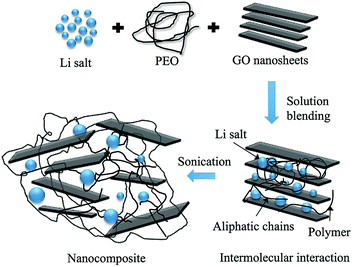
High performance solid polymer electrolyte with graphene oxide nanosheets†
Mengying Yuana,
Jeremy Erdmanab,
Changyu Tangc and
Haleh Ardebili*a
aMechanical Engineering Department, University of Houston, Houston, Texas 77004, USA. E-mail: hardebili@uh.edu
bTrinity University, One Trinity Place, San Antonio, Texas 78212, USA
cChengdu Green Energy and Green Manufacturing Technology R&D Center, 2nd Tengfei Rd. No. 355, Southwest Airport Economic Development Zone, Chengdu, 610207, China
First published on 31st October 2014
Abstract
Two dimensional graphene oxide (GO) sheets with high surface area and excellent mechanical properties are introduced into a solid polyethylene oxide/lithium salt electrolyte. Nearly two orders of magnitude improvement in ion conductivity and a 260% increase in the tensile strength of the polymer electrolyte are achieved with only 1 wt% GO content. GO fillers show improved thermo-mechanical stability for the polymer electrolyte and they appear to significantly enhance the performance of the Li ion battery.
Introduction
Conventional lithium-ion batteries containing organic liquid electrolytes are associated with inherent disadvantages of leakage, solid electrolyte interphase (SEI), dendrite growth, electrical shorting, thermal runaway, and in severe cases, catastrophic fire hazards.1–3 Solid polymer electrolytes (SPEs) have emerged as safer alternatives that offer significant benefits including higher thermal and electrochemical stability, suppression of dendrite growth, and thin film manufacturability4 leading to tantalizing applications like flexible and stretchable batteries. However, the low ion conductivity of solid polymer electrolytes, especially at room temperature remains a challenge. The incorporation of ceramic nanofillers (i.e. Al2O3, SiO2, γ-LiAlO2, and TiO2) into a polymer electrolyte has been shown to improve ion conductivity without compromising the mechanical properties, thus making polymer nanocomposite electrolytes (PNEs) very attractive for energy storage applications.5–15 The enhanced properties are generally attributed to the large surface-to-volume ratio, robust mechanical strength, specific surface chemistry, and interfacial effects of the nanofillers. For example, compared to their micro-sized counterparts, nano-sized alumina and silica with large surface densities are more efficient in improving the ionic conductivity of polyethylene oxide (PEO) electrolyte by suppressing the crystallization of PEO.16–18 Our previous work demonstrated that the nano-sized clay-CNT improved both the ionic conductivity and the mechanical strength of the polymer electrolyte.19Two dimensional, single-atomic-thickness graphene oxide (GO) sheets with their ultra-large surface area, and excellent mechanical and electrical insulating properties, can be promising filler candidates for improving the ionic conductivity and mechanical properties of polymer electrolytes.20–23 Few studies have investigated the incorporation of GO sheets in PEO host.24–26
In this study, we have fabricated solid PEO–LiClO4–GO polymer nanocomposite electrolyte (Scheme 1) by solution blending and evaporation casting and investigated the properties of the electrolyte. The resultant composite electrolyte with 1 wt% GO shows about two orders of magnitude enhancement in ion conductivity (∼10−5 S cm−1) compared to that of pure polymer electrolyte fabricated in our lab (∼10−7 S cm−1). The enhancement of ion conductivity with the addition of GO fillers can be mainly attributed to the reduced crystallinity and increase in polymer chain mobility as indicated by the lower Tg measurements of the GO filled polymer electrolyte, potential formation of GO ion transport channels, and increase in salt dissociation. Furthermore, the tensile strength of the polymer composite electrolyte was found to increase by 260% compared to that of pure polymer electrolyte which can be attributed to the superior mechanical properties of the GO sheets and the strong interaction between the GO and the surrounding PEO host. Moreover, GO fillers appear to improve the thermo-mechanical stability of polymer electrolyte and overall, the Li ion battery with GO filled polymer electrolyte shows enhanced performance.
Results and discussion
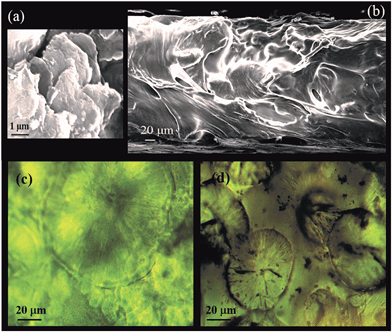
Scanning electron microscopy (SEM) was used to observe the morphology of GO nanosheets (Fig. 1a) and the PEO/GO composite electrolyte (Fig. 1b). Fig. 1a reveals the shape and size of the GO layers. The cross-sectional image of PEO electrolyte with 0.5 wt% GO content (Fig. 1b) shows a wave-like morphology and the GO sheets appear to be well integrated within the polymer matrix. With further increase in GO content (5 wt%) the GO nanosheets form aggregates in the matrix due to their large surface energy and close proximity.The 2-D GO sheet has a number of oxygenated functionalities including epoxy, hydroxyl, and carboxyl groups, which can exhibit good affinity for Li+ transport. As the GO content increases (1 wt%) the GO sheets can interconnect to form a network that can facilitate continuous ion conducting channels within the polymer composite electrolyte.27–30 Our experimental data shows about two orders of magnitude enhancement of ionic conductivity at ambient temperature with only 1 wt% GO fillers. In addition to the potential formation of GO ion conducting network, the large enhancement in ionic conductivity of the PEO/GO electrolyte can be attributed to (i) higher Li ion mobility associated with higher polymer segmental mobility due to free-volume expansion and reduced crystallinity of the polymer matrix and (ii) increase in mobile carrier concentration due to filler-induced dissociation of the Li salt.31
Polarized light microscopy (PLM) was used to examine the crystallinity of the PEO/GO electrolyte films. Spherical crystals (spherulites) can be seen in the PLM images of pure PEO electrolyte as shown in Fig. 1c indicating the semi-crystalline nature of PEO. Fig. 1d shows the PLM image of PEO/GO with 5% GO content. The addition of GO filler appears to decrease the size and number of the spherical crystals. The gradual reduction in the size of the PEO crystals can be further observed from the PLM images of the PEO composites (0.5%, 1%, 5%) provided in Fig. S1.†
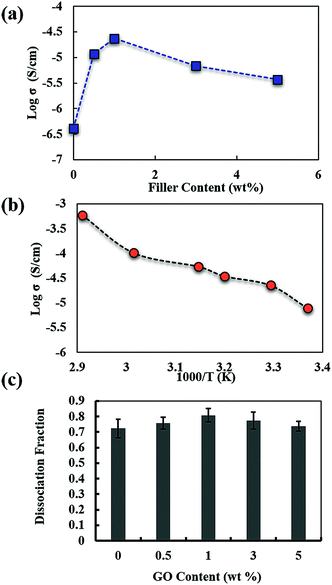
Fig. 2a shows the ionic conductivity of PEO electrolytes obtained from Nyquist plots (Fig. S2†) with various GO contents at room temperature. The ionic conductivity of the PEO composite electrolyte is observed to increase with GO content, and reaches a maximum conductivity at 1 wt% GO content of about 2 × 10−5 S cm−1 showing close to two orders of magnitude conductivity enhancement relative to that of pure PEO electrolyte. As the GO content increases beyond 1%, the ionic conductivity of the composite electrolyte appears to decline. Fig. 2b depicts the temperature dependency of ionic conductivity of PEO with 1 wt% GO. To investigate the effectiveness of GO in the presence of small amount of plasticizer, the ion conductivities of pure and 1% GO filled PEO, both with 5% plasticizer (LiPF6 in ethylene carbonate (EC) + dimethyl carbonate (DMC) + diethyl carbonate (DEC)), were evaluated (Fig. S5†). The ion conductivity of the plasticized PEO/GO (1 × 10−4 S cm−1) shows two orders of magnitude improvement compared to that of unfilled plasticized PEO (4 × 10−6 S cm−1).
 | ||
| Fig. 2 (a) Ionic conductivity (σ) at room temperature (b) temperature dependence of ion conductivity (1 wt% GO content) and (c) Li salt dissociation fractions of polymer electrolyte films. | ||
The ionic conductivity of an electrolyte is related to the number of the charge carriers (ni), ionic charge (zi), and ion mobility (μi) in the electrolyte expressed as follows:32
| σ = Σniziμi | (1) |
In the polymer electrolyte, ni corresponds to the concentration of free ions involved in the ionic transport and μi (ion mobility) is strongly influenced by the polymer chain segmental mobility. Thus, it is necessary to analyse the mechanisms of the ionic conductivity enhancement in the GO-filled PEO electrolyte with respect to the latter two factors, namely, the concentration of the free ions and the ion mobility.
Fig. 2c shows the fraction of free ClO4−1 calculated from the Fourier transform infrared (FTIR) spectra for pure PEO and its nanocomposites of various GO contents. The method of calculation of dissociation fraction is discussed in the Experimental section and the FTIR spectra are shown in Fig. S4.† It can be seen from Fig. 2c that the dissociated lithium salt ions in pure PEO count for about 70%. With the incorporation of GO sheets in the polymer electrolyte, the free ions increase to a maximum of 80% at 1 wt% GO content. This suggests that the GO sheets can effectively facilitate the dissociation of lithium salt by weakening the bond between the contact ion pairs, resulting in the increased charge carrier concentration. There are no obvious changes in the degree of lithium salt dissociation upon further increase in GO content beyond 1 wt%.
The mobility of the polymer chains was investigated by thermal analysis of solid PEO–LiClO4–GO using differential scanning calorimetry (DSC). The DSC curves are shown in Fig. S3.† The melting point (Tm) and the glass transition temperature (Tg) of the polymer electrolyte films are shown in Fig. 3a. The crystalline fraction (χc) shown in Fig. 3b is calculated by the following equation:33
| χc (%) = (ΔHm/H0m) × 100 | (2) |
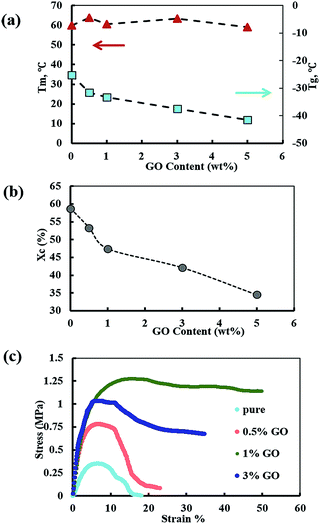 | ||
| Fig. 3 (a) Melting point (Tm) and glass transition temperature (Tg) (b) crystalline fraction (χc) and (c) stress–strain curves of polymer electrolyte films. | ||
Based on the DSC results, the melting temperature and crystallinity of the nanocomposite electrolyte decrease with the addition of GO nanosheets. This suggests that the crystallization of the PEO chains can be effectively disrupted by the presence of GO nanosheets with ultra-large surface area. In addition, the oxygenated functionalities on GO sheets also can play a key role in the formation of strong interaction between GO and PEO.35 The tertiary alcohols and epoxy (1,2-ethers) on the GO basal planes can interact with the PEO ether groups by forming hydrogen bonding.
Upon the addition of lithium salt, the Tg of pure PEO film increased from −60 to −20 °C. This phenomenon is referred to as the “salt effect” and is attributed to the formation of complexes between PEO and ClO4−1.7 Upon the addition of GO nanosheets, the Tg of the polymer electrolyte decreases. A minimum Tg of about −35 °C is reached at 5 wt% of GO content, which is much lower than the Tg at 1 wt% filled electrolyte. The compact molecular packing and crystallization of the solid PEO are greatly disturbed by the presence of GO sheets leading to the lower Tg values. In general, a low Tg indicates increase in free volume and larger amorphous regions in the polymer matrix and subsequent higher polymer chain mobility. Due to intimate relations between ion conductivity, polymer segmental mobility, free volume and Tg, a maximum ionic conductivity is expected to be realized at the lowest Tg which is at 5 wt% GO content in our polymer nanocomposite system. However, the maximum ionic conductivity of PEO electrolyte was observed at 1 wt% GO and beyond this content, the ion conductivity declines. This suggests adverse filler effects at higher GO contents that can counteract the influence of reduced Tg and the associated increase in polymer chain mobility. These adverse effects can consist of filler aggregation, diffusion tortuosity, and ion trapping.36
The stress–strain curves for the polymer electrolytes are presented in Fig. 3c. The 1 wt% GO electrolyte film shows an ultimate tensile strength of 1.27 MPa indicating more than 260% improvement in the tensile strength of the pure polymer electrolyte (0.35 MPa). Stress–strain measurements show a large enhancement of the Young's modulus and of the yield-point stress when passing from filler-free to nanocomposite polymer electrolytes. Here, the active nanocomposite particles serve as both filler and “tie molecules,” thus improving the adhesion between the polymer chains. The PEO/GO composite membranes at lower GO contents (0.5 and 1 wt%) exhibit excellent tensile strength and % elongation properties.
Fig. 4 shows the thermo-gravimetric analysis (TGA) of pure and GO filled polymer electrolyte where no apparent difference can be observed. Fig. 5 shows the thermal expansion of pure and 1% GO filled PEO. GO fillers seem to improve the thermo-mechanical stability of polymer electrolyte attributed to GO's strong “tie molecules”.
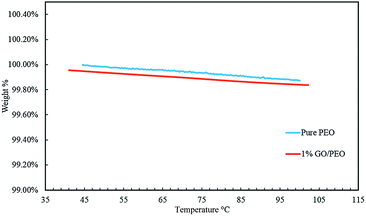 | ||
| Fig. 4 The thermogravimetric analysis (TGA) of pure PEO and GO filled PEO polymer electrolyte films. | ||
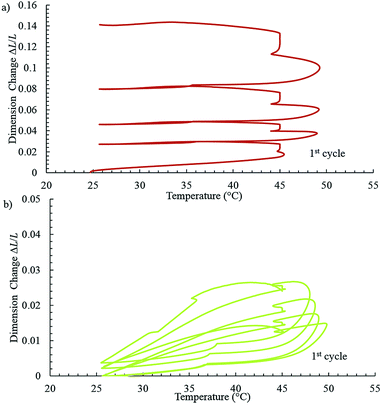 | ||
| Fig. 5 The thermo-mechanical analysis (TMA) of (a) pure PEO and (b) PEO/GO polymer electrolyte films. | ||
The Li ion coin cell battery performance with GO filled polymer electrolyte is presented in Fig. 6. The battery with pure solid polymer electrolyte shows poor capacity attributed mainly to the poor ionic conductivity of the electrolyte. The GO fillers in the electrolyte appear to noticeably improve the performance of the battery where the area capacity reaches 0.17 mA h cm−2. The reasonable value of the surface area capacity of the battery demonstrates the effectiveness of the PEO/GO polymer electrolyte for thin film batteries. The mass density of the cathode material is 0.012 g cm−2. Since the battery contains a commercial electrode that is more compatible with liquid than solid electrolyte, the mass capacity of the battery is relatively low. This capacity can be significantly improved by using a more suitable (thinner and lower density) electrode. The design and optimization of electrode material that is compatible with solid polymer electrolyte is beyond the scope of this study, and can be pursued in future studies for the effective utilization of the GO filled polymer electrolyte in thin film battery applications.
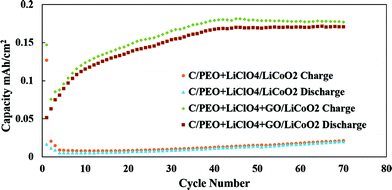 | ||
| Fig. 6 Capacity versus cycle number of C/PEO + LiClO4/LiCoO2 and C/PEO + LiClO4 + GO/LiCoO2 cells cycling. | ||
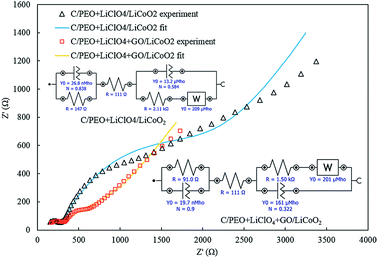
The impedance spectroscopy of the battery with pure and GO filled polymer electrolyte is shown in Fig. 7. The impedance spectroscopy is fitted with equivalent circuits as shown in the inset of Fig. 7 revealing that the GO fillers can lead to internal impedance improvements including enhancement in charge transfer and interfacial conductivity.
The redox activity of the GO filler was investigated using cyclic voltammetry (CV). The CV curves of both the pure PEO and PEO/1% GO electrolyte based coin cells with Li cobalt oxide as the working electrode versus the lithium metal as the reference electrode were obtained with a scan rate of 0.05 and 0.1 mV s−1, respectively (Fig. S6†). The redox peaks appear to be the same for both pure and filled PEO indicating the redox inactivity of GO in the PEO electrolyte. Furthermore, the electronic conductivity of the PEO/1% GO was evaluated using DC polarization method (Fig. S7 and Table S1†) measured to be about 5.6 × 10−8 S cm−1 demonstrating suitability as a battery electrolyte.
In summary, a novel solid polymer nanocomposite electrolyte with 2D graphene oxide nanosheets was fabricated using solution blending and evaporation casting. We demonstrated a significant enhancement, nearly two orders of magnitude, in ion conductivity with only 1 wt% GO. Furthermore, the electrolyte exhibits excellent mechanical properties with over 260% increase in tensile strength. Battery performance appears to be noticeably improved with GO filled polymer electrolyte. GO fillers also show to enhance the thermo-mechanical stability of the polymer electrolyte. Electrochemical, mechanical and thermal characterizations provided fundamental science insights into the mechanisms of enhancement of the polymer electrolyte properties. The novel PEO/GO electrolyte can be a promising electrolyte for next generation safer Li ion batteries and enable special applications such as flexible and stretchable batteries.
Experimental
Preparation of PEO/GO composite films
The Li salt powder (0.3 g, Sigma-Aldrich) and the PEO powder (2 g, Mw = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich) were dissolved in 30 mL acetonitrile in separate bottles. Graphene oxide (GO) powder with layer dimensions of about 0.5–5 microns in length and width and 1.1 ± 0.2 nm in thickness was purchased from Graphene Supermarket. Appropriate amounts of GO (0.5, 1, 3, 5, and 8 wt%) was added to the bottles and stirred for 6 hours. Then, the solution from both bottles with PEO, GO and Li salt were mixed together and stirred for another 3 hours to obtain the PEO/GO/Li salt solution. The mixed solution was sonicated using a Branson 3510 Sonicator for 25 min and the PEO/GO solutions were poured into different Teflon Petri dishes and kept in the oven at 50 °C for 24 h until the membranes were dry.
000, Sigma-Aldrich) were dissolved in 30 mL acetonitrile in separate bottles. Graphene oxide (GO) powder with layer dimensions of about 0.5–5 microns in length and width and 1.1 ± 0.2 nm in thickness was purchased from Graphene Supermarket. Appropriate amounts of GO (0.5, 1, 3, 5, and 8 wt%) was added to the bottles and stirred for 6 hours. Then, the solution from both bottles with PEO, GO and Li salt were mixed together and stirred for another 3 hours to obtain the PEO/GO/Li salt solution. The mixed solution was sonicated using a Branson 3510 Sonicator for 25 min and the PEO/GO solutions were poured into different Teflon Petri dishes and kept in the oven at 50 °C for 24 h until the membranes were dry.
Morphological characterization
The morphologies of the polymer/GO films were observed by scanning electron microscopy (SEM) using LEO 1525 under an acceleration voltage of 15 kV. The samples were coated with gold to increase the electrical conduction. Polarized light microscopy (PLM) was used to investigate the crystallinity of the PEO films carried out with Advanced EPI Trinocular Infinity Polarizing Microscope 50×-1600×.Ion conductivity characterization
The ion conductivities were determined using complex impedance method carried out by Metrohm Autolab. The samples were sandwiched between two stainless steel electrode discs and the complex impedance spectra were obtained with the frequency response analysis (FRA) module of the Autolab in the frequency range of 1 Hz to 1 MHz.Salt dissociation measurement
The Li salt dissociation fraction was obtained using the Fourier transform infrared (FTIR) Agilent Technologies Cary 630. The spectra were collected with MicroLab software and analyzed using Resolutions Pro. The dissociation fraction was obtained as the ratio of the areas under the peaks located in two specific ranges, 620–624 cm−1 range representing the dissociated “free” ClO4−1 ions and 630–635 cm−1 range representing the ion-pair LiClO4.37,38Mechanical testing
The tensile stress–strain measurements were carried out using the Dynamic Mechanical Analysis (DMA) Model Q800 from TA Instruments at room temperature. The preload force was 0.001 N and the tensile rate was 2 N min−1. The specimen dimensions were 18.00 mm in length and 13.00 mm in width.Thermal characterization
The thermal properties of PEO/GO electrolyte films were obtained using TA Instruments Q2000 Differential scanning calorimetry (DSC) under nitrogen atmosphere. The weight of the sample and that of the Tzero aluminum container pans and lids were taken before and after each thermal scan. The samples were heated to 200 °C, cooled to −90 °C and then heated to 200 °C again with heating and cooling rates of 10 °C min−1 and 5 °C min−1. The glass transition temperature was determined as the mid-point of the step transition from the second heating. The melting and crystallization temperatures (when observed) were defined as the maxima of the melting endotherms and crystallization exotherms, respectively.Thermogravimetric analysis (TGA)
Pure and 1% GO composite PEO samples were tested with the TA Instruments TGA in the temperature region of 35 to 100 °C. The temperature scanning rate was 10 °C min−1.Thermomechanical analysis (TMA)
Pure and 1% Go composite PEO were tested using the TA Instruments TMA to investigate their thermal stability and expansion. A probe force of 0.05 N was applied and the temperature was varied between 25 and 45. Five heat and cool cycles were conducted to assess thermal stability. The temperature scan rate was 3 °C min−1.Li ion battery assembly and testing
Coin cell batteries were assembled inside the glove box with graphite (anode), pure and composite solid PEO electrolyte and Li cobalt oxide (cathode). The anode, cathode and current collector materials were purchased from MTI. A drop of plasticizer (LiPF6 in ethylene carbonate (EC) + dimethyl carbonate (DMC) + diethyl carbonate (DEC)) was deposited on the surface of each electrode during assembly to enhance interfacial contact. The battery components were assembled layer by layer and sealed using a coin cell-crimping machine. The batteries were then subjected to multiple charge–discharge cycles and their capacities were measured using Arbin equipment. Impedance spectroscopy of the batteries was conducted using the frequency response analysis (FRA) module of the Autolab with frequency ranging from 1 Hz to 1 MHz.Electronic characterization of PEO/GO
The ionic (tion)/electronic (tele) transport numbers of the pure PEO and PEO/1% GO electrolytes were measured using DC polarization method.24,39,40 A constant voltage of 50 mV was applied across the electrolyte sandwiched between two blocking stainless-steel electrodes, and the polarization current as a function of time was monitored for 1 hour at RT. The electronic conductivity of the PEO/GO was calculated using the electronic transport number and the respective ionic conductivity obtained from impedance spectra.Redox characterization of PEO/GO
Cyclic voltammetry (CV) measurements were performed on both the pure and composite PEO films with 1% GO using Metrohm Autolab at RT. Coin cells were assembled with the pure and GO filled PEO films as electrolytes, Li cobalt oxide as the working electrode and lithium metal as the reference electrode. The voltage scanning rates used were 0.1 mV s−1 and 0.05 mV s−1 for PEO/GO and pure PEO electrolyte based cells, respectively.Acknowledgements
We acknowledge financial support from NSF CAREER (CMMI-1254477) and TcSUH and NSF (EEC 1261981).References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- W. H. Meyer, Adv. Mater., 1998, 10, 439 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303 CrossRef CAS.
- K. S. Ji, H. S. Moon, J. W. Kim and J. W. Park, J. Power Sources, 2003, 117, 124 CrossRef CAS.
- G. Jiang, S. Maeda, Y. Saito, S. Tanase and T. Sakai, J. Electrochem. Soc., 2005, 152, A767 CrossRef CAS PubMed.
- J. H. Ahn, G. X. Wang, H. K. Liu and S. X. Dou, J. Power Sources, 2003, 119, 422 CrossRef.
- P. P. Prosini, S. Passerini, R. Vellone and W. H. Smyrl, J. Power Sources, 1998, 75, 73 CrossRef CAS.
- G. B. Appetecchi, S. Scaccia and S. Passerini, J. Electrochem. Soc., 2000, 147, 4448 CrossRef CAS PubMed.
- M. C. Borghini, M. Mastragostino, S. Passerini and B. Scrosati, J. Electrochem. Soc., 1995, 142, 2118 CrossRef CAS PubMed.
- H. Y. Sun, H. J. Sohn, O. Yamamoto, Y. Takeda and N. Imanishi, J. Electrochem. Soc., 1999, 146, 1672 CrossRef CAS PubMed.
- H. T. Liao and C. S. Wu, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 4272 CrossRef CAS.
- P. D. Yang, D. Y. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature, 1998, 396, 152 CrossRef CAS PubMed.
- S. H. Chung, Y. Wang, L. Persi, F. Croce, S. G. Greenbaum, B. Scrosati and E. Plichta, J. Power Sources, 2001, 97–98, 644 CrossRef CAS.
- F. Croce and B. Scrosati, J. Power Sources, 1993, 43, 9 CrossRef CAS.
- F. Croce, F. Bonino, S. Panero and B. Scrosati, Philos. Mag. B, 1989, 59, 161 CAS.
- B. Kumar and L. G. Scanlon, J. Power Sources, 1994, 52, 261 CrossRef CAS.
- J. J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. X. Wei, ACS Nano, 2010, 4, 5019 CrossRef CAS PubMed.
- C. Tang, K. Hackenberg, Q. Fu, P. M. Ajayan and H. Ardebili, Nano Lett., 2012, 12, 1152 CrossRef CAS PubMed.
- N. A. Kotov, I. Dékány and J. H. Fendler, Adv. Mater., 1996, 8, 637 CrossRef CAS.
- T. Cassagneau and J. H. Fendler, Adv. Mater., 1998, 10, 877 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771 CrossRef CAS.
- J. T. Paci, T. Belytschko and G. C. Schatz, J. Phys. Chem. C, 2007, 111, 18099 CAS.
- J. Shim, D. Kim, H. J. Kim, J. H. Lee, J. Baik and J. Lee, J. Mater. Chem. A, 2014, 2, 13873 CAS.
- S. Gao, J. Zhong, G. Xue and B. Wang, J. Membr. Sci., 2014, 470, 316 CrossRef CAS PubMed.
- M. Akhtar, S. Kwon, F. J. Stadler and O. B. Yang, Nanoscale, 2013, 5, 5403 RSC.
- Y. Cao, C. Xu, X. Wu, X. Wang, L. Xing and K. Scott, J. Power Sources, 2011, 196, 8377 CrossRef CAS PubMed.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 2392 CrossRef CAS PubMed.
- D. Chen, L. Tang and J. Li, Chem. Soc. Rev., 2010, 39, 3157 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- Q. Li, E. Wood and H. Ardebili, Appl. Phys. Lett., 2013, 102, 243903 CrossRef PubMed.
- B. Scrosati, Applications of electroactive polymers, Chapman & Hall, London, 1993, p. 77 Search PubMed.
- Z. H. Li, H. P. Zhang, P. Zhang, G. C. Li, Y. P. Wu and X. D. Zhou, J. Membr. Sci., 2008, 322, 416 CrossRef CAS PubMed.
- X. Li and S. L. Hsu, J. Polym. Sci., Polym. Phys. Ed., 1984, 22, 1331 CrossRef CAS.
- F. Barroso-Bujans, F. Fernandez-Alonso, J. A. Pomposo, E. Enciso, J. L. G. Fierro and J. Colmenero, Carbon, 2012, 50, 5232 CrossRef CAS PubMed.
- F. Croce, G. B. Appetecchi, L. Persi and B. Scrosati, Nature, 1998, 394, 456 CrossRef CAS PubMed.
- A. Naji, J. Ghanbaja, B. Humbert, P. Willmann and D. Billaud, J. Power Sources, 1996, 63, 33 CrossRef CAS.
- Y. T. Chen, Y. C. Chuang, J. H. Su, H. C. Yu and Y. W. Chen-Yang, J. Power Sources, 2011, 196, 2802 CrossRef CAS PubMed.
- D. Pérez-Coll and G. C. Mather, Solid State Ionics, 2010, 181, 20 CrossRef PubMed.
- S. R. Mohapatra, A. K. Thakur and R. N. P. Choudhary, J. Power Sources, 2009, 191, 601 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Polarized light microscopy (PLM), complex impedance spectra, differential scanning calorimetry (DSC), fourier transform infrared (FTIR) spectra, cyclic voltammetry (CV), polarization current curves, ionic/electronic transport numbers and electronic conductivities table. See DOI: 10.1039/c4ra07919a |
| This journal is © The Royal Society of Chemistry 2014 |