Carbon nanotube–polyaniline core–shell nanostructured hydrogel for electrochemical energy storage†
Po-Yen Chenab,
Noémie-Manuelle Dorval Courchesneab,
Md Nasim Hyderab,
Jifa Qibc,
Angela M. Belcher*bcd and
Paula T. Hammond*ab
aDepartment of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: hammond@mit.edu
bThe David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: belcher@mit.edu
cDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
dDepartment of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
First published on 14th April 2015
Abstract
Conductive polymer hydrogels, which synergize the advantageous features of hydrogels and conductive materials, have been utilized in many electrochemical energy storage applications. Here, we introduce phytic acid as (1) a dispersing agent for pristine multi-walled carbon nanotubes (MWNTs) in aqueous solution containing aniline and as (2) a gelator to form polyaniline (PANI)-based hydrogels after polymerization. The PANI-based hydrogels exhibit nanowire-based mesoporous networks with high surface area and electrical conductivity. The nanostructured core (MWNT)–shell (PANI) hydrogels show an improvement on the electrical conductivity from 0.21 to 1.54 S cm−1 as the loading of MWNTs increases from 0 to 5.0 wt%. The conducting nanowire-based networks with MWNT loadings of 3.0 wt% in the hydrogel provide efficient electron transport pathways that exhibit a maximal specific capacity of 609 F g−1. The scalable and facile synthesis demonstrates excellent electrochemical performance, rendering it attractive for sensing, energy conversion, and energy storage applications.
Introduction
Conductive polymers have received tremendous interest due to their widespread applications from energy storage devices to bioelectronics and medical applications.1–5 Hydrogels of polyaniline (PANI), one of the most interesting electroactive polymers, receive significant attention due to facile processability, environmental stability, high electrical conductivity and redox behavior.6–9 To further improve the electrochemical properties (i.e., electrical and ionic conductivities) of PANI-based nanostructures, incorporating carbon nanomaterials has been demonstrated as a promising approach.10–12 Among various carbon nanomaterials, pristine multi-walled carbon nanotubes (MWNTs) have long been considered as ideal components for electrochemical energy storage devices due to their high electrical conductivity and aspect ratio.13,14Incorporating pristine MWNTs into PANI-based hydrogels is often challenged by the formation of non-homogeneous suspensions, and has a propensity to lead to aggregation of both the PANI nanostructures and MWNTs, resulting in the nanocomposites displaying undesired physicochemical properties. While MWNTs have been incorporated in PANI-based dried thin films using various assembly techniques, including colloidal mixture,15 interfacial polymerization,16 electrostatic deposition,13,14 electropolymerization,17 direct polymerization on MWNT supports,18 and enzymatic synthesis,19 these approaches cannot be applied adequately to incorporate MWNTs into hydrated PANI gels. In addition, even though the MWNT aggregation in conductive hydrogels might be addressed by chemical modification of MWNTs, or by adding binders, these methods often lead to further deterioration of the electronic properties or non-uniform distribution of these materials in the nanocomposites. Therefore, an efficient approach is highly desirable to incorporate pristine MWNTs into the PANI-based hydrogels homogeneously.
Phytic acid, an abundant natural product in plant tissues, is an excellent solvent to dissolve aniline and to disperse pristine carbon nanotubes in aqueous solution homogeneously.20,21 Recently, phytic acid has also been used as a gelator by Boa's group due to its excellent hydrogen bonding ability for conjugated polymers.3 Herein, we have synergistically utilized the solvation and gelation properties of phytic acid to design conductive hydrogels with core (MWNT)–shell (PANI) nanostructures. After the phytic acid solution dissolves aniline and disperses MWNT homogenously, the oxidative initiator is introduced into the mixed solution and the emeraldine PANI is then polymerized and coated on the wall surface of MWNTs to form high aspect ratio MWNT–PANI nanostructures. Also, the incorporated phytic acid serves as a dopant for as-synthesized PANI and forms hydrogen bonding between MWNT–PANI nanostructures to achieve conductive hydrogels. The resulting binder-free nanowire (NW)-based mesoporous MWNT–PANI networks provide high surface area and directional pathways for efficient electron transport. Compared to the hydrogels of PANI-only, the MWNT–PANI nanocomposites show an improvement on the electrical conductivity from 0.21 to 1.54 S cm−1 as the loading of MWNTs increases from 0 to 5.0 wt%. In addition, the phytic acid-mediated polymerization allows efficient utilization and dispersion of pristine MWNTs within PANI nanostructures to improve the specific capacity from 460 to 609 F g−1 with a low loading of pristine MWNTs (less than 3.0 wt%). The assembly of MWNT–PANI conductive polymer hydrogels promises the fabrication of three-dimensional (3D) conducting nanocomposites with great potential in sensing, energy conversion, and energy storage applications.
Results and discussion
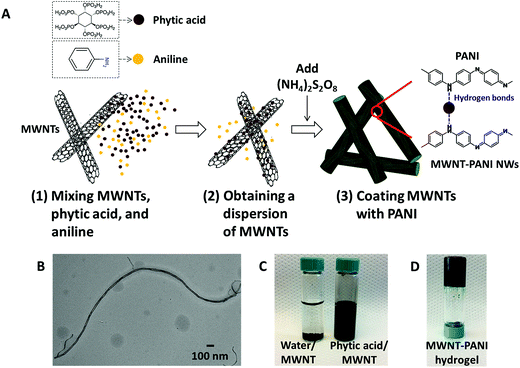
The fabrication process for the MWNT–PANI conductive polymer hydrogels is schematically illustrated in Fig. 1A. First, aniline is dissolved in a 50 wt% aqueous solution of phytic acid and then sonicated for an hour. After the mixture of aniline and phytic acid is formed, a given amount of pristine MWNTs (diameter ∼ 15 nm, transmission electron microscopy (TEM) image in Fig. 1B) is added, homogenized, ultrasonicated, and centrifuged to achieve a well-dispersed solution containing MWNTs, aniline, and phytic acid. The phytic acid-mediated approach greatly improves the dispersion of pristine MWNTs in aqueous solution (illustration shown in Fig. 1C).20,21 Phytic acid acts as a capping agent to inhibit MWNT aggregation by binding to aromatic carbon rings on the surface of pristine MWNTs via secondary interactions, and by providing a highly hydrated interface through the bound water molecules associated with its six phosphorous acid groups. After the oxidative initiator (i.e., ammonium persulfate (NH4)2S2O8) is added to the phytic acid solution containing MWNTs and aniline, the polymerization of PANI (described in eqn (1)) is indicated by a solution color change from dark brown (color of phytic acid and MWNTs) to dark green (color of emeraldine PANI).22,23
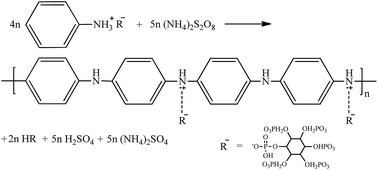 | (1) |
After the polymerization, each phytate moiety can interact with multiple PANI chains by doping the lone pair nitrogen of the polymer (Fig. 1A), and by creating a hydrogen bond with neighboring phytic acid molecule thus resulting in the formation of a mesh-like hydrated fibrous network (Fig. 1D). While the hydrogen bonding allows for the gel to maintain its stability, the doping of PANI creates electron hopping along the backbone of the PANI nanofibers to impart electronic conductivity.
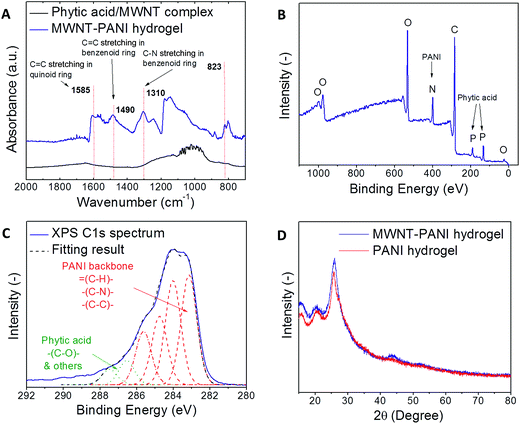
The formation of PANI was confirmed by Fourier transform infrared (FTIR) measurements (Fig. 2A); the spectrum of the MWNT–PANI hydrogel has three peaks at 1310 cm−1, 1490 cm−1 and 1585 cm−1, which are attributed to C–N stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the benzenoid ring, and C
C stretching in the benzenoid ring, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the quinoid ring, respectively.3,10 Furthermore, the nitrogen and phosphorous peaks in the X-ray photoelectron spectrometer (XPS) survey scan, shown in Fig. 2B, indicate the presence of PANI in the emeraldine state doped by phytic acid. In addition, the different carbon bonds composing the PANI backbone can be identified in the XPS C1s scan (Fig. 2C). These include C–N, C–C, and
C stretching in the quinoid ring, respectively.3,10 Furthermore, the nitrogen and phosphorous peaks in the X-ray photoelectron spectrometer (XPS) survey scan, shown in Fig. 2B, indicate the presence of PANI in the emeraldine state doped by phytic acid. In addition, the different carbon bonds composing the PANI backbone can be identified in the XPS C1s scan (Fig. 2C). These include C–N, C–C, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– moieties originated from the backbone of PANI. Carbon–oxygen groups and other carbon chemistries at higher binding energies belong to the phytic acid. The X-ray diffraction (XRD) patterns of PANI-only and MWNT–PANI thin films are shown in Fig. 2D to distinguish the solid-state crystalline structure. The XRD patterns of PANI-only and MWNT–PANI samples both show three distinct peaks at 2θ = 15°, 22°, and 26°. The peaks in XRD patterns indicate that there is some degrees of molecular arrangement in the polymer chain, which can enhance the electronic conductivity depend on the level of doping. The peak at 22° is due to the periodicity of the polymer chains where the PANI chains are parallel to each other, and the peak at 26° is attributed to the periodicity of the polymer chains, where the chains are arranged in a perpendicular manner. It is also worth noting that the XRD pattern of MWNT–PANI sample reveals the presence of two peaks around 26° (overlapped with the peak from PANI) and 43° corresponding to the interlayer spacing of the nanotube (d002) and the d100 reflection of the carbon atoms, respectively. The nearly identical XRD patterns between the PANI and MWNT–PANI indicate that the crystalline structures of PANI coating in both samples are similar.
CH– moieties originated from the backbone of PANI. Carbon–oxygen groups and other carbon chemistries at higher binding energies belong to the phytic acid. The X-ray diffraction (XRD) patterns of PANI-only and MWNT–PANI thin films are shown in Fig. 2D to distinguish the solid-state crystalline structure. The XRD patterns of PANI-only and MWNT–PANI samples both show three distinct peaks at 2θ = 15°, 22°, and 26°. The peaks in XRD patterns indicate that there is some degrees of molecular arrangement in the polymer chain, which can enhance the electronic conductivity depend on the level of doping. The peak at 22° is due to the periodicity of the polymer chains where the PANI chains are parallel to each other, and the peak at 26° is attributed to the periodicity of the polymer chains, where the chains are arranged in a perpendicular manner. It is also worth noting that the XRD pattern of MWNT–PANI sample reveals the presence of two peaks around 26° (overlapped with the peak from PANI) and 43° corresponding to the interlayer spacing of the nanotube (d002) and the d100 reflection of the carbon atoms, respectively. The nearly identical XRD patterns between the PANI and MWNT–PANI indicate that the crystalline structures of PANI coating in both samples are similar.
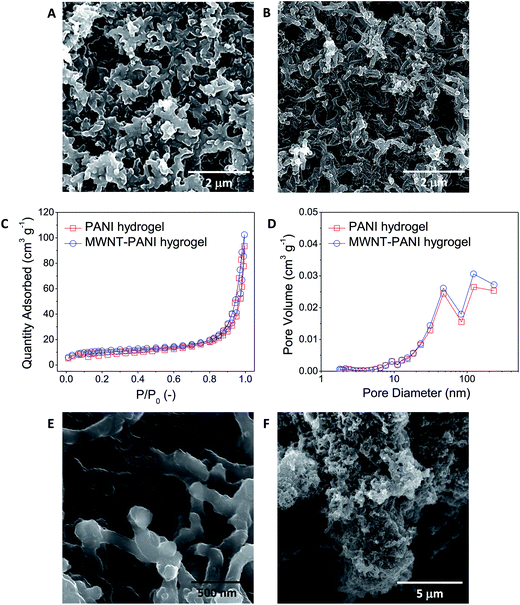
The morphology of thin films are examined by scanning electron microscope (SEM), and the images are shown in Fig. 3A and B, respectively. Fig. 3A and S1A–C† demonstrate that the PANI-only hydrogels generate a uniform mesoporous network composed of interconnected PANI NWs, and the average dimensions of the PANI-only NWs are 100–200 nm in diameter and 500–700 nm in length. During the polymerization process of MWNT–PANI hydrogels, one-dimensional (1D) MWNT walls act as nucleation sites for the growth of the PANI chains, which contribute to the facile synthesis of core–shell nanostructures of MWNT and PANI. Compared to PANI-only hydrogels, the MWNT–PANI hydrogels exhibit more interconnected networks composed of NWs with higher aspect ratio (Fig. 3B, 100–200 nm in diameter and 2–3 μm in length).
To investigate the specific surface area and pore size distribution of the PANI-based films, Brunauer–Emmett–Teller (BET) surface area analysis and Barrett–Joyner–Halenda (BJH) pore size distribution analysis were conducted on both PANI-only and MWNT–PANI samples, and the corresponding results are shown in Fig. 3C and D, respectively. From nitrogen adsorption/desorption isotherms shown in Fig. 3C, it can be observed that the quantity of nitrogen adsorbed onto the surface of PANI and MWNT–PANI thin films is similar. The measured BET specific surface areas of PANI-only and MWNT–PANI samples are 35.1 m2 g−1 and 39.7 m2 g−1, respectively. Fig. 3D shows the pore size distribution plots calculated using the BJH equation from the desorption branch of the isotherms. The MWNT–PANI network exhibits similar distribution curves with slightly higher pore volume than the PANI-only sample. The nitrogen adsorption/desorption isotherms and pore size distribution measurements indicate that both PANI-only and MWNT–PANI samples have porous structures mainly composed of mesopores with an average pore diameter of around 80 nm. With interconnected mesopores, the liquid electrolyte can penetrate into the structure and maximize interfacial contact with the electrolyte, thereby increasing the mobility of redox couples and minimizing back-recombination. In addition, the porosities (Fig. S2A and B†) of PANI-only and MWNT–PANI thin films are estimated by analyzing SEM images (Fig. 3A and B) with ImageJ software. The overall porosities of PANI-only and MWNT–PANI thin films are 36.1% and 41.7%, respectively. With 10% larger specific surface area and smaller porosity in MWNT–PANI networks, the MWNT–PANI hydrogel provides more active sites that can interact with the electrolyte, leading to higher electrochemical activities. These porous and interconnected NW-based networks with well-developed mesopores (Fig. 3E) persist across the entire film (thickness > 20 μm) and provide directional pathways for efficient electron transport throughout the porous electrode (Fig. 3F). Such mesoporous architectures with interconnected pores maximize the interfacial contact with the electrolyte, thereby increasing the mobility of the redox couples and minimizing electron recombination.
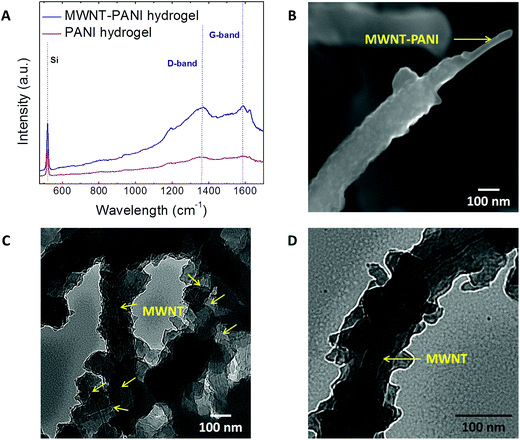
The presence of MWNTs is confirmed by Raman spectroscopy (Fig. 4A); the D-band peaks at ∼1350 cm−1 and G-band peaks at ∼1600 cm−1 from MWNTs are observed in the MWNT–PANI hydrogels. To further investigate the inner structure of the MWNT–PANI nanocomposites, the MWNT–PANI NW-based complexes were examined under SEM, and Fig. 4B clearly shows that MWNTs are being utilized as scaffolds to enable the templated polymerization of PANI to form core (MWNT)–shell (PANI) nanostructures. In addition, the MWNT–PANI nanostructures are observed via TEM (shown in Fig. 4C and D) and demonstrate that pristine MWNTs are embedded inside PANI NWs without severe aggregation of the MWNTs. The well-dispersed and embedded MWNTs are thus able to maximize the contact area with PANI coating.
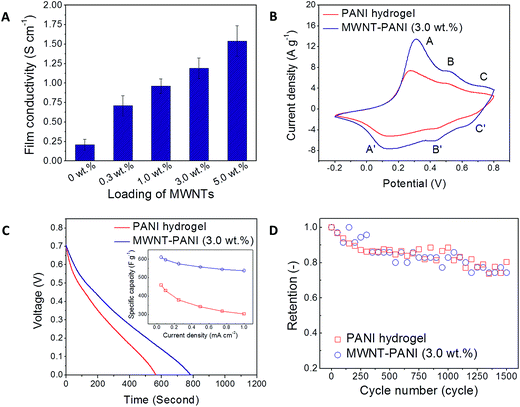
The incorporation of highly conductive MWNTs enhances the electrical conductivity of thin films, and the improvement correlates strongly with the loading of MWNTs in the nanocomposites (Fig. 5A). A remarkable improvement on the electrical conductivity from 0.21 to 1.54 S cm−1 was observed as the loading of MWNTs was increased from 0 to 5.0 wt%. It is noted that when the loading of MWNTs goes above 5.0 wt%, the doctor-bladed thin films begin to form large aggregates, and their surface becomes rougher, and the variation in conductivity increases (Fig. S3†). Therefore, the loading of MWNTs is not increased beyond 5.0 wt%, and here, the MWNT–PANI composite film with a 3.0 wt% loading of MWNTs is chosen as a reference film that displays high conductivity, smooth surface, and uniform morphology.
To evaluate the electrochemical behavior of both PANI-only and MWNT–PANI hydrogels, cyclic voltammetry (CV) measurements are performed in the potential range of −0.2 V to 0.8 V (vs. Ag/AgCl reference electrode) in 1.0 M sulfuric acid (H2SO4) electrolyte with Pt mesh as the counter electrode. In Fig. S4A and B,† both PANI-only and MWNT–PANI hydrogels remain stable for a wide range of scan rates from 5 to 100 mV s−1. In Fig. 5B, three characteristic pairs of redox peaks are evident for all of the PANI-based films, and are labeled as A/A′, B/B′ and C/C′. The A/A′ and C/C′ peaks are associated with the redox behavior of PANI as it is cycled through the leucoemeraldine and pernigraniline states; the weak B/B′ peaks are attributed to the double electron redox transition between the benzoquinone and hydroquinone through the protonation/deprotonation of PANI.15 The MWNT–PANI thin film (loading ∼ 3.0 wt%) exhibits higher current density than PANI alone (scan rate 10 mV s−1). Furthermore, the galvanostatic charging and discharging behaviors of both thin films are measured from 0 to 0.8 V at a discharging current of 0.1 mA cm−2 in a 1.0 M H2SO4 electrolyte (shown in Fig. 5C). The specific capacitance value, CS (F g−1), of the nanocomposites is estimated from the discharging data according to eqn (2) and the results are presented in the inset of Fig. 5C.
| CS = I × Δt/(m × ΔV) | (2) |
Experimental
Materials
50 wt% phytic acid aqueous solution, aniline, (NH4)2S2O8, and H2SO4 were purchased from Sigma-Aldrich. Fluorine-dope tin oxide (FTO) glass (TEC 15, thickness = 2.2 mm) was purchased from Pilkington. Pristine multi-walled carbon nanotubes (MWNTs, outer diameter ∼ 8–15 nm, length ∼ 10–50 μm) were purchased from CheapTube. All water was deionized (18.2 MΩ, mill-Q pore). All reagents were used as received and without further purification.Generation of PANI-based hydrogels and thin films
Solution A was prepared by mixing 1.842 mL (2 mmol) 50 wt% aqueous solution of phytic acid and 0.458 mL (5 mmol) aniline. The mixed solution were sonicated for half an hour and stirred overnight. Pristine MWNTs were first mixed with solution A at a certain weight fraction, and the solution, containing phytic acid, aniline, and MWNTs, was homogenized for 1 hour and ultrasonicated for 1 hour at 40% amplitude. The resulting solution was centrifuged at 3000 rpm. for 10 minutes to get rid of large aggregates and to obtain well-dispersed MWNT–aniline–phytic acid solution (solution B). 0.286 g (NH4)2S2O8 (1.25 mmol) was dissolved in 1 mL milli-Q water and 1 mL 50 wt% phytic acid aqueous solution (solution C). To synthesize the PANI-only hydrogels, solution A was mixed with solution C quickly without shaking; to synthesize the MWNT–PANI hydrogels, solution B was mixed with solution C. The solutions were held at 4 °C overnight to complete the reaction. After polymerization, the PANI-based hydrogels were dialyzed (dialysis tube, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MW cutoff) with milli-Q water and stored in room temperature. PANI-based thin films were fabricated by a doctor-blading process, and the thickness of the thin film was controlled by the tape thickness. The coated thin films were air-dried first and heated to 80 °C to enhance the adhesion between the PANI thin film and the FTO glass.
000 MW cutoff) with milli-Q water and stored in room temperature. PANI-based thin films were fabricated by a doctor-blading process, and the thickness of the thin film was controlled by the tape thickness. The coated thin films were air-dried first and heated to 80 °C to enhance the adhesion between the PANI thin film and the FTO glass.
Characterization of PANI-based thin films
Phytic acid–MWNT complex and MWNT–PANI samples were placed on transparent IR cards (International Crystal Laboratories, 19 mm KBr aperture IR card). A JASCO 4100 FTIR spectrometer was used for data collection. All spectra were obtained in absorbance mode with a resolution of 1 cm−1, using 100 accumulation scans. XRD patterns were collected (PANanalytical Multipurpose Diffractometer, Cu Kα radiation operated at 45 kV and 40 mA) using a step size of 0.02° with 6.0° per minute scan speeds under the following settings: 1° of anti-scatter slit, 6 mm of irradiated length of automatic mode and 0.04 rad of Soller slit. XPS (PHI Versa-Probe II) with a scanning monochromated Al source (1100 eV survey scan; 50 W; spot size, 200 μm) was used to quantify the surface composition of the thin films. Surface morphology and interior structure of the MWNT–PANI thin films were investigated using a SEM (Helios Nanolab 600 Dual Beam Focused Ion Beam System) operating at 10.0 kV for medium and high resolution imaging. TEM observations of the synthesized MWNT–PANI nanocomposites were performed using a JEOL 2010TEM with an accelerating voltage of 200 kV. TEM samples were prepared by directly dropping the solutions onto carbon supported copper or nickel grids. The surface chemistry of the PANI-only and MWNT–PANI thin films were analyzed using a Raman Spectrometer (Kaiser Optical System) operating with a 785 nm laser for excitation to confirm the existence of MWNTs. Electrical conductivities were measured for films deposited on glass slides by using a standard four-point probe configuration (Jandel General Purpose System). A series of 4 or 5 measurements were taken on each film, and the measurements were averaged to give the final reported value with the standard deviation as an error range. The thickness of the PANI-based thin films was determined using a Veeco Dektak 150 profilometer. The surface area of the materials was measured by the BET technique, using an ASAP 2020 BET and monitoring nitrogen gas adsorption and desorption.Electrochemical measurement of PANI-based thin films
A three-electrode cell was employed for the electrochemical measurements in the aqueous-cell test, where an Ag/AgCl electrode (BASi) (3 M NaCl) and a Pt wire were used as the reference and counter electrode, respectively. The PANI-only and MWNT–PANI thin films were used as the working electrodes. Cyclic voltammetry was performed between −0.2 and 0.8 V (vs. Ag/AgCl) at room temperature in 1.0 M H2SO4 solution using a potentiostat/galvanostat (Princeton Applied Research Model 263A). The weight of films deposited onto the substrate was measured by microbalance (Discovery microbalance in OHAUS).Conclusions
In conclusion, we have demonstrated a facile phytic acid-mediated synthesis technique to prepare a highly porous 3D core (MWNT)–shell (PANI) conductive hydrogels with enhanced electrical conductivity and electrochemical activity. Phytic acid serves as (1) an excellent solvent for non-functionalized dispersions of pristine MWNTs in aqueous solution and (2) an excellent dopant/gelator to crosslink PANI NWs into hydrogels. The affinity of phytic acid to MWNTs enables the formation of well-dispersed MWNT–phytic acid complexes. The polymerization of PANI onto the surface of MWNTs enables the synthesis of MWNT–PANI composite hydrogels, and the as-fabricated nanostructured thin films form mesoporous networks with high surface area and directional pathways for efficient electron transport. The incorporation of MWNTs leads to improved electrical conductivity from 0.21 to 1.54 S cm−1 as the loading of MWNTs increases to 5.0 wt%. By improving the electrical conductivity, a maximal specific capacity of 609 F g−1 is attained with 3.0 wt% loading of MWNTs in the nanocomposite. Compared to the MWNT–PANI nanocomposites reported in the literature (see Table S1†), the phytic acid-mediated hydrogel synthesis and assembly of NW-based electrodes not only demonstrate efficient electrochemical activities but open a low-cost route for electrode fabrication that can be translated to large-scale production for applications related to solar cells, pseudocapacitors, batteries, and sensors.Author contributions
P.Y.C., A.M.B. and P.T.H. conceived the idea and designed the experiments. P.Y.C. performed the synthesis, fabrication, characterization, and electrochemical testing. N.M.D.C. performed TEM, XPS and porosity characterizations. J.Q. performed XRD analysis. P.Y.C., N.M.D.C., M.N.H., A.M.B. and P.T.H. co-wrote the paper and all authors discussed the results and commented on the manuscript.Funding sources
This work was supported by Eni, S.p.A (Italy) through the MIT Energy Initiative Program, by NSF for the Center for Chemical Innovation under the NSF Center CHE-1305124, and by Institute for Collaborative Biotechnologies from the U.S. Army Research Office. P.Y.C. and N.M.D.C. acknowledge support from the MIT Energy Initiative Eni-MIT Energy Fellowship. M.N.H. is thankful for the support of a postdoctoral fellowship and N.M.D.C. is thankful for a postgraduate scholarship both from the Natural Sciences and Engineering Research Council (NSERC) of Canada.Acknowledgements
The authors wish to dedicate this paper to the memory of Officer Sean Collier, for his caring service to the MIT community and for his sacrifice.Notes and references
- M. N. Hyder, N. J. Shah and P. T. Hammond, in Multilayer Thin Films, Wiley-VCH, 2012, pp. 393–435 Search PubMed.
- Y. Lu, W. He, T. Cao, H. Guo, Y. Zhang, Q. Li, Z. Shao, Y. Cui and X. Zhang, Sci. Rep., 2014, 4, 5792 CAS.
- L. Pan, G. Yu, D. Zhai, H. R. Lee, W. Zhao, N. Liu, H. Wang, B. C. K. Tee, Y. Shi, Y. Cui and Z. Bao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef CAS PubMed.
- D. Zhai, B. Liu, Y. Shi, L. Pan, Y. Wang, W. Li, R. Zhang and G. Yu, ACS Nano, 2013, 7, 3540–3546 CrossRef CAS PubMed.
- Y. Zhao, B. Liu, L. Pan and G. Yu, Energy Environ. Sci., 2013, 6, 2856–2870 CAS.
- J.-W. Jeon, J. O'Neal, L. Shao and J. L. Lutkenhaus, ACS Appl. Mater. Interfaces, 2013, 5, 10127–10136 CAS.
- H.-W. Park, T. Kim, J. Huh, M. Kang, J. E. Lee and H. Yoon, ACS Nano, 2012, 6, 7624–7633 CrossRef CAS PubMed.
- J. Tarver and Y.-L. Loo, Thin Solid Films, 2013, 539, 303–308 CrossRef CAS PubMed.
- K. Wang, J. Huang and Z. Wei, J. Phys. Chem. C, 2010, 114, 8062–8067 CAS.
- P.-Y. Chen, M. N. Hyder, D. Mackanic, N.-M. D. Courchesne, J. Qi, M. T. Klug, A. M. Belcher and P. T. Hammond, Adv. Mater., 2014, 26, 5101–5107 CrossRef CAS PubMed.
- J. Hur, K. Im, S. W. Kim, U. J. Kim, J. Lee, S. Hwang, J. Song, S. Kim, S. Hwang and N. Park, J. Mater. Chem. A, 2013, 1, 14460–14466 CAS.
- N. A. Kumar, H.-J. Choi, Y. R. Shin, D. W. Chang, L. Dai and J.-B. Baek, ACS Nano, 2012, 6, 1715–1723 CrossRef CAS PubMed.
- M. N. Hyder, R. Kavian, Z. Sultana, K. Saetia, P.-Y. Chen, S. W. Lee, Y. Shao-Horn and P. T. Hammond, Chem. Mater., 2014, 26, 5310–5318 CrossRef CAS.
- M. N. Hyder, S. W. Lee, F. Cebeci, D. J. Schmidt, Y. Shao-Horn and P. T. Hammond, ACS Nano, 2011, 5, 8552–8561 CrossRef CAS PubMed.
- C.-M. Chang, C.-J. Weng, C.-M. Chien, T.-L. Chuang, T.-Y. Lee, J.-M. Yeh and Y. Wei, J. Mater. Chem. A, 2013, 1, 14719–14728 CAS.
- S. R. Sivakkumar, W. J. Kim, J.-A. Choi, D. R. MacFarlane, M. Forsyth and D.-W. Kim, J. Power Sources, 2007, 171, 1062–1068 CrossRef CAS PubMed.
- H. Wei, H. Gu, J. Guo, S. Wei and Z. Guo, J. Electrochem. Soc., 2013, 160, G3038–G3045 CrossRef CAS PubMed.
- H. Zhang, G. Cao, W. Wang, K. Yuan, B. Xu, W. Zhang, J. Cheng and Y. Yang, Electrochim. Acta, 2009, 54, 1153–1159 CrossRef CAS PubMed.
- G. Otrokhov, D. Pankratov, G. Shumakovich, M. Khlupova, Y. Zeifman, I. Vasil'eva, O. Morozova and A. Yaropolov, Electrochim. Acta, 2014, 123, 151–157 CrossRef CAS PubMed.
- S. Jo, H. Jeong, S. R. Bae and S. Jeon, Microchem. J., 2008, 88, 1–6 CrossRef CAS PubMed.
- G. Xiaoyu, M. Yun, Y. Pingping, W. Ying and Y. Haifeng, Mater. Res. Express, 2014, 1, 025403 CrossRef.
- J.-C. Chiang and A. G. MacDiarmid, Synth. Met., 1986, 13, 193–205 CrossRef CAS.
- I. Sapurina and J. Stejskal, Polym. Int., 2008, 57, 1295–1325 CrossRef CAS PubMed.
- J.-W. Jeon, R. Sharma, P. Meduri, B. W. Arey, H. T. Schaef, J. L. Lutkenhaus, J. P. Lemmon, P. K. Thallapally, M. I. Nandasiri, B. P. McGrail and S. K. Nune, ACS Appl. Mater. Interfaces, 2014, 6, 7214–7222 CAS.
- J. Yan, T. Wei, Z. Fan, W. Qian, M. Zhang, X. Shen and F. Wei, J. Power Sources, 2010, 195, 3041–3045 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Top-down and cross-sectional SEM images of the PANI-only thin film, illustration of the large aggregation of MWNTs in the doctor-bladed MWNT–PANI thin film (loading of MWNTs ∼ 10.0 wt%), CV curves of the PANI-only and MWNT–PANI (loading of MWNTs ∼ 3.0 wt%) thin films at scan rates ranging from 1 mV s−1 to 100 mV s−1, summary of the electrochemical parameters from this work and the literature. See DOI: 10.1039/c5ra02944a |
| This journal is © The Royal Society of Chemistry 2015 |