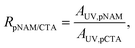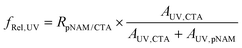Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
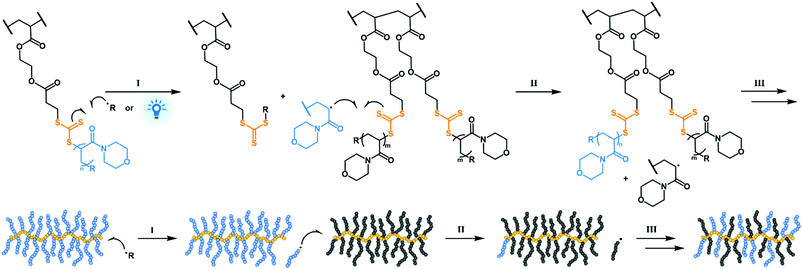
Putting the RAFT in GRAFT: intermolecular graft exchange between bottlebrush polymers using reversible addition–fragmentation chain transfer†
Satu
Häkkinen
 a,
Billy
Dyer
a,
Andrew
Kerr
a and
Sébastien
Perrier
a,
Billy
Dyer
a,
Andrew
Kerr
a and
Sébastien
Perrier
 *ab
*ab
aDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, UK. E-mail: s.perrier@warwick.ac.uk
bWarwick Medical School, University of Warwick, Coventry CV4 7AL, UK
First published on 20th December 2021
Abstract
A versatile synthetic methodology is presented for the preparation of graft copolymers with mixed graft distributions using reversible addition–fragmentation chain transfer (RAFT). The approach harnesses the ability of Z group-tethered grafts to fragment off the backbone to facilitate intermolecular graft exchange reactions between distinct starting materials.
The discovery and industrious development of controlled polymerisation techniques have allowed the construction of macromolecules with intricate architectures and functionalities.1 Today, architectural design is an elemental step in adjusting polymer properties to suit its application. Further exploration of new synthetic routes is needed to expand the horizons of functional polymers.2,3
Branched architectures have been widely studied for their intriguing solution and bulk properties arising from their high density, reduced interchain penetration and entanglements, and conformational constraints imposed by their branching points.4,5 Amongst them, graft copolymers continue to be of interest to material scientists.6–8 The field has stemmed three synthetic strategies for their preparation, all of which may be used in conjunction with one or more polymerisation techniques. These include polymerisation of macromonomers (grafting through), conjugation of grafts to a substrate (grafting to), and polymerisation of grafts from initiating sites on a substrate (grafting from).7
The grafting from approach is a versatile synthetic strategy from a chemical and a mechanistic standpoint when conducted as a RAFT polymerisation.9–12 In RAFT grafting from polymerisations initiating sites (i.e., RAFT agents) may be tethered to a substrate via the reinitiating R group or the stabilising Z group, giving rise to two mechanistically different reactions with each having their benefits and limitations.9 In the R group approach propagating graft radicals remain covalently bound to the substrate, resulting in similar reaction mechanics to grafting from polymerisations conducted with other controlled polymerisation techniques. The Z group reaction mechanism is unique to RAFT polymerisation and akin to reversible grafting to radical reactions. In the Z group approach – also known as the transfer to approach13 – grafts fragment off their graft sites to propagate and may diffuse freely in the reaction medium. We hypothesised that the graft fragmentation should lead to intermolecular graft exchange if graft radicals were able to diffuse away from their original graft sites and close to those on another molecule (Scheme 1). Building on this feature, the Z group approach could be used to exchange distinct grafts in a mixture of graft copolymers to yield hybrid products, giving access to heterograft structures in a simple manner. While densely grafted heterograft copolymers may also be conveniently produced via the grafting through strategy, these polymerisations can suffer from poor control when conducted using RAFT and targeting long backbones due to steric hindrance congestion near the propagating and dormant chain-ends.14 It is therefore useful to find alternative ways to achieve these structures.
We present two convenient routes through which graft copolymers with mixed graft distributions – such as heterograft copolymers – may be prepared using the Z group mechanism. The first route involves mixing two or more structurally different graft copolymers in solution and subsequent initiation to induce graft interchange. The exchange may also be conducted using a mixture of graft copolymers and linear polymers capable of forming a chain radical. In the second approach a linear polymer with chain transfer agent-functionalised side groups (pCTA) and a linear polymer with a RAFT end-group are reacted to graft the linear chains to the pCTA using radical reactions.
A library of graft copolymers was prepared for the graft exchange study using a three-step synthetic protocol. In short, RAFT polymerisation and post-modification of poly(2-hydroxyethyl acrylate) (pHEA) was conducted to give a functionalised pHEA with 3-((((1-methoxy-1-oxopropan-2-yl)thio)carbonothioyl)thio)propanoic acid (MPPATC) tethered to the hydroxy groups via the Z group. Using such pCTAs as photoiniferters,15 a series of graft copolymers was prepared by polymerising 4-acryloylmorpholine (NAM) via the Z group approach under blue light irradiation. The products were isolated through repeated precipitations to remove any residual monomer and terminated linear polymers and characterised with size-exclusion chromatography (SEC) and 1H NMR spectroscopy. A selection of linear polymers was prepared via photoiniferter RAFT polymerisations of NAM to study the linear chain grafting approach. (ESI†: full synthesis and characterisation details of all materials.)
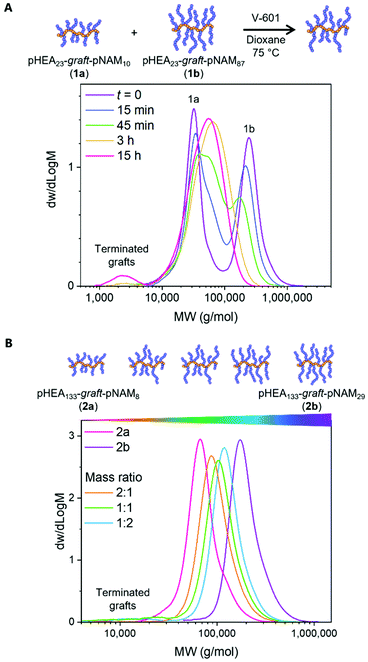
To test our hypothesis of intermolecular graft exchange taking place upon fragmentation of Z group tethered grafts, a reaction was conducted between pHEA23-graft-pNAM10 and pHEA23-graft-NAM87 under typical RAFT polymerisation conditions using an equal mass of each polymer and dimethyl 2,2′-azobis(2-methylpropionate) (V-601) initiator ([CTA]/[I]0 = 20) at 75 °C in dioxane. The polymers comprised identical backbones (DP 23) but different graft lengths (DPs 10 and 87) and were therefore expected to exhibit a unimodal molecular weight distribution (MWD) upon a successful exchange. Samples were withdrawn throughout the reaction for SEC analysis. The chromatogram of a sample taken before initiation showed two distinct MWDs, corresponding to pHEA23-graft-pNAM10 with short grafts at the lower end and pHEA23-graft-pNAM87 with longer grafts at the higher end of the molecular weight range (Fig. 1A). Changes in the MWDs after 15 minutes indicated a successful initiation of intermolecular graft exchange. This change became more apparent with increasing reaction time as the larger and smaller species continued to shift towards lower and higher molecular weights, respectively. After 3 h the exchange was sufficient to result in a nearly uniform MWD with Mn,SEC = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600, Đ = 1.46, and a subtle peak split still visible. Roughly 4 wt% of terminated grafts were formed over 15 h, corresponding to the expected 5 mol% termination due to the added initiator16 and resulting in a reduced grafting density. The main distribution exhibited subtle asymmetry after the reaction due to different degrees of graft termination in the preparation of the starting materials (Table S3†), which lead to the two polymers having slightly different grafting densities and therefore different chain volumes despite having indistinguishable graft length distributions after the exchange. Overall, the data indicated a successful graft exchange.
600, Đ = 1.46, and a subtle peak split still visible. Roughly 4 wt% of terminated grafts were formed over 15 h, corresponding to the expected 5 mol% termination due to the added initiator16 and resulting in a reduced grafting density. The main distribution exhibited subtle asymmetry after the reaction due to different degrees of graft termination in the preparation of the starting materials (Table S3†), which lead to the two polymers having slightly different grafting densities and therefore different chain volumes despite having indistinguishable graft length distributions after the exchange. Overall, the data indicated a successful graft exchange.
The reaction was repeated in the absence of exogenous initiator by employing the side group trithiocarbonate as a photoiniferter.15 A blue light induced reaction between pHEA23-graft-pNAM10 and pHEA23-graft-pNAM87 at 40–50 °C showed a nearly identical transformation of the MWD over 15 h, however the apparent rate of graft exchange was slower than in the initiator-driven reaction (Fig. S12†). The slower reaction rate was ascribed to a reduced frequency of successful graft detachment events due to a slower rate of radical formation, cage reactions, and/or slower radical diffusion. Repeated attempts at 20–30 °C resulted in no apparent exchange over 15 h, likely due to a reduced bond dissociation and/or diffusivity.
One of the advantages of the presented synthetic strategy is that the graft distribution may be adjusted with reaction stoichiometry. This modularity could be particularly advantageous in studies involving large polymer libraries in which one or multiple properties, such as polymer aspect ratio, rigidity, charge density, or a functionality are systematically varied. To this end, photoiniferter graft exchange reactions were conducted between pHEA133-graft-pNAM8 and pHEA133-graft-pNAM29 in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratios of the two polymers to yield three products with distinct hydrodynamic volumes (Fig. 1B). Some termination was observed (≤7 wt%), the amount of which increased with increasing average graft length. A reaction between graft copolymers with both different backbone and graft lengths, pHEA23-graft-pNAM10 and pHEA300-graft-pNAM51, resulted in a mixture of polymers with similar graft size distributions but dissimilar hydrodynamic volumes (Fig. S10 and S11†). While these reactions were performed in the absence of monomer, the exchange could alternatively be carried out as a block extension of the original grafts.
2 mass ratios of the two polymers to yield three products with distinct hydrodynamic volumes (Fig. 1B). Some termination was observed (≤7 wt%), the amount of which increased with increasing average graft length. A reaction between graft copolymers with both different backbone and graft lengths, pHEA23-graft-pNAM10 and pHEA300-graft-pNAM51, resulted in a mixture of polymers with similar graft size distributions but dissimilar hydrodynamic volumes (Fig. S10 and S11†). While these reactions were performed in the absence of monomer, the exchange could alternatively be carried out as a block extension of the original grafts.
Grafts may also be exchanged for linear chains capable of forming a chain radical, providing a versatile functionalisation strategy. This was demonstrated by exchanging grafts of pHEA23-graft-pNAM10 for pyrene-functional linear pNAM15 under blue light irradiation. Equimolar amounts of linear chains and grafts were used in an attempt to exchange 50% of the original grafts for pyrene-functional grafts. The reaction was monitored by SEC using UV detection at a 265 nm wavelength, at which pyrene has a strong absorption but trithiocarbonate groups absorb only weakly. The data showed a gradual increase in the UV absorption of the graft copolymer relative to the linear polymer, reaching 45% of total absorption over 24 h and confirming a successful functionalisation of the bottlebrush polymer with a fluorescent probe (Fig. 2).
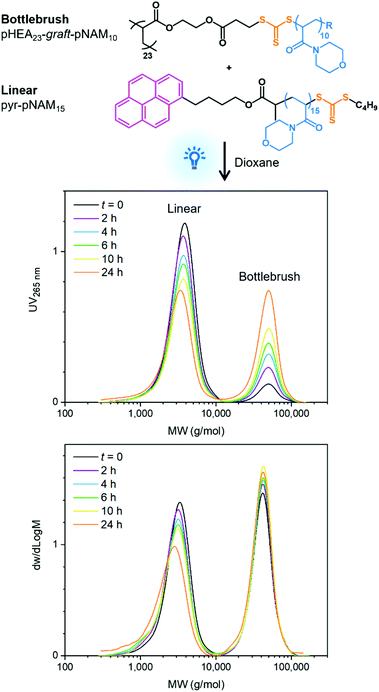 | ||
Fig. 2 SEC data shows exchange of pHEA23-graft-pNAM10 grafts for pyrene-functional linear pNAM15 chains. Analysis was conducted in CHCl3 with DRI and UV265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm detection and PMMA calibration. nm detection and PMMA calibration. | ||
The Z group approach reaction mechanism was also used to access the graft copolymer architecture by reacting linear pNAM chains with a pCTA. Using this strategy, graft copolymers may be prepared from a mixture of linear polymers in a similar fashion to previously reported grafting to reactions with polymeric radicals.17 Due to the ability of grafts to continuously fragment off the backbone, the reaction was expected to reach an equilibrium wherein the addition and fragmentation of grafts takes place at equal rates. Therefore, the reactions would result in a mixture of graft copolymers and linear chains which would need to be separated to isolate the desired product. The ratio of linear chains to backbone CTAs in the reaction was expected to determine the number of linear chains attached to the backbone.
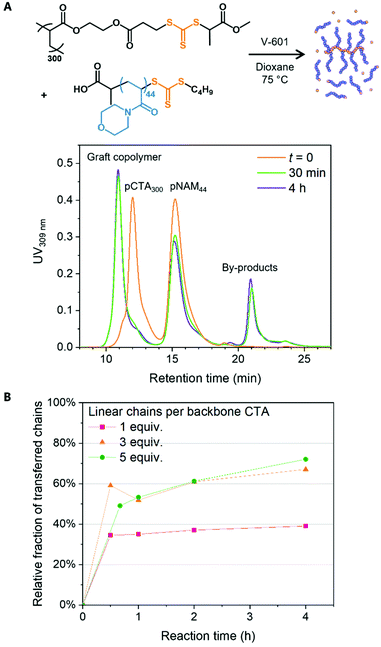
To this end, pNAM44 was reacted with pCTA300 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of linear chains to backbone CTAs in the presence of V-601 ([CTA]0/[I]0 = 20) in dioxane at 75 °C. SEC indicated a rapid increase in the hydrodynamic volume of pCTA300 within the first 30 min as linear chains were grafted to the polymer (Fig. 3A). The reaction was fast, and samples taken at longer reaction times indicated very little change in the MWD as the reaction reached an equilibrium. The fraction of grafted chains was estimated by monitoring the formation of UV-active, end-group-derived by-products resulting from chain grafting to the backbone. The initial molar ratio of pNAM44 to backbone CTAs was estimated from their respective peak areas AUV,pNAM and AUV,pCTA as
1 molar ratio of linear chains to backbone CTAs in the presence of V-601 ([CTA]0/[I]0 = 20) in dioxane at 75 °C. SEC indicated a rapid increase in the hydrodynamic volume of pCTA300 within the first 30 min as linear chains were grafted to the polymer (Fig. 3A). The reaction was fast, and samples taken at longer reaction times indicated very little change in the MWD as the reaction reached an equilibrium. The fraction of grafted chains was estimated by monitoring the formation of UV-active, end-group-derived by-products resulting from chain grafting to the backbone. The initial molar ratio of pNAM44 to backbone CTAs was estimated from their respective peak areas AUV,pNAM and AUV,pCTA as
However, the molar absorptivities of backbone CTAs and pNAM44 were not known to be equal and the relative areas were only used as an approximation.18 The relative fraction of grafted linear chains, which is descriptive of the grafting density, was calculated using the peak area of the by-products (AUV,CTA) as
An excess of linear chains was used in subsequent reactions to target higher grafting densities. Steric shielding effects near the reactive sites were anticipated to set a practical upper limit for the number of grafts per backbone. Grafting was carried out using 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios of pNAM44 to backbone CTAs, expecting to reach fRel,UV = 75% and 83%, respectively, but observing 67% and 72% after 4 h (Fig. 3B). The data suggested that even with a large excess of linear polymer roughly 30% of backbone repeating units remained without a graft.
1 molar ratios of pNAM44 to backbone CTAs, expecting to reach fRel,UV = 75% and 83%, respectively, but observing 67% and 72% after 4 h (Fig. 3B). The data suggested that even with a large excess of linear polymer roughly 30% of backbone repeating units remained without a graft.
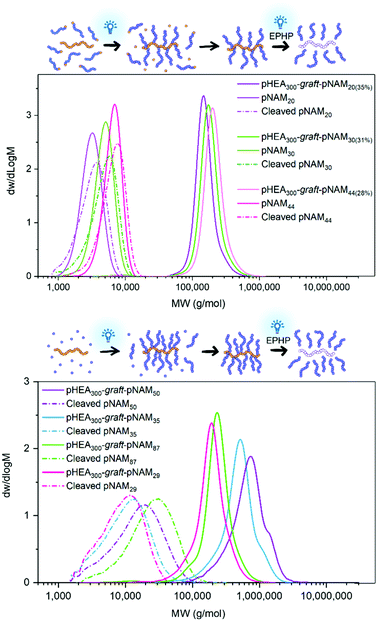
While this approach limits the achievable grafting density, it gives an excellent control over graft dispersity. Three grafting reactions between pCTA300 and pNAM (DP 20, 30, and 44) were conducted to compare the MWDs of the grafted chains after cleaving them off the backbone. Grafting was performed under blue light irradiation over 1.5 h with an equimolar ratio of linear chains to backbone CTAs, resulting in a 30–35% grafting density. After isolating the graft copolymers from linear chains through repeated precipitations, the grafts were fragmented off the backbone with blue light irradiation in the presence of 1-ethylpiperidine hypophosphite19 and analysed by SEC (Fig. 4). The cleaved grafts retained the molecular weight and dispersity of the original linear polymer well, and a clear distinction could be made between the three graft lengths after cleavage. In contrast, grafts polymerised through the Z group approach exhibited much higher dispersities (Đ = 1.58–1.98) regardless of the backbone or graft length. The poor control was to be expected due to chain transfer to the graft sites being hindered by steric shielding effects and the fast propagation rate of NAM.16,20 The new approach seems beneficial when low dispersity grafts are preferred over high grafting densities or when the selected materials cannot be synthesised through grafting from polymerisations. The strategy is applicable to linear polymers prepared through other polymerisation methods, provided that they carry a suitable radical-forming functionality.
In conclusion, the Z group approach was used to realise two new synthetic strategies that may be adapted as a convenient way to construct heterograft copolymers and other mixed graft distributions. In applying Z group grafting strategies, attention should be paid to the radical flux to minimise graft termination which may generally be expected to increase with increasing graft length due to steric shielding effects. Parameters such as reaction temperature, viscosity, concentration,21 and solvent quality22 may be used to optimise each system. In reactions employing various monomer families (e.g., methacrylic and acrylic monomers) the relative reactivities and radical stabilities of each should be taken into consideration. The versatile UV absorption characteristics and reactivities of RAFT agents may be explored further to develop more elegant grafting strategies. The scope of our work may be expanded beyond RAFT chemistry by employing polymers prepared through other polymerisation techniques carrying radical forming functionalities.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Lubrizol is acknowledged for the provision of scholarships (SH and AK).Notes and references
- R. B. Grubbs and R. H. Grubbs, Macromolecules, 2017, 50, 6979 CrossRef CAS.
- A. S. Abd-El-Aziz, M. Antonietti, C. Barner-Kowollik, W. H. Binder, A. Böker, C. Boyer, M. R. Buchmeiser, S. Z. D. Cheng, F. D'Agosto, G. Floudas, H. Frey, G. Galli, J. Genzer, L. Hartmann, R. Hoogenboom, T. Ishizone, D. L. Kaplan, M. Leclerc, A. Lendlein, B. Liu, T. E. Long, S. Ludwigs, J. F. Lutz, K. Matyjaszewski, M. A. R. Meier, K. Müllen, M. Müllner, B. Rieger, T. P. Russell, D. A. Savin, A. D. Schlüter, U. S. Schubert, S. Seiffert, K. Severing, J. B. P. Soares, M. Staffilani, B. S. Sumerlin, Y. Sun, B. Z. Tang, C. Tang, P. Théato, N. Tirelli, O. K. C. Tsui, M. M. Unterlass, P. Vana, B. Voit, S. Vyazovkin, C. Weder, U. Wiesner, W. Y. Wong, C. Wu, Y. Yagci, J. Yuan and G. Zhang, Macromol. Chem. Phys., 2020, 221, 2000216 CrossRef CAS.
- K. Parkatzidis, H. S. Wang, N. P. Truong and A. Anastasaki, Chem, 2020, 6, 1575 CAS.
- S. E. Seo and C. J. Hawker, Macromolecules, 2020, 53, 3257 CrossRef CAS.
- G. Polymeropoulos, G. Zapsas, K. Ntetsikas, P. Bilalis, Y. Gnanou and N. Hadjichristidis, Macromolecules, 2017, 50, 1253 CrossRef CAS.
- M. Müllner and A. H. E. Müller, Polymer, 2016, 98, 389 CrossRef.
- G. Xie, M. R. Martinez, M. Olszewski, S. S. Sheiko and K. Matyjaszewski, Biomacromolecules, 2019, 20, 27 CrossRef CAS.
- Z. Li, M. Tang, S. Liang, M. Zhang, G. M. Biesold, Y. He, S.-M. Hao, W. Choi, Y. Liu, J. Peng and Z. Lin, Prog. Polym. Sci., 2021, 116, 101387 CrossRef CAS.
- J. C. Foster, S. C. Radzinski and J. B. Matson, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2865 CrossRef CAS.
- A. Kerr, M. Hartlieb, J. Sanchis, T. Smith and S. Perrier, Chem. Commun., 2017, 53, 11901 RSC.
- S. Shanmugam, J. Cuthbert, T. Kowalewski, C. Boyer and K. Matyjaszewski, Macromolecules, 2018, 51, 7776 CrossRef CAS.
- J. Tanaka, S. Häkkinen, P. T. Boeck, Y. Cong, S. Perrier, S. S. Sheiko and W. You, Angew. Chem., 2020, 59, 7203 CrossRef CAS PubMed.
- B. S. Sumerlin, ACS Macro Lett., 2012, 1, 141 CrossRef CAS.
- T. G. Floyd, S. Häkkinen, S. C. L. Hall, R. M. Dalgliesh, A.-C. Lehnen, M. Hartlieb and S. Perrier, Macromolecules, 2021, 54, 9461 CrossRef CAS.
- M. Chen, M. Zhong and J. A. Johnson, Chem. Rev., 2016, 116, 10167 CrossRef CAS PubMed.
- S. Perrier, Macromolecules, 2017, 50, 7433 CrossRef CAS.
- G. Wang and J. Huang, Polym. Chem., 2014, 5, 277 RSC.
- K. Skrabania, A. Miasnikova, A. M. Bivigou-Koumba, D. Zehm and A. Laschewsky, Polym. Chem., 2011, 2, 2074 RSC.
- R. N. Carmean, C. A. Figg, G. M. Scheutz, T. Kubo and B. S. Sumerlin, ACS Macro Lett., 2017, 6, 185 CrossRef CAS.
- M. G. Fröhlich, P. Vana and G. Zifferer, Macromol. Theory Simul., 2007, 16, 610 CrossRef.
- M. M. Nardai and G. Zifferer, Polymer, 2013, 54, 4183 CrossRef CAS PubMed.
- M. G. Fröhlich, M. M. Nardai, N. Förster, P. Vana and G. Zifferer, Polymer, 2010, 51, 5122 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterisation data. See DOI: 10.1039/d1py01510a |
| This journal is © The Royal Society of Chemistry 2022 |