Open Access Article
Open Access ArticleChiral induction in the crystallization of KIO3 and LiIO3: the role of amino acids in controlling the chirality of inorganic crystals†
Matan Oliel * and
Yitzhak Mastai
* and
Yitzhak Mastai
Department of Chemistry, Institute for Nanotechnology and Advanced Materials, Bar-Ilan University, Ramat-Gan 5290002, Israel. E-mail: Yitzhak.Mastai@biu.ac.il; matanoliel5@gmail.com
First published on 5th March 2025
Abstract
Chiral induction in crystals has attracted significant attention due to its implications for developing chiral materials and understanding mechanisms of symmetry-breaking enantioselective crystallization of naturally occurring chiral minerals. Despite its potential use in chiral discrimination, this area remains largely unexplored. Here, we investigate chiral induction during crystallization of naturally occurring chiral KIO3 and LiIO3 minerals using arginine and alanine as chiral inducers. The chiral nature of the crystallization and the effect of the chiral inducers were examined using circular dichroism, polarimetry, and low-frequency Raman spectroscopy. The impact of chiral molecules on the rate and final crystal structure was studied by electron microscopy including SEM and TEM. We demonstrate that it is possible to control the chirality with chiral exogenous molecules, mainly amino acids. Understanding chiral induction in crystal growth may open avenues for controlled assembly of chiral materials and development of novel functional materials with unique properties.
Introduction
Chirality is often associated with organic molecules; however, many inorganic compounds also exhibit chirality in their crystal structures. This chirality in inorganic crystals generally results from a chiral arrangement of non-centrosymmetric metal chelates or oxides. For instance, quartz (SiO2) demonstrates chirality in its α polymorph due to the chiral arrangement of SiO2 units.Studies on the chirality of chiral inorganic crystals are surprisingly limited.1 The surface of chiral inorganic crystals holds significant promise for various applications including chiral discrimination,2–5 chiral sensing6 and enantioselective catalysis.7,8 Understanding how surface chirality is influenced by chiral induction and controlling chirality in these materials is crucial, as chiral selectivity occurs primarily on the material surface.9
In recent years, chirality has been found to play an important role in nanotechnology.10–12 Chirality plays a useful role in many nano-systems, such as chiroptical molecular switches,13–15 molecular motors, chiral nanosurfaces16–26 and chiral nanoparticles.27–39 Overall, the areas of chiral nanoscience and nanotechnology show exceptional promise for further developments in areas such as catalysts, bio-recognition and chiral separation processes.40
One good example of chiral surfaces is chiral selectivity on metal surfaces that are well-established, in particular with cubic closed-packed (CCP) metals.41 Crystal planes such as Cu (6,4,3) and Au (3,2,1) exhibit chiral properties despite the lack of chirality of bulk metals. These surface features yield exceptional enantiomeric excess (e.e) during adsorption of small chiral molecules.4,42
In recent decades, remarkable strides have been made in exploring the nature of inorganic chiral surfaces and systems. Pioneering research led by the Gellman group unveiled the chirality of high-Miller-index planes in metallic surfaces, revealing their enantiospecific adsorption in the presence of chiral molecular probes.6,17 Research led by Hazen and collaborators emphasized the significance of chiral inorganic crystal surfaces, in particular in mineral-catalyzed organic synthesis and the interactions between biomolecules and mineral surfaces.1,43,44 In a series of publications, Hazen et al. carefully documented numerous common rock-forming minerals, predominantly chiral oxides and silicates, which exhibit chiral crystal surfaces, showcasing the chiral selection of amino acids on these surfaces.45,46
However, naturally occurring chiral minerals including quartz (SiO2),47 monohydrocalcite (CaCO3·H2O),48 wulfingite (ε-Zn(OH)2)49 and α-HgS50,51 have received little attention. Some chiral minerals are believed to contribute to the development of homochirality in biological molecules through an extensive process of chiral discrimination.52,53 Studying the influence of chiral amino acids on the formation of chiral minerals and replicating the natural conditions in which this occurs provides valuable insights into these processes. This research can also elucidate mechanisms that control chiral biomolecules. While some chiral minerals form the chiral phase in a straightforward manner, the crystallization of certain chiral crystals is more complex. For example, berlinite, the quartz-like chiral form of AlPO4, requires high annealing temperatures to transform into its chiral phase.54,55 These extreme conditions present challenges for analysis of chiral induction.
To address this issue, we sought chiral inorganic systems that crystallize under relatively simple conditions, mainly at low temperature and in aqueous solutions. Potassium iodate (KIO3)56,57 and lithium iodate (LiIO3)58,59 are perfect examples. KIO3 and LiIO3 are naturally occurring chiral minerals with space groups of P1 and P6322, respectively, which are easy to crystallize, and their chiral phases are stable at room temperature (RT). As water-soluble salts, their chirality and chiral induction have apparently not been investigated.
Here, we present our research on the control of chirality of KIO3 and LiIO3 crystals by chiral induction with amino acids. The crystal morphology and structural changes were confirmed by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and high-resolution scanning electron microscopy (HR-SEM). Moreover, the chirality was analyzed by circular dichroism (CD) spectroscopy of crystal pellets and using an optical polarizer for low-frequency Raman (LFR). The enantioselectivity and chiral discrimination were proved by CD and polarimetry by performing chiral adsorption experiments.
Experimental methods
Materials
The materials used in this research are detailed in the ESI (S1).†Preparation of chiral-induced crystals
Chiral-induced KIO3 crystals were synthesized by recrystallization using a slow cooling method. Supersaturated solutions in double distilled water (DDW) were prepared, heated to 50 °C and stirred until complete dissolution. After dissolution, 10% (moles/to KIO3 moles) of L/D-Arg were added and the solution was stirred for 15 min and spontaneously cooled to RT by turning off the hot plate and keeping the solution on it to allow as slow a cooling process as possible and then the solution was refrigerated overnight. The white crystals were filtered in a vacuum, washed several times with ethanol to remove Arg traces and dried at RT.The chiral-induced LiIO3 crystals were synthesized by a slow evaporation method using the following reaction:
| Li2CO3 + 2HIO3 → 2LiIO3 + H2O + CO2 |
In a typical case, 0.5 g of lithium carbonate (Li2CO3) and 2.375 g of iodic acid (HIO3) were dissolved in 20 mL of DDW. The solutions were put in an oil bath and heated to 60 °C under stirring at pH 10 to allow the reaction to occur. The basic conditions were achieved by adding lithium hydroxide (LiOH). After dissolution, 60.6 mg of L/D-Ala (10% moles of Li2CO3) were added and the solution was covered by pierced aluminum foil to prevent contamination and allow evaporation. The stirring was stopped after 24 h and the heating was maintained at 60 °C in the oil bath to maintain constant temperature until complete evaporation. To remove residual Ala, clear white crystals were soaked for 24 hours in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeOH/DDW in a glass beaker. The cleaned crystals were filtered and dried in an oven at 50 °C.
1 MeOH/DDW in a glass beaker. The cleaned crystals were filtered and dried in an oven at 50 °C.
Characterization methods
The crystallographic structures of KIO3 and LiIO3 were determined by XRD using a Bruker AXS D8 Advance diffractometer with Cu Kα (λ = 1.5418 Å) operating at 40 kV/40 mA, in the range from 10 to 80°.FTIR was performed using a Thermo Scientific Nicolet iS10 spectrometer equipped with a Smart iTR attenuated total reflectance sampler with a single bounce diamond crystal. Data were collected in the 530–4000 cm−1 range at a spectral resolution of 4 cm−1 and analyzed using OMNIC software.
HR-SEM images were taken using a field-emission FEI (Helios 600) instrument. Samples were sputtered with gold.
KIO3 and LiIO3 crystals’ chirality measurements
Pellets (KBr, 300 mg, 5/10% L/D-induced Li/KIO3) were prepared (mixed and pressed under 10 ton for 3 min) and measured by CD in a designated holder at different orientations at RT with a Chirascan spectrometer (Applied Photophysics, UK).LFR was carried out on an inVia Raman microscope (Renishaw, UK) at RT under an optical microscope with a 50× objective. The samples were excited using a 514 nm laser source with a P-polarization (vertical) ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.5 mW, 40 s) and spectra were acquired using a polarizer with 1800 L mm−1 grating in the range of 100–1500 cm−1.
1 (0.5 mW, 40 s) and spectra were acquired using a polarizer with 1800 L mm−1 grating in the range of 100–1500 cm−1.
Chiral adsorption onto KIO3 and LiIO3 crystals
Tartaric acid (TA) was chosen due to its solubility in ethanol in which KIO3 and LiIO3 are insoluble. Chiral adsorption measurements were carried out with CD by preparing a 2.5 mM solution of D/L-TA or DL-TA in ethanol and adding L-Arg-induced KIO3 crystals or L/D-Ala-induced LiIO3 crystals in several concentrations. The crystals were kept suspended in solution overnight using a rotary suspension mixer and filtered out using a 0.2 mm filter. The filtered solutions were taken for CD measurement and compared to pure D/L-TA or DL-TA solutions.Chiral adsorption using a polarimeter was performed by preparing a 5 wt% solution of D/L-TA in ethanol and adding L-Arg-induced KIO3 or L-Ala-induced LiIO3 crystals in several concentrations. The crystals were kept suspended in solution overnight using a rotary suspension mixer and filtered out using a 0.2 mm filter. The filtered solutions were taken for polarimeter measurement and compared to pure D/L-TA solutions. The solutions were measured at RT using a Jasco P-2000 polarimeter.
The selectivity was calculated by measuring the polarization ratio of the enantiomer (adsorption peak maxima) with respect to the pure enantiomer.
For the selectivity, measurement by mass change was carried out by preparing a 20 mM of D/L-TA solution in ethanol and adding D-Ala-induced LiIO3 at a concentration of 32 mg mL−1. The crystals were kept suspended in solution overnight using a rotary suspension mixer and filtered out using a 0.2 mm filter. The filtered solutions were evaporated and the mass change calculated and compared to the mass of D/L-TA before the adsorption.
Results and discussion
Characterization
First, we studied the changes in the structure and morphology by XRD and FTIR following chiral induction using amino acids. In all samples, the main crystalline structure of KIO3 at RT was the stable triclinic phase with chiral space group P1 (COD ID: 4318187) and the main diffraction peak at 2θ = 28.2° corresponding to its (2, −2, 2) plane (Fig. 1I). The cell units a, b, and c are 8.923, 8.942, and 7.709 Å, respectively, and α, β, and γ are 54.4, 125.3, and 90.6°, respectively. The unchanged diffractions indicate that the crystalline structure is maintained. LiIO3 displays a crystalline structure that fits the RT stable hexagonal phase with chiral space group P6322 (COD ID: 1529711). The key diffraction peak at 25.5° corresponds to the (1, 0, 1) plane (Fig. 1II). The unit cell dimensions were a = 5.478 Å, b = 5.478 Å, and c = 5.17 Å with α = β = 90° and γ = 120°. Similar to KIO3, the consistent results indicate that the structure was maintained. | ||
| Fig. 1 XRD of KIO3 (I) and LiIO3 crystals (II) – control (black), L-Arg-induced (red) and D-Arg-induced (blue) displaying the main diffraction planes of triclinic KIO3 and hexagonal LiIO3. | ||
We also characterized the crystals by FTIR to check for structural changes or traces of amino acid. Both pristine and chiral-induced KIO3 showed the same spectra and indicated two peaks, close to each other, with a broad peak at 722 cm−1 and a very small peak at 797 cm−1. Both peaks represent the vibration modes of the oxygen–iodine bonds (Fig. S2I†). Similarly, the LiIO3 crystals showed two peaks belonging to the O–I vibration modes and no difference between the pristine and chiral-induced crystals (Fig. S2II†). However, we noticed a redshift at 763 and 860 cm−1 probably due to the effect of the cation on the vibration mode energies in the lattice. Moreover, the control LiIO3 sample also has a broad absorbance peak between 1400, and 1700 cm−1 associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O indicating some unreacted Li2CO3.
O indicating some unreacted Li2CO3.
Morphology and surface analysis
The morphology was examined by HR-SEM. The control KIO3 crystals exhibit a square shape and smooth surface (Fig. 2I and IV); however, with the amino acid the morphology and surface change. The L-Arg-induced KIO3 crystals maintain a square shape albeit with a rough surface (Fig. 2II and V), while the D-Arg-induced KIO3 crystals are hexagonal with a smooth surface similar to the control (Fig. 2III and VI). | ||
| Fig. 2 HR-SEM images of KIO3 crystals – control (I, IV/10, 20 μm), L-Arg-induced KIO3 (II, V/10, 50 μm) and D-Arg-induced KIO3 (III, VI/30, 50 μm). | ||
Fig. 3 presents HR-SEM images of LiIO3 crystals. Pristine LiIO3 shows an uneven morphology and very rough surface (I and IV). In contrast, L-Ala-induced LiIO3 crystals have a more hexagonal shape with a smoother surface (II and V). D-Ala-induced LiIO3 crystals have an uneven morphology similar to pristine LiIO3 with a rougher surface, which is smoother than the surface of pristine LiIO3 (III and VI).
 | ||
| Fig. 3 HR-SEM images of LiIO3 crystals – control (I, IV/10, 200 μm), L-Ala-induced LiIO3 (II, V/20, 100 μm) and D-Ala-induced LiIO3 (III, VI/50, 100 μm). | ||
Direct chirality measurements
To prove the chiral preference, we measured the crystals with CD and LFR. For CD measurement, L/D-Arg-induced KIO3 and L/D-Ala-induced LiIO3 pellets were prepared and measured at different angles. The CD measurement of the chiral-induced KIO3 crystals shows two chiral signal areas: a wide one from 525 to 275 nm, and a narrow one below 525 nm.Moreover, a positive signal is seen for the D-Arg-induced crystals and a negative one (anticlockwise direction polarization) for L-Arg-induced KIO3 (Fig. 4I). In contrast, LiO3 (Fig. 4II) presents opposite signals – positive for L-Ala-induced LiIO3 and negative for D-Ala-induced LiIO3. LiIO3 crystals also display a wide chiral signal from 600 to 400 nm. Both CD spectra show the optical activity of the iodate crystals proving their chiral preference.
While this measure does not provide absolute chirality, the relative purity can be obtained by the most frequent polarizations. The polarization of L-Ala-induced LiIO3 is +4 mdeg vs. −6 mdeg for D-Ala-induced LiIO3, thus the latter crystals are 1.5 times purer. L-Arg-induced KIO3 is 1.2 times purer than the D-Arg-induced crystals (−15/+12.5 mdeg for L/D-Arg-induced KIO3).
The chirality was also measured by low frequency Raman spectroscopy (LFR),60 a new technique that allows direct measurement of the chirality employing the low-frequency region of the spectrum, which highlights lattice-level interactions and global molecular fluctuations. These lower frequencies are associated with vibrations from weaker bonds and long-range interactions making them highly sensitive to changes in polarization. The enantiomers were excited using fixed polarized light and collected using a movable optical polarizer. The polarized light can be horizontal (S-Pol) or vertical (P-Sol). One enantiomer gives a stronger signal for S-Pol, while the other gives a stronger signal for P-Pol.
Aviv et al. presented a method that differentiates racemic and enantiopure crystals of amino acids. Intensity changes and wavenumber shifts were seen in single crystals of arginine, aspartic acid and valine.61 Nematsov et al. used LFR to analyze the chiral purity of crystals and showed specific vibrational mode changes as a function of the enantiomeric purity.62 Moreover, they investigated the dependence of the signal intensity on the orientation of L-Ala single crystals showing correlation between signal intensities with the proximity between the incident beam direction and the orientations of the intermolecular interactions.63 This makes LFR a powerful method to prove chiral preference by direct measurement.
As iodate salts, the Raman spectra of KIO3 and LiIO3 are quite similar (Fig. S3†) and can be divided into three areas: 700–800 cm−1 (iodate vibrational modes), 300–400 cm−1 (free translational motion of the iodate anion) and 100–200 cm−1 (lattice vibrational modes).
We used the LFR method to examine the effect of KIO3 and LiIO3 chiral-induced crystals on the collection of light through a polarizer at different angles (S-Pol and P-Pol), where the enantiomers of the crystal under test are supposed to give opposite effects to each other in intensities at same angles. Moreover, in order to prove the effect precisely, the comparison is of the angles for each crystal separately when the light is collected from the same point in the sample in order to neutralize as many factors as possible that could affect the measurements.
Fig. 5I presents the LFR of chiral-induced crystals. Higher intensity is evident for the L-Ala-induced KIO3 crystals when the light is collected at P-Pol with the maximum intensity ratio P-Pol/S-Pol of 1.225 at 303 cm−1. In contrast, D-Ala-induced LiIO3 crystals produce higher intensity when the light is collected at S-Pol with the maximum S-Pol/P-Pol intensity ratio at 329 cm−1 of 1.247 (Fig. 5II).
 | ||
| Fig. 5 LFR spectra of L-Arg-induced KIO3 (I) and D-Ala-induced LiIO3 (II). Black/red curves show signals excited using S/P-Pol. | ||
The LFR results support previous measurements. Iodate crystals with higher P-Pol intensity are more prone to be L type, while those with higher S-Pol intensity tend to be D type crystals. Similar results were obtained for D-Arg-induced KIO3 and L-Ala-induced LiIO3 (Fig. S4†). LFR signifies long-range interactions where orientation-dependent polarizable interactions prevail. Such interactions are highly responsive to the symmetry of the excitation cone induced by the polarizer and thus provide insight into the crystal chirality.
After proving the chiral preference of our crystals, we used chiral adsorption methods to check their stereoselectivity and chiral discrimination.
Chiral selectivity measurements
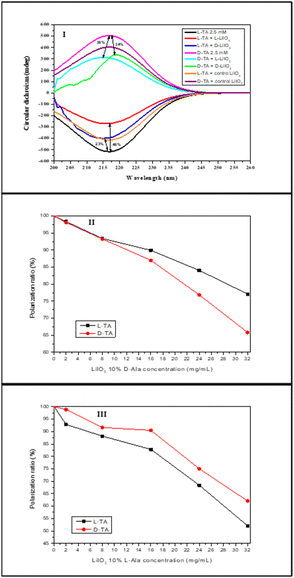
The adsorption of chiral molecules onto chiral surfaces is a standard way of measuring chirality in solids. Here, we measured the chiral induction in L-Arg-induced KIO3 and L/D-Ala-induced LiIO3 using CD and polarimetry. In the CD adsorption experiment, a 2.5 mM solution of pure L/D-TA was adsorbed at RT onto different powder concentrations. Fig. 6I presents the CD adsorption at 32 mg mL−1. The control group which contains the KIO3 without a chiral inducer showed adsorption of ∼5% for both L- and D-TA without any selectivity at all (green and cyan curves, respectively). However, the addition of L-Arg to the KIO3 crystals led to adsorption of 7% for L-TA (red curve) compared to pure L-TA (black curve) and better adsorption of (18%) onto D-TA (pink curve) compared to pure D-TA (blue curve). Fig. 6II shows the selectivity of both enantiomers to L-Arg-induced KIO3; we see better selectivity at all concentrations to D-TA (up to 11%). Moreover, the difference between the enantiomers increases with increasing concentrations of L-Arg-induced KIO3 crystals. | ||
| Fig. 6 Selective chiral adsorption of TA on chiral L-Arg-induced KIO3 crystals: CD spectra of L/D-TA adsorbed onto L-Arg-induced KIO3 (I) and selectivity of L/D-TA at different concentrations (II). | ||
Fig. 7I illustrates the CD adsorption at a concentration of 32 mg mL−1. Like KIO3, the control group of un-induced LiIO3 doesn't display discernible selectivity with an adsorption of about 20% for both L- and D-TA (orange and purple curves, respectively). L-Ala-induced LiIO3 demonstrates enhanced adsorption for L-TA (48%, red curve) compared to pure L-TA (black), while D-TA exhibits 38% absorption (cyan). Superior selectivity is seen at all concentrations, which increases with the crystal concentration up to 10% (Fig. 7III). D-Ala-induced LiIO3 on the other hand shows better adsorption for D-TA (34%, green curve) compared to pure D-TA (pink), while L-TA exhibits 23% absorption onto D-Ala-induced LiIO3 (blue). D-Ala-induced LiIO3 crystals show better selectivity for D-TA at all concentrations, with quite similar values up to 11% (Fig. 7II).
To verify that the results obtained indeed show selectivity of the crystals, two additional measurements were made: measuring the mass change of the TA enantiomer that did not adsorb onto the crystals after evaporation of the solvent, and measuring the circular dichroism of the racemic solution of TA in order to identify any chiral sign which points to selectivity of the crystals.
Table 1 shows the results for the mass changes of the TA enantiomer that did not adsorb onto the D-Ala-induced LiIO3 crystals after filtration of the crystals and evaporation of the solvent. We can see that the D-TA mass decreased more (25%) than the L-TA mass (15%) which means that the D-Ala-induced LiIO3 crystals are selective in favor of D-TA. Moreover, we notice that the selective percentages are similar to the selectivity we obtained from the CD measurements (11% and 10% respectively).
| Crystal | D-Ala-LiIO3 | |
|---|---|---|
| Tested enantiomer | L-TA | D-TA |
| Vial mass (mg) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683.5 683.5 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 753.1 753.1 |
| Total mass (mg) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693.7 693.7 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762.1 762.1 |
| Unadsorbed TA mass (mg) | 10.2 | 9 |
| Decrease from total mass (%) | 15 | 25 |
| Selectivity (%) | 10 in favor of D-TA | |
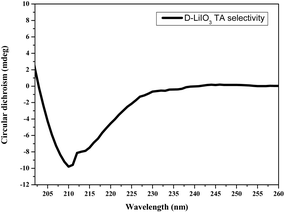
Fig. 8 presents the circular dichroism measurement of the racemic solution of TA that did not adsorb onto the D-Ala-induced LiIO3 crystals. The measurement shows negative signals of the solution that indicate higher presence for the L-TA enantiomer which means that the D-Ala-induced LiIO3 crystals are chiral selective in favor of D-TA.
We also examined the selective chiral adsorption on the crystal surfaces by using a polarimeter. The polarimeter adsorption experiment included pure L/D-TA 5 wt% solution that adsorbed onto L-Arg-induced KIO3 crystal powder concentrations (2, 4, and 8 mg mL−1) at RT. Fig. 9 presents the selectivity of both TA enantiomers to the L-Arg-induced KIO3 crystals, and like the CD measurements, we can see better selectivity for the D-TA enantiomer for all concentrations. The L-Arg-induced KIO3 shows a maximum adsorption of 7% for L-TA at the most concentrated KIO3 compared to D-TA at the same crystal concentration with a maximum adsorption of 24%, therefore, the maximum selectivity we got was 17%. Moreover, we saw by polarimeter that the selectivity improvement as function of L-Arg-induced KIO3 crystals’ concentration increase. From the results obtained it can be noted that there is a difference of 6% between the maximum selectivity of L-Arg-induced KIO3 we got from the CD measurements and that from the polarimeter. The difference can be explained by the lower sensitivity of the polarimeter which required more concentrated solutions for measurements that affect the amount of the TA adsorbed onto the L-Arg-induced KIO3.
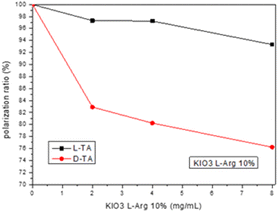 | ||
| Fig. 9 Chiral selective adsorption of TA on L-Arg-induced KIO3 crystals examined using a polarimeter. | ||
Chiral separation by crystallization64–67 is a well-established method whereby a chiral substance such as a chiral resolving agent or solvent is introduced in the crystallization of a racemic mixture. This method typically involves the chiral agent interacting differently with the crystals of each enantiomer during crystallization. In our case, the chiral separation is likely due to the interaction of amino acids with the ionic crystal surfaces in a chiral-specific manner. This interaction can influence the nucleation and growth processes by preferentially stabilizing one enantiomer over another. A kinetic study assessed the impact of amino acids on crystallization and chiral separation. Fig. 9 shows the results for the samples collected at various stages of crystallization.
The normal crystallization of KIO3 after 4 h shows crystals with a uniform rhombus shape and relatively uniform size of 4.5 μm (Fig. 10I). After 12 h, crystals of various sizes were obtained resembling a dodecahedron structure with 25 μm size (Fig. 10IV). The chiral-induced crystallization presents a different phenomenon: L-Arg-induced KIO3 shows an arrangement of square crystals on top of each other that resembles terraces (Fig. 10II and V), while D-Arg-induced KIO3 shows such an arrangement after 12 h (Fig. 10VI) and a trapezoid structure with an average size of 4 μm after 4 h (Fig. 10III).
It should be noted that we investigated the effect of amino acid concentration on both the crystallization process and chiral induction in the crystals. A series of crystallization experiments were conducted at varying amino acid concentrations, ranging from 1% to 10% by weight relative to the crystal-forming ions. It was found that at concentrations below 10%, no chiral induction was observed. However, starting at 10% (w/w) and above, chiral induction effects were detected and maintained. At higher amino acid concentrations, no further enhancement in chiral induction was observed.
Our results suggest that amino acids can promote the formation of mesocrystals68,69 by facilitating the organization of nanocrystals into ordered aggregates. Mesocrystals are crystalline materials consisting of nanometer-sized building blocks often referred to as “nanocrystals” arranged in a regular, repeating pattern. Unlike traditional crystals, composed of a continuous lattice of atoms or molecules, mesocrystals are made up of smaller, ordered nanocrystals that can be connected in various ways. Overall, it appears that during nucleation, amino acids preferentially bind to one chiral face of the crystal, influencing the nucleation rate, resulting in the observed chiral separation.
Conclusions
Chiral induction of the naturally occurring chiral iodate salt minerals KIO3 and LiIO3 was achieved using chiral alanine and arginine. The resultant crystals varied in morphology, size and surface roughness in contrast to the uniform crystallization obtained without chiral induction. The most prominent change was in the KIO3 crystals, which transformed from a square to hexagonal/deformed shape in D/L-Arg-induced KIO3. Both chiral-induced LiIO3 crystals exhibited smoother surfaces. L-Ala-induced LiIO3 retained a hexagonal shape, whereas D-Ala-induced LiIO3 displayed an uneven morphology.The effect of the chiral induction on KIO3 kinetics was investigated by crystallization at different times. The presence of the chiral inducing molecules was found to affect the arrangement of the KIO3 crystals by creating mesocrystals with a terrace arrangement.
The chiral preference of our crystals was confirmed by various direct measurements including CD of crystal pellets and LFR powder spectroscopy. Significantly, both methods were found to be applicable for direct measurement of the crystal chirality.
The enantioselectivity was also assessed by chiral adsorption measurements on the crystal surface using CD spectroscopy and polarimetry. While L-Arg-induced KIO3 crystals showed higher selectivity for D-TA, L-Ala-induced LiIO3 showed higher selectivity for L-TA. These findings indicate that L/D-induced crystals do not necessarily improve selectivity for L/D-enantiomers; better improvement may be obtained for the other enantiomers. It should be noted that while our study primarily focuses on chiral induction, we recognize that the reversibility of this process is a crucial factor in understanding the underlying mechanisms. Many chiral crystallization studies have shown that chiral induction using chiral molecules can, in some cases, be reversible under specific conditions, such as changes in pH, temperature, or solvent environment. However, in our experiments, once chirality was induced in the crystallized products, no spontaneous reversal was observed under the experimental conditions used.
Overall, this study provides insight into the chirality of iodate salt crystals within their chiral space group. Study of the chirality and chiral discrimination of naturally occurring chiral minerals such as KIO3 and LiIO3 is important for understanding the origin of biochemical homochirality and life. Integration of chiral amino acids in the crystallization of naturally occurring chiral minerals is anticipated to provide deeper insight and a more nuanced understanding of the intricate interactions between chiral molecules and crystal surfaces. This approach holds promise for elucidating mechanisms underlying chiral discrimination in inorganic crystals as a holistic process. Utilizing chiral recognition on inorganic surfaces offers advantages across various applications, from asymmetric autocatalysis to chiral sensing and optical functionalities.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Experimental data, including raw spectra and additional supplementary information, are included in the manuscript and its ESI.† Data not included in the manuscript are available upon request, and all relevant materials will be made accessible to qualified researchers for non-commercial purposes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MO acknowledges the Institute for Nanotechnology and Advanced Materials at Bar-Ilan University for his Bar-Ilan President's PhD Scholarship.Notes and references
- D. Aquilano, F. Otálora, L. Pastero and J. M. García-Ruiz, Prog. Cryst. Growth Charact. Mater., 2016, 62(2), 227–251 CrossRef CAS.
- W. Xu, M. Cheng, S. Zhang, Q. Wu, Z. Liu, M. K. Dhinakaran, F. Liang, E. G. Kovaleva and H. Li, Chem. Commun., 2021, 57(61), 7480–7492 RSC.
- N. Nandi and D. Vollhardt, Curr. Opin. Colloid Interface Sci., 2008, 13(1–2), 40–46 CrossRef CAS.
- D. S. Sholl, A. Asthagiri and T. D. Power, J. Phys. Chem. B, 2001, 105(21), 4771–4782 CrossRef CAS.
- J. I. Putman and D. W. Armstrong, Chirality, 2022, 34(10), 1338–1354 CrossRef CAS PubMed.
- J. D. Horvath and A. J. Gellman, J. Am. Chem. Soc., 2002, 124(10), 2384–2392 CrossRef CAS PubMed.
- C. E. Song and S. G. Lee, Chem. Rev., 2002, 102(10), 3495–3524 CrossRef CAS PubMed.
- A. Matsumoto, Y. Kaimori, M. Uchida, H. Omori, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2017, 56(2), 545–548 CrossRef CAS.
- T. Cao, Y. Li, L. Tian, H. Liang and K. Qin, ACS Appl. Nano Mater., 2018, 1(2), 759–767 CrossRef CAS.
- D. B. Amabilino, Chem. Soc. Rev., 2009, 38, 669–670 RSC.
- D. S. Bag, T. C. Shami and K. U. B. Rao, Sci. J., 2008, 58, 626–635 Search PubMed.
- J. Zhang, M. T. Albelda, Y. Liu and J. W. Canary, Chirality, 2005, 17, 404–418 CrossRef CAS PubMed.
- B. L. Feringa, N. P. M. Huck and A. M. Schoevaars, Adv. Mater., 1996, 8, 681–684 CrossRef CAS.
- B. L. Feringa, R. A. van Delden, N. Koumura and E. M. Geertsema, Chem. Rev., 2000, 100, 1789–1816 CrossRef CAS PubMed.
- B. L. Feringa, Acc. Chem. Res., 2001, 34, 504–513 CrossRef CAS PubMed.
- X. Y. Zhao, S. S. Perry, J. D. Horvath and A. J. Gellman, Surf. Sci., 2004, 563, 217–224 CrossRef CAS.
- C. F. McFadden, P. S. Cremer and A. J. Gellman, Langmuir, 1996, 12, 2483–2487 CrossRef CAS.
- K. H. Ernst, Curr. Opin. Colloid Interface Sci., 2008, 13, 54–59 CrossRef CAS.
- A. J. Gellman, ACS Nano, 2010, 4, 5–10 CrossRef CAS PubMed.
- A. Kuhnle, T. R. Linderoth and F. Besenbacher, Top. Catal., 2011, 54, 1384–1391 CrossRef.
- S. M. Barlow and R. Raval, Surf. Sci. Rep., 2003, 50, 201–341 CrossRef CAS.
- Y. Mastai, Chem. Soc. Rev., 2009, 38, 772–780 RSC.
- L. Thomsen, A. Tadich, D. P. Riley, B. C. C. Cowie and M. J. Gladys, J. Phys. Chem. C, 2012, 116, 9472–9480 CrossRef CAS.
- R. Oda, F. Artzner, M. Laguerre and I. Huc, J. Am. Chem. Soc., 2008, 130, 14705–14712 CrossRef CAS.
- A. E. Baber, A. J. Gellman, D. S. Sholl and E. C. H. Sykes, J. Phys. Chem. C, 2008, 112, 11086–11089 CrossRef CAS.
- C. Roth and K. H. Ernst, Top. Catal., 2011, 54, 1378–1383 CrossRef CAS.
- G. Shemer, O. Krichevski, G. Markovich, T. Molotsky, I. Lubitz and A. B. Kotlyar, J. Am. Chem. Soc., 2006, 128, 11006–11007 CrossRef CAS.
- C. Gautier and T. Burgi, J. Am. Chem. Soc., 2006, 128, 11079–11087 CrossRef CAS PubMed.
- C. Gautier and T. Burgi, J. Am. Chem. Soc., 2008, 130, 7077–7084 CrossRef CAS PubMed.
- O. Álvarez-Bermúdez, K. Landfester, K. A. I. Zhang and R. Muñoz-Espí, Macromol. Rapid Commun., 2024, 45, 2400615 CrossRef.
- L. C. Preiss, M. Wagner, Y. Mastai, K. Landfester and R. Muñoz-Espí, Macromol. Rapid Commun., 2016, 37, 1421–1426 CrossRef CAS PubMed.
- L. C. Preiss, L. Werber, V. Fischer, S. Hanif, K. Landfester, Y. Mastai and R. Muñoz-Espí, Adv. Mater., 2015, 27, 2728–2732 CrossRef CAS.
- P. Paik, Y. Mastai, I. Kityk, P. Rakus and A. Gedanken, J. Solid State Chem., 2012, 192, 127–131 CrossRef CAS.
- R. Oda, I. Hue, M. C. D. J. Schmutz, S. J. Candau and F. C. MacKintosh, Nature, 1999, 399, 566–569 CrossRef CAS.
- K. Sugiyasu, S. I. Tamaru, M. Takeuchi, D. Berthier, I. Hue, R. Oda and S. Shinkai, Chem. Commun., 2002, 11, 1212–1213 RSC.
- F. Freire, J. M. Seco, E. Quiñoá and R. Riguera, Angew. Chem., Int. Ed., 2011, 50, 11692–11696 CrossRef CAS PubMed.
- F. Freire, J. M. Seco, E. Quiñoá and R. Riguera, J. Am. Chem. Soc., 2012, 134, 19374–19383 CrossRef CAS PubMed.
- M. Lui, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef PubMed.
- L. Zhang, T. Wang, Z. Shen and M. Liu, Adv. Mater., 2016, 28, 1044–1059 CrossRef CAS.
- T. Cao, L. Mao, Y. Qiu, L. Lu, A. Banas, K. Banas, R. E. Simpson and H. C. Chui, Adv. Opt. Mater., 2019, 7(3), 1801172 CrossRef.
- J. Morales-Vidal, N. R. López and M. A. Ortuño, J. Phys. Chem. C, 2019, 123(22), 13758–13764 CrossRef CAS.
- Ž. Šljivančanin, K. V. Gothelf and B. Hammer, J. Am. Chem. Soc., 2002, 124(49), 14789–14794 CrossRef PubMed.
- A. Ben-Moshe, S. G. Wolf, M. B. Sadan, L. Houben, Z. Fan, A. O. Govorov and G. Markovich, Nat. Commun., 2014, 5(1), 1–9 Search PubMed.
- R. M. Hazen and D. A. Sverjensky, Cold Spring Harbor Perspect. Biol., 2010, 2(5), a002162 Search PubMed.
- W. Jiang, M. S. Pacella, D. Athanasiadou, V. Nelea, H. Vali, R. M. Hazen, J. J. Gray and M. D. McKee, Nat. Commun., 2017, 8(1), 1–13 CrossRef PubMed.
- R. M. Hazen and D. S. Sholl, Nat. Mater., 2003, 2(6), 367–374 CrossRef CAS PubMed.
- Y. Tanaka, T. Takeuchi, S. W. Lovesey, K. S. Knight, A. Chainani, Y. Takata, M. Oura, Y. Senba, H. Ohashi and S. Shin, Phys. Rev. Lett., 2008, 100(14), 145502 CrossRef PubMed.
- G. Otis, M. Nassir, M. Zutta, A. Saady, S. Ruthstein and Y. Mastai, Angew. Chem., 2020, 132(47), 21110–21115 CrossRef.
- P. Cintas, Angew. Chem., Int. Ed., 2002, 41(7), 1139–1145 CrossRef CAS.
- A. Ben-Moshe, S. G. Wolf, M. B. Sadan, L. Houben, Z. Fan, A. O. Govorov and G. Markovich, Nat. Commun., 2014, 5(1), 1–9 Search PubMed.
- R. M. Hazen, T. R. Filley and G. A. Goodfriend, Proc. Natl. Acad. Sci. U. S. A., 2001, 98(10), 5487–5490 CrossRef CAS.
- J. Jumas, A. Goiffon, B. Capelle, A. Zarka, J. Doukhan, J. Schwartzel, J. Detaint and E. Philippot, J. Cryst. Growth, 1987, 80(1), 133–148 CrossRef CAS.
- Y. Muraoka and K. Kihara, Phys. Chem. Miner., 1997, 24(4), 243–253 CrossRef CAS.
- P. Prado-Herrero, J. Garcia-Guinea, E. Crespo-Feo and V. Correcher, Phase Transitions, 2010, 83(6), 440–449 CrossRef CAS.
- L. Bayarjargal, L. Wiehl and A. Friedrich, et al, J. Phys.: Condens. Matter, 2012, 24(32), 1–11 CrossRef PubMed.
- H. Kasatani, S. Aoyagi, Y. Kuroiwa, K. Yagi, R. Katayama and H. Terauchi, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 199, 49–53 CrossRef CAS.
- L. Liu, R. Q. Wu, Z. H. Ni, Z. X. Shen and Y. P. Feng, J. Phys.: Conf. Ser., 2006, 28(1), 105–109 CrossRef CAS.
- R. Ashok Kumar, R. Ezhil Vizhi, N. Vijayan and D. Rajan Babu, Sch. Res. Lib., 2011, 2(4), 373–383 Search PubMed.
- A. Silambarasan, P. Rajesh and P. Ramasamy, et al, Bull. Mater. Sci., 2017, 40(4), 783–789 CrossRef CAS.
- V. Damle, H. Aviv and Y. R. Tischler, Anal. Chem., 2022, 94, 3188–3193 CrossRef CAS PubMed.
- H. Aviv, I. Nematsov, Y. Mastai and Y. R. Tischler, J. Phys. Chem. A, 2017, 121(41), 7882–7888 CrossRef CAS PubMed.
- I. Nematsov, Y. Mastai, Y. R. Tischler and H. Aviv, ChemPhysChem, 2018, 19(22), 3116–3121 CrossRef PubMed.
- I. Nematsov, H. Aviv, Y. Mastai and Y. R. Tischler, Crystals, 2019, 9(8), 425 CrossRef.
- I. Weissbuch, L. Addadi, M. Lahav and L. Leiserowitz, Science, 1991, 253(5020), 637–645 CrossRef CAS PubMed.
- G. Coquerel, Novel Optical Resolution Technologies, 2007, vol. 269, pp. 1–51 Search PubMed.
- H. Lorenz and A. Seidel-Morgenstern, Angew. Chem., 2014, 53(5), 1218–1250 CrossRef CAS PubMed.
- H. Cölfen and M. Antonietti, Angew. Chem., 2005, 44(35), 5576–5591 CrossRef PubMed.
- M. Niederberger and H. Coelfen, Phys. Chem. Chem. Phys., 2006, 8(28), 3271–3287 RSC.
- X. Xia, J. Tu, Y. Zhang, X. Wang, C. Gu, X. Zhao and H. J. Fan, ACS Nano, 2012, 6(6), 5531–5538 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, and FTIR spectra of L/D-Arg-induced KIO3 and L/D-Ala-induced LiIO3 crystals. See DOI: https://doi.org/10.1039/d4na01006j |
| This journal is © The Royal Society of Chemistry 2025 |