispersibility, or fluorescence emission (Fig. 3b and c), which further demonstrates the strong adsorption capacity of PFGA and its outstanding stability, and the fact that PFGA-CsPbBr3 PNCs retain their excellent optical properties even after long-term storage and multiple washing. Compared with the other ligands for the synthesis of CsPbX3 PNCs (especially for CsPbBr3 PNCs) summarized in Table S2,† PFGA served as a powerful ligand for the synthesis of CsPbBr3 PNCs in polar solvents with high stability and PLQY, compared with the other ligands.
The susceptibility of the intrinsic ionic structure of PNCs to ionic attack and the tendency of ion migration to occur in a polar environment make the polar stability and dispersion of PFGA-CsPbBr3 PNCs ideal for use as a fluorescent probe for ion detection, especially for the chloride ion (Cl−). Cl− can rapidly and selectively react with the CsPbBr3 PNCs by halogen exchange due to the low vacancy diffusion activation energy of halides (CsPbBr3 and CsPbCl3 have activation energies of 0.25 eV and 0.29 eV, respectively).32 The detection of Cl− is of great significance in practical applications due to its important industrial uses and physiological functions, as highlighted in previous studies (Fig. 3d).33–35 Here, we showcase two common instances of homogeneous and heterogeneous Cl− detection using a PFGA-CsPbBr3 PNC ethanol solution.
To detect Cl− in aqueous solutions, such as urine samples, homogeneous detection can be used. To optimize the system stability and reaction time, several key parameters were adjusted, as shown in Fig. S5 and S6.† The relationship between the PL wavelength and the concentration of Cl− was then examined, as shown in Fig. 3e. The wavelength shift Δλ (where Δλ = λ0 − λ, and λ0 and λ represent the PL wavelength of PNCs before and after the addition of Cl−) displayed a good linear relationship with Cl− concentration ranging from 0–300 mM (Fig. 3f). The XRD pattern in Fig. S7† shows the structure of PFGA-CsPbBr3 PNCs after addition of Cl− and has been investigated, and the relative XRD pattern is shown subsequently. We could find that as Cl− increased, the characteristic peaks of the products still retain the cubic CsPbBr3 phase with a small amount of CsPb2Br5 phase, and all the peaks shifted to higher angles, which might be ascribed to the cell being shrunk upon incorporation of Cl−, which is consistent with the results reported in previous work.2 Besides, the PL lifetime changes of CsPbBr3 PNCs after halide exchange have been investigated (Fig. S8†). Results showed that contrary to previously reported results, the fluorescence lifetime increased with increasing chloride ion concentration, since the proportion of τ3 increased with increasing Cl− concentration, indicating a nonradiative recombination process (such as surface traps or/and bulk defect-related traps).1,2 The prepared system demonstrated good selectivity and anti-interference ability, as shown in Fig. S9.† Direct detection was carried out on urine samples obtained from three healthy volunteers without any pretreatment. The results showed relative standard deviations (RSD) ranging from 1.8% to 2.3% and recovery rates in the range of 94%–106% (Table S3†), indicating the reliability and reproducibility of the detection results. The comparison of chloride sensing in different solvents has been summarized in Table S4.† The results show that the ligand in CsPbBr3 PNCs plays a decisive role in the detection of chloride ions, and when polar-stabilized CsPbBr3 PNCs are used, chloride ions can be detected by homogeneous determination, and in contrast, when non-polar-stabilized CsPbBr3 PNCs are used, chloride ions can only be detected by non-homogeneous determination, and the diffusion of chloride ions needs to be accelerated through magnetic stirring,36 which makes the steps relatively cumbersome.
The ability of PFGA-CsPbBr3 PNCs to qualitatively detect Cl− in solids, such as in river/sea sand, is of great importance in the construction industry. This is because the use of sea sand in construction can lead to the formation of soluble metal ions due to the enrichment of Cl−, which can cause corrosion of stainless steel and ultimately affect the lifespan of the building. The traditional method for detecting Cl− in solids using silver ions is expensive and time-consuming, but PFGA-CsPbBr3 PNCs provide a simple, efficient, and visualized identification platform. When river/sea sand is introduced into an ethanol solution of PFGA-CsPbBr3 PNCs, the system shows no response to river sand (Fig. 3g and h), but sea sand causes a pronounced color change and a wide wavelength blue-shift within minutes (Fig. 3i and Fig. S6†), demonstrating the potential of this system for practical applications.
Conclusions
In summary, we have successfully passivated the surface of CsPbBr3 PNCs through atomic-scale double chelating coordination with Pb2+ between neighbouring [PbBr6]4− unit cells using the stapled ligand PFGA. This has resulted in precise passivation of the CsPbBr3 PNC surface and strong bonding energy. Both theoretical and experimental results have confirmed the size matching and double-chelated mechanism of PFGA, which has enabled excellent optical performance of the PFGA-CsPbBr3 PNCs under a single acid as ligand. Specifically, we have demonstrated the ability of PFGA-CsPbBr3 PNCs to serve as robust fluorescent probes for real-time and on-site detection of Cl−. In addition, we have shown that homogeneous detection of chlorine in urine can be achieved using an ethanol solution of PFGA-CsPbBr3 PNCs, with reliable and reproducible results. Furthermore, we have demonstrated that this solution can also rapidly identify river/sea sand heterogeneously within several minutes. Our work sheds light on the spatial correlation between the crystal plane of PNCs and the binding site of a polydentate ligand, and has broadened the sensing field of PNCs in polar media.Author contributions
Tianju Chen: Methodology, investigation, writing – original draft. Qi Yang: Data curation. Peng Zhang: Validation. Ruihao Chen: Supervision, investigation. Yuke Lin: Resources, supervision. Weifang Zhou: Supervision, investigation. Laizhi Sui: Theoretical calculations. Xuan Zhen: Theoretical calculations. Guoliang Chen: Resources, project administration. Feiming Li: Supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 22004055) and Natural Science Foundation of Fujian Province of China (2020J05165).References
- N. F. Maneiro, K. Sun, L. L. Fernández, S. G. Graña, P. M. Buschbaum and L. Polavarapu, Ligand chemistry of inorganic lead halide perovskite nanocrystals, ACS Energy Lett., 2023, 8, 1152–1191 CrossRef.
- Q. A. Akkerman, G. Rainò, M. V. Kovalenko and L. Manna, Genesis, Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals, Nat. Mater., 2018, 17, 394–405 CrossRef CAS PubMed.
- M. Liu, Q. Wan, H. Wang, F. Carulli, X. Sun, W. Zheng, L. Kong, Q. Zhang, C. Zhang, Q. Zhang, S. Brovelli and L. Li, Suppression of temperature quenching in perovskite nanocrystals for efficient and thermally stable light-emitting diodes, Nat. Photonics, 2021, 15, 379–385 CrossRef CAS.
- L. Jing, Q. Xie, H. Li, K. Li, H. Yang, P. L. P. Ng, S. Li, Y. Li, E. H. T. Teo, X. Wang and P.-Y. Chen, Multigenerational crumpling of 2D materials for anticounterfeiting patterns with deep learning authentication, Matter, 2020, 3, 2160–2180 CrossRef.
- Y. H. Hsieh, B.-W. Hsu, K.-N. Peng, K.-W. Lee, C. W. Chu, S.-W. Chang, H.-W. Lin, T.-J. Yen and Y.-J. Lu, Perovskite quantum dot lasing in a gap-plasmon nanocavity with ultralow threshold, ACS Nano, 2020, 14, 11670–11676 CrossRef CAS PubMed.
- S. Li, D. Lei, W. Ren, X. Guo, S. Wu, Y. Zhu, A. L. Rogach, M. Chhowalla and A. K. Y. Jen, Water-resistant perovskite nanodots enable robust two-photon lasing in aqueous environment, Nat. Commun., 2020, 11, 1192 CrossRef CAS PubMed.
- M. Xia, S. Zhu, J. Luo, Y. Xu, P. Tian, G. Niu and J. Tang, Ultrastable perovskite nanocrystals in all-Inorganic transparent Matrix for high-Speed underwater wireless optical communication, Adv. Opt. Mater., 2021, 9, 2002239 CrossRef CAS.
- X. Pan, J. Zhang, H. Zhou, R. Liu, D. Wu, R. Wang, L. Shen, L. Tao, J. Zhang and H. Wang, A nano-micro engineering nanofiber for electromagnetic absorber, green shielding and sensor, Nano-Micro Lett., 2021, 13, 70 CrossRef PubMed.
- H. Zhang, Z. Yang, M. Zhou, L. Zhao, T. Jiang, H. Yang, X. Yu, J. Qiu, Y. Yang and X. Xu, Reproducible X-ray imaging with a perovskite nanocrystal scintillator embedded in a transparent amorphous network structure, Adv. Mater., 2021, 33, 2102529 CrossRef CAS PubMed.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang, Z. Yi, J. Li, X. Xie, Y. Wang, Y. Li, D. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. Yang, W. Huang and X. Liu, All-inorganic perovskite nanocrystal scintillators, Nature, 2018, 561, 88–93 CrossRef CAS PubMed.
- W. Sun, R. Yun, Y. Liu, X. Zhang, M. Yuan, L. Zhang and X. Li, Ligands in Lead Halide perovskite nanocrystals: from synthesis to optoelectronic applications, Small, 2023, 19, 2205950 CrossRef CAS PubMed.
- Y. Chen, N. Wei, Y. Miao, H. Chen, M. Ren, X. Liu and Y. Zhao, Inorganic CsPbBr3 perovskite nanocrystals as interfacial ion reservoirs to stabilize FAPbI3 perovskite for efficient photovoltaics, Adv. Energy Mater., 2022, 12, 2200203 CrossRef CAS.
- D. Liu, Y. Guo, M. Que, X. Yin, J. Liu, H. C. Zhang and W. Que, Metal halide perovskite nanocrystals: application in high-performance photodetectors, Mater. Adv., 2021, 2, 856–879 RSC.
- C. Kang, I. Dursun, G. Liu, L. Sinatra, X. Sun, M. Kong, J. Pan, P. Maity, E. Ooi, T. Ng, O. Mohammed, O. Bakr and B. Ooi, High-speed colour-converting photodetector with all-inorganic CsPbBr3 perovskite nanocrystals for ultraviolet light communication, Light: Sci. Appl., 2019, 8, 94 CrossRef PubMed.
- L. L. Zheng, K. Y. Jiang, X. L. Li, P. B. Hong, K. Chen, H. Zhang, Y. B. Song and B. B. Luo, Water-assisted preparation of ethanol-dispersed CsPbBr3 perovskite nanocrystals and emissive gel, J. Colloid Interface Sci., 2021, 598, 166–171 CrossRef CAS PubMed.
- E. Yassitepe, Z. Yang, O. Voznyy, Y. Kim, G. Walters, J. A. Castañeda, P. Kanjanaboos, M. Yuan, X. Gong, F. Fan, J. Pan, S. Hoogland, R. Comin, O. M. Bakr, L. A. Padilha, A. F. Nogueira and E. H. Sargent, Amine-free synthesis of cesium lead halide perovskite quantum dots for efficient light-emitting diodes, Adv. Funct. Mater., 2016, 26(47), 8757–8763 CrossRef CAS.
- Y. Tan, Y. Zou, L. Wu, Q. Huang, D. Yang, M. Chen, M. Ban, C. Wu, T. Wu, S. Bai, T. Song, Q. Zhang and B. Sun, Highly luminescent and stable perovskite nanocrystals with octylphosphonic acid as a ligand for efficient light-emitting diodes, ACS Appl. Mater. Interfaces, 2018, 10(4), 3784–3792 CrossRef CAS PubMed.
- B. Zhang, L. Goldoni, J. Zito, Z. Dang, G. Almeida, F. Zaccaria, J. de Wit, I. Infante, L. De Trizio and L. Manna, Alkyl phosphonic acids deliver CsPbBr3 nanocrystals with high photoluminescence quantum yield and truncated octahedron shape, Chem. Mater., 2019, 31(21), 9140–9147 CrossRef CAS.
- H. Wang, N. Sui, X. Bai, Y. Zhang, Q. Rice, F. J. Seo, Q. Zhang, V. L. Colvin and W. W. Yu, Emission recovery and stability enhancement of inorganic perovskite quantum dots, J. Phys. Chem. Lett., 2018, 9(15), 4166–4173 CrossRef CAS PubMed.
- C. Lu, H. Li, K. Kolodziejski, C. Dun, W. Huang, D. Carroll and S. M. Geyer, Enhanced stabilization of inorganic cesium lead triiodide (CsPbI3) perovskite quantum dots with tri-octylphosphine, Nano Res., 2018, 11(2), 762–768 CrossRef CAS.
- F. Liu, Y. Zhang, C. Ding, K. Kawabata, Y. Yoshihara, T. Toyoda, S. Hayase, T. Minemoto, R. Wang and Q. Shen, Trioctylphosphine oxide acts as alkahest for SnX2/PbX2: A general synthetic route to perovskite ASnxPb1−xX3 (A=Cs, FA, MA; X=Cl, Br, I) quantum dots, Chem. Mater., 2020, 32(3), 1089–1100 CrossRef CAS.
- G. Almeida, O. J. Ashton, L. Goldoni, D. Maggioni, U. Petralanda, N. Mishra, Q. A. Akkerman, I. Infante, H. J. Snaith and L. Manna, The phosphine oxide route toward lead halide perovskite nanocrystals, J. Am. Chem. Soc., 2018, 140(44), 14878–14886 CrossRef PubMed.
- D. Yang, X. Li, W. Zhou, S. Zhang, C. Meng, Y. Wu, Y. Wang and H. Zeng, CsPbBr3 quantum dots 2.0: benzenesulfonic acid equivalent ligand awakens complete purification, Adv. Mater., 2019, 31(30), e1900767 CrossRef PubMed.
- J. Pan, Y. Shang, J. Yin, M. De Bastiani, W. Peng, I. Dursun, L. Sinatra, A. M. El-Zohry, M. N. Hedhili, A.-H. Emwas, O. F. Mohammed, Z. Ning and O. M. Bakr, Bidentate ligand-passivated CsPbI3 perovskite nanocrystals for stable near-unity photoluminescence quantum yield and efficient red light-emitting diodes, J. Am. Chem. Soc., 2018, 140(2), 562–565 CrossRef CAS PubMed.
- F. Krieg, Q. K. Ong, M. Burian, G. Rainò, D. Naumenko, H. Amenitsch, A. Süess, M. J. Grotevent, F. Krumeich, M. I. Bodnarchuk, I. Shorubalko, F. Stellacci and M. V. Kovalenko, Stable ultraconcentrated and ultradilute colloids of CsPbX3 (X=Cl, Br) nanocrystals using natural lecithin as a capping ligand, J. Am. Chem. Soc., 2019, 141(50), 19839–19849 CrossRef CAS PubMed.
- Y. Hassan, J. H. Park, M. L. Crawford, A. Sadhanala, J. Lee, J. C. Sadighian, E. Mosconi, R. Shivanna, E. Radicchi, M. Jeong, C. Yang, H. Choi, S. H. Park, M. H. Song, F. De Angelis, C. Y. Wong, R. H. Friend, B. R. Lee and H. J. Snaith, Ligand-engineered bandgap stability in mixed-halide perovskite LEDs, Nature, 2021, 591(7848), 72–77 CrossRef CAS PubMed.
- G. B. Deacon and R. J. Phillips, Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination, Coord. Chem. Rev., 1980, 33(3), 227–250 CrossRef CAS.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes, Adv. Funct. Mater., 2016, 26(15), 2435–2445 CrossRef CAS.
- Y. Wu, C. Wei, X. Li, Y. Li, S. Qiu, W. Shen, B. Cai, Z. Sun, D. Yang, Z. Deng and H. Zeng, In situ passivation of PbBr64− octahedra toward blue luminescent CsPbBr3 nanoplatelets with near 100% absolute quantum yield, ACS Energy Lett., 2018, 3(9), 2030–2037 CrossRef CAS.
- X. Wu, M. T. Trinh, D. Niesner, H. Zhu, Z. Norman, J. S. Owen, O. Yaffe, B. J. Kudisch and X. Y. Zhu, Trap states in lead iodide perovskites, J. Am. Chem. Soc., 2015, 137(5), 2089–2096 CrossRef CAS PubMed.
- A. Ito, K. Kamogawa, H. Sakai, K. Hamano, Y. Kondo, N. Yoshino and M. Abe, Micelle aggregating condition of fluorocarbon−hydrocarbon hybrid surfactants in aqueous solution, Langmuir, 1997, 13(11), 2935–2942 CrossRef CAS.
- Y. Huang, Y. Feng, F. Li, F. Lin, Y. Wang, X. Chen and R. Xie, Sensing studies and applications based on metal halide perovskite materials: current advances and future perspectives, Trends Anal. Chem., 2021, 134, 116127 CrossRef CAS.
- S. Cinti, L. Fiore, R. Massoud, C. Cortese, D. Moscone, G. Palleschi and F. Arduini, Low-cost and reagent-free paper-based device to detect chloride ions in serum and sweat, Talanta, 2018, 179, 186–192 CrossRef CAS PubMed.
- X. He, Y. Gu, B. Yu, Z. Liu, K. Zhu, N. Wu, X. Zhao, Y. Wei, J. Zhou and Y. Song, Multi-mode structural-color anti-counterfeiting labels based on physically unclonable amorphous photonic structures with convenient artificial intelligence authentication, J. Mater. Chem. C, 2019, 7(45), 14069–14074 RSC.
- M. Jin, L. Jiang, M. Lu and S. Bai, Monitoring chloride ion penetration in concrete structure based on the conductivity of graphene/cement composite, Constr. Build. Mater., 2017, 136, 394–404 CrossRef CAS.
- F. M. Li, Y. F. Feng, Y. P. Huang, Q. H. Yao, G. H. Huang, Y. M. Zhu and X. Chen, Colorimetric sensing of chloride in sweat based on fluorescence wavelength shift via halide exchange of CsPbBr3 perovskite nanocrystals, Microchim. Acta, 2021, 188, 2–8 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis, DFT computation details and characterization of the PNCs; parameters of chloride sensing. See DOI: https://doi.org/10.1039/d3qi00719g |
| This journal is © the Partner Organisations 2023 |