Emily K. Kirkeby and Andrew G. Roberts
Chem. Commun., 2020,56, 9118-9121
DOI:
10.1039/D0CC03503C,
Communication
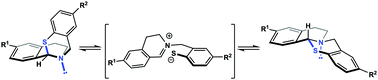
We report the synthesis, characterization and comparison of a series of electronically perturbed, cyclic N,S-acetals. Inspired by electrophilic auxiliaries utilized for amine capture and concomitant peptide ligation, we studied these N,S-acetal systems and evaluated their propensity to generate zwitterionic intermediates in situ. Certain N,S-acetals in this study exhibit structurally dynamic properties through a solvent and pH-dependent ability to ring-open and ring-close via C1–S bond ionization at room temperature.