Organic–inorganic hybrid nanomaterials prepared via polymerization-induced self-assembly: recent developments and future opportunities
Bing
Niu
 a,
Ying
Chen
b,
Li
Zhang
ab and
Jianbo
Tan
a,
Ying
Chen
b,
Li
Zhang
ab and
Jianbo
Tan
 *ab
*ab
aDepartment of Polymeric Materials and Engineering, School of Materials and Energy, Guangdong University of Technology, Guangzhou 510006, China. E-mail: tanjianbo@gdut.edu.cn
bGuangdong Provincial Key Laboratory of Functional Soft Condensed Matter, Guangzhou 510006, China
First published on 13th April 2022
Abstract
Organic–inorganic hybrid nanomaterials are an important class of functional materials that find applications in many areas. Cooperative self-assembly of inorganic nanoparticles and block copolymers in solution is one of the most widely employed approaches for preparing organic–inorganic hybrid nanomaterials with precise structures and properties. However, this method usually encounters problems with low solids contents (<1% w/w) and multi-step processes, and is difficult to implement on a large scale. Over the past decade or so, the development of polymerization-induced self-assembly (PISA) has enabled the preparation of concentrated block copolymer nanomaterials (10–50% w/w solids) with a diverse set of morphologies. This review focuses on recent developments in the preparation of organic–inorganic hybrid nanomaterials via PISA including: (i) post-modification of block copolymer nanoparticles, (ii) in situ encapsulation of inorganic nanoparticles into vesicles, (iii) cooperative self-assembly of inorganic nanoparticles and polymers. By highlighting these important developments, the current challenges and future opportunities of organic–inorganic hybrid nanomaterials prepared via PISA are also provided.
1. Introduction
Nanoscience has made significant progress over the past twenty years, and researchers have been able to control the preparation of nanomaterials with various structures such as nanospheres, nano-mesoporous materials, nanowires, hollow nanotubes, core–shell structural nanomaterials and so on.1–10 Since the size of nanomaterials is close to the wavelength of light and the coherence length of electrons, materials at the nanoscale exhibit different properties compared to bulk materials.11 Hybrid nanomaterials are emerging multifunctional nanomaterials that usually integrate with two or more dissimilar materials,12,13 and typically they are inorganic components and organic components. Inorganic components usually include gold, silica, iron oxide, quantum dots, etc.,14 while organic components usually include ligands, biomolecules, polymers, etc.15,16The development of self-assembly techniques provides more opportunities for diverse applications of nanomaterials. Self-assembly usually refers to basic units (i.e., nanomaterials and block copolymers) that spontaneously form an ordered structure through the interaction of non-covalent bonds (i.e., hydrogen bonding, hydrophobic interactions, and π–π interactions).17–21 In particular, the cooperative self-assembly of polymers and inorganic nanoparticles in solution has proved to be one of the most efficient methods to prepare organic–inorganic hybrid nanomaterials with unique structures and properties.22–30 However, the cooperative self-assembly method is usually conducted in a highly dilute solution (<1% w/w) with multi-step processes, which is not beneficial for the large-scale preparation and application of organic–inorganic hybrid nanomaterials.
Over the past decade or so, the development of polymerization-induced self-assembly (PISA) has enabled the preparation of concentrated block copolymer nanomaterials (10–50% w/w solids) with a diverse set of morphologies including spheres, worms, vesicles, large-compound vesicles, nanotubes, etc.31–82 During PISA, the formation and in situ self-assembly of block copolymers occur at the same time, making the mechanism of PISA much more complicated than the traditional self-assembly method. A variety of polymerization techniques has been introduced into PISA including reversible addition–fragmentation chain transfer (RAFT) polymerization,31,35,83 atom transfer radical polymerization (ATRP),84,85 nitroxide mediated polymerization (NMP),86,87 ring-opening metathesis polymerization (ROMP),88,89 ring-opening polymerization (ROP),90,91 living anionic polymerization (LAP),92 and organotellurium-mediated radical polymerization (TERP).93 Numerous PISA reviews on various topics have been published over the past several years.94–115 Over the past ten years, PISA has been employed to prepare organic–inorganic hybrid nanomaterials at high solids contents, and it can overcome the problems of the traditional cooperative self-assembly method. In this review, we summarize the recent developments in the preparation of organic–inorganic hybrid nanomaterials based on PISA including the post-modification method via either in situ reduction or absorption, in situ encapsulation of inorganic nanoparticles into vesicles, and cooperative self-assembly of inorganic nanoparticles and polymers. Finally, the current challenges and future opportunities are also provided.
2. Post-modification method
In PISA, functional groups could be introduced to the surface and inner regions of block copolymer nano-objects using functional macro-CTAs and functional monomers, respectively.107 The presence of these functional groups can be used to anchor inorganic nanoparticles via either in situ reduction or absorption, allowing the large-scale preparation of organic–inorganic hybrid nanomaterials with a diverse set of morphologies. Table 1 summarizes organic–inorganic hybrid nanomaterials prepared by the post-modification method.| Entry | Block copolymer | Functional group | Inorganic component | Morphology | Ref. |
|---|---|---|---|---|---|
| S: Spheres, W: Worms, and V: Vesicles. | |||||
| 1 | POEGMA-PAEMA | β-Ketoester group | Ag | S, V | 116 |
| 2 | PHPMA-P(AEMA-co-IBOMA) | β-Ketoester group | Tb, Ho | V | 117 |
| 3 | PMAA-PBzMA | Carboxyl group | SiO2 | V | 118 |
| 4 | POEGMA-b-PMAA-b-PSt | Carboxyl group | Fe2O3 | W, V | 119 |
| 5 | PAA-PMMA | Carboxyl group | LaF3:Eu | S | 120 |
| 6 | PAA-PSt | Carboxyl group | Ag | V | 121 |
| 7 | POEGMA-PDMAEMA-PSt | Tertiary amine group | Au | S, W, V | 122 |
| 8 | PDMAEMA-PSt | Tertiary amine group | SiO2 | W | 123 |
| 9 | PDMAEMA-PSt | Tertiary amine group | Au, SiO2, TiO2 | S | 124 |
| 10 | PDMAEMA-PBzMA | Tertiary amine group | SiO2 | S | 125 |
| 11 | PGMA-P(HPMA-co-DMAEMA) | Tertiary amine group | SiO2 | V | 126 |
| 12 | PHPMA-PDEMA | Tertiary amine group | Au | S, W, V | 127 |
| 13 | mPEG-b-PDMAEMA-b-PHPMA | Tertiary amine group | Au, Pd | S | 128 |
| 14 | β-CD-POEGMA-PHPMA | β-CD | Au | S, W, V | 129 |
| 15 | (PCMS-g-P4VP)-b-PSt | Pyridine group | Au | S | 130 |
| 16 | mPEG-P(St-co-4VP) | Pyridine group | Ag | V | 131 |
| 17 | EDA/mPEG-PGMA | Amine group | Ag | V | 132 |
| 18 | Succinic acid/POEOMA-b-P(PHFEMA-co-GMA) | Carboxyl group | Fe3O4 | S | 133 |
| 19 | PMPS-PBzMA | Alkoxysilane | SiO2 | S, W, V | 134 |
| 20 | mPEG-PtBA | Trithiocarbonate group | Ag | V | 135 |
| 21 | Segmented PDMA-PSt | Trithiocarbonate group | Ag | V | 136 |
The An group116 prepared β-ketoester functional nanospheres and vesicles by thermally initiated RAFT dispersion polymerization of 2-(acetoacetoxy)ethyl methacrylate (AEMA) at high monomer concentrations (20–30%). Taking advantage of the unique structure of the β-ketoester group that can be used to achieve metal complexation, silver nanoparticles could be formed within the block copolymer nano-objects via in situ reduction (Fig. 1). Recently, block copolymer nano-objects with embedded β-ketoester groups have also been prepared by the photoinitiated RAFT dispersion polymerization of AEMA and isobornyl methacrylate (IBOMA) at low temperatures.117 The β-ketoester-functionalized block copolymer nano-objects were then cross-linked with a certain amount of ethylenediamine and the unreacted β-ketoester groups were further used to coordinate lanthanide ions in a good solvent (tetrahydrofuran in this case). Lanthanide-doped block copolymer vesicles with luminescence and magnetic properties were successfully prepared.
 | ||
| Fig. 1 (A and B) Organic–inorganic hybrid materials were prepared by in situ formation of Ag nanoparticles within POEGMA-PAEMA vesicles. (C) TEM image of the prepared Ag-loaded vesicles. Reproduced with permission from ref. 116. Copyright © 2014, American Chemical Society. | ||
Semsarilar et al.118 reported the efficient synthesis of block copolymer vesicles using a poly(methacrylic acid) (PMAA) macromolecular chain transfer agent (macro-CTA). After mixing alumina-coated silica nanoparticles (Ludox CL) with the anionic vesicles, the vesicles armored with different payloads of silica nanoparticles could be obtained. Karagoz et al.119 synthesized poly(oligoethylene glycol methacrylate)-b-poly(methacrylic acid)-b-polystyrene (POEGMA-b-PMAA-b-PSt) triblock copolymer nano-objects with worm-like and vesicular morphologies. Carboxyl groups on the surface of block copolymer nano-objects were used to complex an iron ion mixture. Iron oxide nanoparticles/nanocomposites exhibiting high transverse relativities were prepared by alkaline coprecipitation of FeII and FeIII salts (Fig. 2). Yu et al.120 prepared monodisperse poly(methyl methacrylate) (PMMA) microspheres by photoinitiated RAFT dispersion polymerization using poly(acrylic acid) (PAA) as the macro-CTA. Europium-doped lanthanum fluoride nanoparticles (LaF3:Eu) with photoluminescence properties have been employed to prepare various functional materials.137 LaF3:Eu/PMMA nanocomposites were then prepared by depositing LaF3:Eu nanoparticles onto PMMA microspheres. It was found that increasing the number of carboxyl groups on the surface promoted the formation of hollow LaF3:Eu/PMMA nanocomposites. After dissolving LaF3:Eu nanoparticles in HNO3, the hollow LaF3:Eu/PMMA nanocomposites transformed into solid microspheres with smooth surfaces again. Tan et al.121 reported a one-pot synthesis of PAA-PSt or PAA-P(St-co-AA) block copolymer nano-objects with various morphologies. Taking advantage of the interaction between Ag nanoparticles and the carboxyl group as well as the unique properties (i.e. catalytic and antibacterial properties) of Ag nanoparticles,138,139 the carboxyl-functional block copolymer nano-objects were further used as substrates to prepare Ag/polymer nanocomposites. The obtained Ag/polymer nanocomposites can be used to reduce 4-nitrophenol in the presence of NaBH4.
 | ||
| Fig. 2 Schematic illustration of the iron complexation and iron oxide formation in POEGMA-b-PMAA-b-PSt triblock copolymer vesicles. Reproduced with permission from ref. 119. Copyright © 2014, American Chemical Society. | ||
Au nanoparticles have broad applications in catalysis, biological imaging, and energy storage due to their good biocompatibility and high photothermal conversion efficiency.140–142 Bleach et al.122 synthesized poly(oligoethylene glycol methacrylate)-b-poly(2-(dimethylamino)ethyl methacrylate)-b-polystyrene (POEGMA-PDMAEMA-PSt) triblock copolymer nano-objects by RAFT dispersion polymerization with tertiary amine groups located on the corona. Au/polymer nanocomposites were prepared by the in situ reduction of HAuCl4 using NaBH4. The composition of Au nanoparticles in these Au/polymer nanocomposites could be further tuned by changing the concentration of chloroauric acid. Silica nanotubes have important applications in adsorption, catalysis, medicine and other fields due to their large specific surface areas, high aspect ratios and hollow structures. However, the large-scale preparation of silica nanotubes is challenging.143–146 Zhang et al.123 synthesized a series of PDMAEMA-PSt diblock copolymer nanowires by RAFT dispersion polymerization using a PDMAEMA macro-CTA. Then, the PDMEAMA corona on the surface of nanowires was used to catalyze the condensation polymerization of tetraethyl orthosilicate (TEOS), allowing the preparation of silica/polymer hybrid nanowires. After the calcination of these hybrid nanowires at 500 °C, silica nanotubes could be obtained (Fig. 3). Zhang et al.124 also employed PISA-made PDMAEMA-PSt block copolymer nanoparticles as templates to prepare various inorganic–organic hybrid nanoparticles. Recently, our group126 prepared CO2-responsive cross-linked vesicles by aqueous photoinitiated RAFT dispersion polymerization of 2-hydroxypropyl methacrylate (HPMA) and DMAEMA. Taking advantage of the interaction between silica nanoparticles and tertiary amine groups, hybrid vesicles were obtained by mixing silica nanoparticles with the vesicles. The payload of silica nanoparticles could be significantly increased after protonating the tertiary amine group via CO2 treatment.
 | ||
| Fig. 3 Schematic illustration of the synthesis of PDMAEMA-PSt block copolymer nanowires and their application in the preparation of silica/PDMAEMA-PSt hybrid nanowires and silica nanotubes. Reproduced with permission from ref. 123. Copyright © 2014, Royal Society of Chemistry. | ||
Huang et al.127 reported the synthesis of photocrosslinkable and amine-containing block copolymer nano-objects by the RAFT dispersion polymerization of 2-((3-(4-(diethylamino)phenyl)acryloyl)oxy)ethyl methacrylate (DEMA). The block copolymer nano-objects could be crosslinked via UV irradiation and further employed as templates for the in situ preparation of Au/polymer hybrid nanomaterials. Wan et al.128 synthesized mPEG-PDMAEMA-PHPMA triblock copolymer nano-objects with tertiary amine groups on the surface by ultrasound-initiated RAFT dispersion polymerization (Fig. 4). The obtained amine-functionalized block copolymer nano-objects were further employed as scaffolds for in situ reduction of metal ions (including Au and Pd ions) by radicals via sonolysis of H2O. The Au/polymer and Pd/polymer hybrid nanomaterials formed showed excellent catalytic properties on aerobic alcohol oxidation and the Suzuki–Miyaura cross-coupling reaction, respectively.
 | ||
| Fig. 4 Schematic illustration of the synthesis of mPEG-PDMAEMA-PHPMA triblock copolymer nano-objects by sono-RAFT PISA, and the preparation of Au and Pd nanocomposites by ultrasound. Reproduced with permission from ref. 128. Copyright © 2021, Royal Society of Chemistry. | ||
Shi et al.130 synthesized multicompartment nanoparticles of the brush block terpolymer of [poly(p-chloromethylstyrene)-graft-poly(4-vinylpyridine)]-block-polystyrene (PCMS-g-P4VP)-b-PSt via RAFT dispersion polymerization of St (Fig. 5). Au nanoparticles were immobilized on the PCMS-g-P4VP domains to form Au/polymer hybrid nanomaterials. The obtained Au/polymer hybrid nanomaterials exhibited high catalytic efficiency in the oxidation of aerobic alcohols. Huang et al.131 prepared large compound vesicles by the RAFT dispersion polymerization of St and 4VP mediated by a binary mixture of a macro-CTA and a CTA. Ag nanoparticles were attached to the large compound vesicles via the in situ reduction of AgNO3 using NaBH4. The obtained Ag/polymer hybrid nanomaterials also exhibited excellent catalytic properties by reducing methylene blue with NaBH4.
 | ||
| Fig. 5 Schematic illustration of the synthesis of (PCMS-g-P4VP)-b-PSt nanoparticles by PISA and the preparation of Au nanocomposites. Reproduced with permission from ref. 130. Copyright © 2015, American Chemical Society. | ||
Tan et al.132 reported the first PISA formulation of glycidyl methacrylate (GlyMA) to prepare epoxy-functionalized block copolymer nano-objects with various morphologies at room temperature. Cross-linked and amine-functionalized block copolymer nano-objects could be obtained by treatment with excess ethylene diamine (EDA) via the epoxy-amine chemistry. The obtained amine-functionalized block copolymer nano-objects were further used as templates to prepare Ag/polymer hybrid nanomaterials via in situ reduction of AgNO3 that exhibited good catalytic properties. Shi et al.133 developed initiators for continuous activator regeneration (ICAR) ATRP-based dispersion polymerization of 2-(perfluorohexyl)ethyl methacrylate (PFHEMA) and GlyMA for the synthesis of epoxy-functionalized block copolymer nano-objects (Fig. 6). After the introduction of carboxyl groups into the core-forming block via epoxy-thiol chemistry, Fe2+ and Fe3+ ions were loaded into the incorporated carboxyl groups, and NH3·H2O was added to form Fe3O4 nanoparticles within the block copolymer nano-objects.
 | ||
| Fig. 6 Preparation scheme of Fe3O4 nanoparticles within the block copolymer nano-objects. Reproduced with permission from ref. 133. Copyright © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
It is well known that RAFT groups (e.g., trithiocarbonate group) have strong interactions with metal ions, which can be used to anchor heavy metal nanoparticles onto block copolymer nano-objects prepared by RAFT-mediated PISA.147,148 However, RAFT groups usually embed inside block copolymer nano-objects in most RAFT-mediated PISA formulations due to the use of R-type macro-RAFT agents (the solvophilic block is connected to the fragmenting group of the RAFT agent), which makes it difficult to prepare organic–inorganic hybrid nanomaterials based on the RAFT groups. To overcome this problem, one attractive strategy is the utilization of a Z-type macro-RAFT agent (the solvophilic block is connected to the non-fragmenting group of the RAFT agent) in RAFT-mediated PISA. Our group135 developed the first well-controlled Z-RAFT-mediated dispersion polymerization using a Z-type macro-RAFT agent. In this formulation, all RAFT groups were located on the surface of block copolymer nano-objects. Ag/polymer hybrid nanomaterials were successfully prepared by attaching Ag nanoparticles on the block copolymer nano-objects via the in situ reduction of AgNO3 in the presence of poly(N-vinylpyrrolidone). Very recently, we synthesized segmented macro-RAFT agents using an asymmetric difunctional small molecular RAFT agent.136 These segmented macro-RAFT agents were used to mediate the RAFT dispersion polymerization of St and various block copolymer nano-objects were obtained. Due to the unique structure of the segmented macro-RAFT agent, a certain number of RAFT groups could be introduced to the surfaces of block copolymer nano-objects. These block copolymer nano-objects were also used as scaffolds to prepare Ag/polymer hybrid nanomaterials via the in situ reduction of AgNO3 (Fig. 7). As a control experiment, block copolymer nano-objects prepared by RAFT dispersion polymerization using a traditional macro-RAFT agent were also employed to prepare Ag/polymer hybrid nanomaterials. However, no silver nanoparticles were observed on the block copolymer nano-objects due to the absence of RAFT groups on the surface.
 | ||
| Fig. 7 (a) Schematic illustration of the in situ reduction of AgNO3 on the surface of block copolymer nano-objects. (b and c) TEM images of Ag hybrid vesicles prepared via in situ reduction of AgNO3. (d) UV-vis spectra of methylene blue reduced by NaBH4 using the Ag hybrid vesicles. Reproduced with permission from ref. 136. Copyright © 2022, American Chemical Society. | ||
3. In situ encapsulation of inorganic nanoparticles into vesicles
Block copolymer vesicles are hollow nanospheres with a bilayer structure that have broad applications in drug delivery, nanoreactors, Pickering emulsions, catalysis, sensing, and so on.149–151 In the morphological phase diagram of PISA, the phase of vesicles usually occupies a very large region at high solids contents.152–154 There is a natural fit for the efficient preparation of vesicles by PISA at high solids contents. Moreover, the Armes group155 reported that vesicles evolved from anisotropic worms during PISA and a “jellyfish” intermediate morphology is usually observed. Therefore, it is possible to prepare inorganic nanoparticle-loaded hybrid vesicles by encapsulating nanoparticles into vesicles via PISA.Our group156,157 reported the first in situ encapsulation of inorganic nanoparticles into vesicles by PISA. Silica nanoparticles were added at the beginning of aqueous photoinitiated RAFT dispersion polymerization of 2-hydropropyl methacrylate (HPMA) at room temperature using a monomethoxy poly(ethylene glycol) (mPEG) macro-CTA (Fig. 8). Transmission electron microscopy (TEM) characterization showed that the presence of silica nanoparticles had no influence on the formation of the vesicular morphology. Unloaded silica nanoparticles could be removed via several centrifugation–resuspension cycles. Taking advantage of the high electron contrast of silica nanoparticles, TEM analysis confirmed the successful formation of silica nanoparticle-loaded hybrid vesicles. Silica nanoparticle-loaded hybrid vesicles can also be prepared by enzyme-initiated aqueous RAFT dispersion polymerization at room temperature.158 Almost at the same time, the Armes group159 also reported the successful preparation of silica nanoparticle-loaded hybrid vesicles by the aqueous RAFT dispersion polymerization of HPMA using a poly(glycerol methacrylate) (PGMA) macro-CTA. TEM, cryo-TEM, small-angle X-ray scattering (SAXS), and disc centrifuge sedimentometry (DCP) were employed to characterize the silica nanoparticle-loaded vesicles formed. They demonstrated that the loading efficiency determined by TGA was consistent with that determined by either SAXS or DCP. Ding et al.160 synthesized tubular Ag/polymer hybrid nanomaterials by the RAFT dispersion polymerization of St in poly(ethylene glycol) using a poly(N-isopropylacrylamide) macro-CTA. They found that adding Ag nanoparticles in RAFT dispersion polymerization had little effect on the polymerization kinetics. In contrast, the addition of Ag nanoparticles had a significant effect on the morphology of Ag/polymer hybrid nanomaterials (Fig. 9).
 | ||
| Fig. 8 (A and B) Schematic illustration of the preparation of hybrid vesicles loaded with silica nanoparticles via aqueous photo-PISA of HPMA. (C) TEM images of unpurified and purified mPEG113-PHPMA365 hybrid vesicles prepared via aqueous photo-PISA of HPMA by adding different amounts of silica sol. Reproduced with permission from ref. 157. Copyright © 2017, Royal Society of Chemistry. | ||
 | ||
| Fig. 9 Schematic illustration of the synthesis of coil–coil diblock copolymer nanotubes and tubular Ag/polymer nanocomposites. Reproduced with permission from ref. 160. Copyright © 2017, American Chemical Society. | ||
For some specific applications, it is necessary to release inorganic nanoparticles from vesicles under mild conditions. For example, the controlled release of silica nanoparticles from polymer vesicles has potential to repair living tissues or hydrogels via a self-repair mechanism.161 To achieve this goal, we copolymerized a certain amount of DMAEMA with HPMA in aqueous photoinitiated RAFT dispersion polymerization to obtain silica nanoparticle-loaded hybrid vesicles.157 After treatment with CO2 at room temperature for 2 min, the vesicular membrane disassembled into dissolved block copolymers due to the protonation of the tertiary amine, leading to the release of silica nanoparticles from vesicles. Taking advantage of the thermo-responsive property of PHPMA, the Armes group159 induced the morphology change from vesicles to worms or spheres after incubating the silica nanoparticle-loaded PGMA-PHPMA vesicles at low temperatures (0–10 °C), leading to the release of silica nanoparticles from vesicles. The same group162 further investigated the kinetics of thermally triggered release of silica nanoparticles from vesicles using time resolved SAXS. They found that the payload of silica nanoparticles inside PGMA-PHPMA vesicles could lead to different thermally triggered morphological transitions. Deng et al.163 used the dynamic covalent chemistry of phenylboronic acids with cis-diols to induce vesicle-to-worm/sphere transition, which could also release silica nanoparticles from PGMA-PHPMA vesicles (Fig. 10).
 | ||
| Fig. 10 Release of silica nanoparticles from PGMA-PHPMA vesicles via the dynamic covalent chemistry of phenylboronic acids with cis-diols. Reproduced with permission from ref. 163. Copyright © 2017, American Chemical Society. | ||
In contrast to aqueous RAFT dispersion polymerization, RAFT emulsion polymerization should be a more versatile method that can help prepare block copolymer nano-objects in water using various hydrophobic monomers.96 However, kinetically trapped spheres are usually obtained by RAFT emulsion polymerization. Recently, the Armes group164 and our group165 reported that the aqueous solubility of monomers played an important role in the morphological evolution from lower-order morphologies to higher-order morphologies during RAFT-mediated emulsion polymerization. Our group166 successfully synthesized silica nanoparticle-loaded hybrid vesicles by adding silica nanoparticles at the beginning of the photoinitiated RAFT emulsion polymerization of tert-butyl acrylate (tBA) at room temperature. The Hawkett group167 encapsulated a titanium dioxide (TiO2) pigment into vesicles by RAFT emulsion polymerization for the preparation of TiO2 nanoparticle-loaded vesicles. 100% loading efficiency was achieved in this formulation and the obtained TiO2 nanoparticle-loaded vesicles were employed as enhanced opacifiers in water-borne painting.
The above examples demonstrated that hybrid vesicles with inorganic nanoparticle loading inside could be conveniently prepared by PISA. The stabilizer block located on the surface of hybrid vesicles enables the distribution of these nanoparticles in different media or substrates. Ning et al.168 prepared silica nanoparticle-loaded poly(methacrylic acid)-poly(benzyl methacrylate) (PMAA-PBzMA) vesicles by PISA in ethanol or ethanol/water. The silica nanoparticle-loaded vesicles were further used to mediate the crystallization of CaCO3, leading to the formation of CaCO3 single crystals with silica nanoparticles embedded inside (Fig. 11). It is noteworthy that the Armes group has recently reviewed the preparation of organic–inorganic hybrid materials by efficient occlusion of block copolymer nanoparticles within inorganic single crystals.115
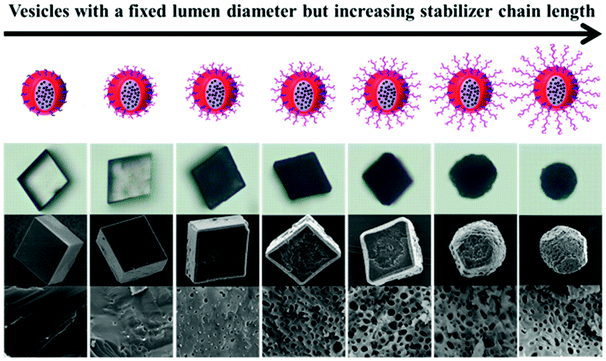 | ||
| Fig. 11 Anionic block copolymer vesicles for efficient occlusion in calcite: the effects of different chain lengths of poly(methacrylic acid) on the CaCO3 crystal morphology. Reproduced with permission from ref. 168. Copyright © 2019, American Chemical Society. | ||
4. Cooperative self-assembly of inorganic nanoparticles and polymers
During PISA, the synthesis and in situ self-assembly of block copolymers occur at the same time. Therefore, the in situ self-assembly process can be precisely controlled by tuning the polymerization kinetics.75 Moreover, the cooperative self-assembly of different types of polymers can be achieved by mixing different macro-CTAs or CTAs, leading to the formation of different types of polymer nanomaterials.47,169–172 The unique features of PISA provide a facile platform to prepare organic–inorganic hybrid nanomaterials using inorganic nanoparticles with macro-CTAs or CTAs on the surface.Zheng et al.173 grafted a poly(2-hydroxyethyl methacrylate) (PHEMA) brush on the surface of silica nanoparticles by RAFT polymerization. Subsequently, RAFT groups at the end of the PHEMA brush were removed, and additional RAFT agents were further modified on the surface of silica nanoparticles. The obtained silica nanoparticle-based macro-CTAs were dispersed in methanol to mediate the polymerization-induced self-assembly of benzyl methacrylate (BzMA). With the continuous increase of the molecular weight of PBzMA, each silica nanoparticle became more and more solvophobic, and cooperative self-assembly occurred to decrease the interfacial energy between the PBzMA chains and the solvent. Finally, worm-like organic–inorganic hybrid nanoparticles were formed (Fig. 12). To improve the stabilizing effect, the same group174 conducted the PISA of BzMA in ethanol using PHPMA-grafted silica nanoparticles instead of PHEMA-grafted silica nanoparticles. In this case, the authors observed the formation of silica nanoparticle vesicles during the PISA process (Fig. 13). However, in these systems, the polymerization system became unstable as the monomer conversion increased, and the final monomer conversion was extremely low (<15%). Moreover, the silica nanoparticles were relatively polydisperse, which is not beneficial for the formation of well-defined organic–inorganic hybrid nanomaterials. Recently, Wang et al.175 proposed a cooperative self-assembly strategy for preparing superstructures of inorganic–organic hybrid nanomaterials. Through DPD simulations, they found that ring, disk, and composite superstructures could be obtained by changing the grafting density of the nanoparticles. Dynamics, thermodynamics, and structural details in the cooperative self-assembly process are also shown.
 | ||
| Fig. 12 (A) Surface-initiated RAFT dispersion polymerization of BzMA and HEMA from SiO2 nanoparticles in methanol. (B) TEM images of the morphologies formed at 0 h, 1.5 h, 2 h, and 4.5 h. Reproduced with permission from ref. 173. Copyright © 2016, Royal Society of Chemistry. | ||
 | ||
| Fig. 13 (A) Synthesis of inorganic nanoparticle vesicles via surface-initiated polymerization-induced self-assembly. (B) TEM images of the cooperative self-assembly at different polymerization times. (C) The mechanisms of cooperative self-assembly. Reproduced with permission from ref. 174. Copyright © 2017, Royal Society of Chemistry. | ||
Recently, Hou et al.176 reported a novel PISA system that was mediated by a mixture of linear macro-CTAs dissolved in the reaction medium and macro-CTAs grafted on the surface of silica nanoparticles (220 nm). As shown in Fig. 14, with the growth of the solvophobic block (PSt in this case), there are two competitive PISA processes that occurred in the system. To lower the interfacial energy, cooperative self-assembly of the block copolymer brush on the surface of silica nanoparticles and the free block copolymers in the reaction medium occurs, leading to the formation of organic–inorganic hybrid nanomaterials with block copolymer micelles attached on the surface of silica nanoparticles. Meanwhile, a certain number of stable block copolymer micelles was also formed during the PISA process, which can be removed via several centrifugation–redispersion cycles. The morphologies of organic–inorganic hybrid nanocomposites can be controlled by changing the molecular weights of the solvophilic block and the solvophobic block. Using a similar strategy, the same group177 performed PISA mediated by a mixture of a linear macro-CTA dissolved in the reaction medium and CTA-grafted silica nanoparticles. In this case, asymmetric organic–organic hybrid nanoparticles with tunable anisotropy were formed.
 | ||
| Fig. 14 (A) Schematic diagram of polymerization-induced surface self-assembly. (B) TEM and (C) high-resolution SEM images of hybrid silica particles. Reproduced with permission from ref. 176. Copyright © 2019, American Chemical Society. | ||
Qiao et al.178,179 reported the first nitroxide-mediated synthesis of silica/polymer nanocomposites by surfactant-free emulsion polymerization of St and n-butyl methacrylate. In this study, a brush-type macro-alkoxyamine initiator composed of poly(ethylene oxide) methacrylate (PEGMA) and a small amount of St was first synthesized and then adsorbed on the surface of silica nanoparticles through hydrogen-bonding interactions. Using these adsorbed macro-alkoxyamine initiators in surfactant-free emulsion polymerization, silica nanoparticles with block copolymer micelles distributed on the surface were formed. The morphologies of the formed organic–inorganic hybrid nanomaterials could be further tuned by changing the macroinitiator concentration or the size of silica nanoparticles. This strategy is also versatile for surfactant-free RAFT-mediated emulsion polymerization using PEG-based macro-RAFT agents.180 It was found that changing the composition of the macro-RAFT agent led to different adsorptions to the silica nanoparticles. Therefore, the morphologies of silica/polymer nanocomposites could be tuned using different macro-RAFT agents. Upadhyaya et al.181 reported the synthesis of PMAA-PMMA diblock copolymers via RAFT-mediated PISA in the presence of Fe2O3 nanoparticles. They found that Fe2O3 nanoparticles were decorated on the block copolymer nanoparticles. The formed hybrid nanoparticles were further used to prepare thin film membranes for water filtration.
Recently, Liu et al.182 synthesized Janus Au@block copolymer nanoparticles by UV-initiated RAFT-mediated PISA (Fig. 15). Firstly, a P4VP-based macro-RAFT agent was synthesized and employed to functionalize the citrate-capped Au nanoparticles. The obtained Au nanoparticles were further used to mediate the RAFT dispersion polymerization of St under UV light irradiation. As the polymerization proceeded, the size of the polymer part of the Janus Au@block copolymer nanoparticles increased.
 | ||
| Fig. 15 Schematic illustration for (a) the synthesis of P4VP-b-PSt block copolymers and (b) the synthesis of Janus Au@block copolymer nanoparticles by UV-initiated PISA. Reproduced with permission from ref. 182. Copyright © 2021, Royal Society of Chemistry. | ||
5. Current challenges and future opportunities
With the development of PISA over the past ten years or so, more and more simple and feasible strategies have been explored for the preparation of organic–inorganic hybrid nanomaterials with diverse morphologies and functionalities. As discussed in the previous sections, there are mainly three strategies for preparing organic–inorganic hybrid nanomaterials based on PISA including the post-modification strategy, in situ encapsulation of inorganic nanoparticles into vesicles, and cooperative self-assembly of inorganic nanoparticles and polymers. Although great progress has been made in the preparation of organic–inorganic hybrid nanomaterials via PISA, there are still many challenges and possible opportunities in this research area.The post-modification method using PISA-made block copolymer nano-objects as scaffolds is a facile and scalable method to prepare organic–inorganic hybrid nanomaterials. However, it is very difficult to precisely control the payload of inorganic nanoparticles, the size of inorganic nanoparticles, and the distribution of inorganic nanoparticles within hybrid nanomaterials. Moreover, inorganic nanoparticles cannot anchor strongly to the polymer scaffolds due to relatively weak interactions. To overcome these problems, the development of novel PISA systems that can form block copolymer nano-objects with precise sizes and functionalities by PISA is desirable. For example, Manners et al.183 successfully developed polymerization-induced crystallization-driven self-assembly (PI-CDSA), allowing the efficient synthesis of uniform fiber-like micelles and block copolymer micelles. The PI-CDSA provides a potential method to control the distribution of inorganic nanoparticles within the hybrid nanomaterials. Moreover, introducing functional groups into block copolymer nano-objects that can strongly anchor to inorganic nanoparticles is also an attractive strategy to enhance the stability of organic–inorganic hybrid nanomaterials.
The in situ encapsulation of inorganic nanoparticles into vesicles via PISA is an attractive strategy to prepare organic–inorganic hybrid nanomaterials that may find applications in some specific areas. Until now, only SiO2 and TiO2 nanoparticles have been encapsulated into vesicles via PISA. Moreover, only limited approaches have been developed to release inorganic nanoparticles from vesicles. To expand the versatility of this strategy, the in situ encapsulation of other inorganic nanoparticles and the development of other stimulus-responsive vesicles that can release inorganic nanoparticles are encouraged.
The cooperative self-assembly of inorganic nanoparticles and polymers by PISA is a potential strategy that can precisely result in the preparation of organic–inorganic hybrid nanomaterials. Due to the intrinsic mechanism of PISA in which polymerization and self-assembly occur simultaneously, the mechanism of cooperative self-assembly of inorganic nanoparticles and polymers in PISA is complicated. One important current state-of-the-art is the detailed investigation of the mechanism of this unique process by various characterization techniques such as TEM, size exclusion chromatography (SEC), SAXS, dynamic light scattering (DLS), TGA, etc. The effects of the reaction parameters on the morphologies of the hybrid nanomaterials should also be studied in detail.
Finally, we note that organic–inorganic hybrid nanomaterials prepared based on PISA have shown potential applications in various areas such as catalysis, functional coating, biomineralization, etc.132,167,168 It would be fascinating to explore the applications of these organic–inorganic hybrid nanomaterials in other areas such as drug delivery, water purification, sensing, stimuli-responsive hydrogels and so on.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge support from the National Natural Science Foundation of China (Grant 22171055 and 21971047), the Guangdong Natural Science Foundation for Distinguished Young Scholar (Grant 2022B1515020078), the Science and Technology Program of Guangzhou (Grant 202102020631), and the Innovation Project of Education Department in Guangdong (Grant 2018KTSCX053). Y. C. acknowledges the support from the Guangdong Special Support Program (2017TX04N371).References
- A. J. Heinrich, W. D. Oliver, L. M. K. Vandersypen, A. Ardavan, R. Sessoli, D. Loss, A. B. Jayich, J. Fernandez-Rossier, A. Laucht and A. Morello, Nat. Nanotechnol., 2021, 16, 1318–1329 CrossRef CAS.
- K. A. Brown, S. Brittman, N. Maccaferri, D. Jariwala and U. Celano, Nano Lett., 2020, 20, 2–10 CrossRef CAS PubMed.
- C. Toumey, Nat. Nanotechnol., 2020, 15, 250–251 CrossRef CAS PubMed.
- C. R. Kagan, L. E. Fernandez, Y. Gogotsi, P. T. Hammond, M. C. Hersam, A. E. Nel, R. M. Penner, C. G. Willson and P. S. Weiss, ACS Nano, 2016, 10, 9093–9103 CrossRef CAS.
- S. Rigo, C. Cai, G. Gunkel-Grabole, L. Maurizi, X. Zhang, J. Xu and C. G. Palivan, Adv. Sci., 2018, 5, 1700892 CrossRef.
- A. P. Alivisatos, ACS Nano, 2008, 2, 1514–1516 CrossRef CAS PubMed.
- A.-A. E. Mel, P.-Y. Tessier, M. Buffiere, E. Gautron, J. Ding, K. Du, C.-H. Choi, S. Konstantinidis, R. Snyders, C. Bittencourt and L. Molina-Luna, Small, 2016, 12, 2885–2892 CrossRef.
- Y. Peng, T. Cullis and B. Inkson, Nano Lett., 2009, 9, 91–96 CrossRef CAS PubMed.
- P. Happel, T. Waag, M. Schimke, S. Schweeberg, A. Muzha, K. Fortak, D. Heesch, L. Klask, M. Pilscheur, F. Hoppe, T. Lenders, J. Meijer, G. Lepperdinger and A. Krueger, Adv. Funct. Mater., 2018, 28, 1802873 CrossRef.
- Y. Jiang, H. Su, W. Wei, Y. Wang, H.-Y. Chen and W. Wang, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 6630–6634 CrossRef CAS.
- D. Guo, G. Xie and J. Luo, J. Phys. D: Appl. Phys., 2013, 47, 013001 CrossRef.
- L. Nicole, C. Laberty-Robert, L. Rozes and C. Sanchez, Nanoscale, 2014, 6, 6267–6292 RSC.
- E. Salimi and M. N. Z. Abidin, Functional Hybrid Nanomaterials for Environmental Remediation, 2021, pp. 56–78 Search PubMed.
- V. P. Ananikov, Nanomaterials, 2019, 9, 1197 CrossRef CAS PubMed.
- N. Zhao, L. Yan, X. Zhao, X. Chen, A. Li, D. Zheng, X. Zhou, X. Dai and F.-J. Xu, Chem. Rev., 2019, 119, 1666–1762 CrossRef CAS.
- H. S. Han, K. Y. Choi, H. Lee, M. Lee, J. Y. An, S. Shin, S. Kwon, D. S. Lee and J. H. Park, ACS Nano, 2016, 10, 10858–10868 CrossRef CAS PubMed.
- S. Yadav, A. K. Sharma and P. Kumar, Front. Bioeng. Biotechnol., 2020, 8, 127 CrossRef PubMed.
- A. C. Mendes, E. T. Baran, R. L. Reis and H. S. Azevedo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 582–612 CAS.
- D. Bobo, K. J. Robinson, J. Islam, K. J. Thurecht and S. R. Corrie, Pharm. Res., 2016, 33, 2373–2387 CrossRef CAS.
- B. S. Bolu, R. Sanyal and A. Sanyal, Molecules, 2018, 23, 1570 CrossRef.
- C. Bissantz, B. Kuhn and M. Stahl, J. Med. Chem., 2010, 53, 5061–5084 CrossRef CAS.
- B. Du, X. Chen, B. Zhao, A. Mei, Q. Wang, J. Xu and Z. Fan, Nanoscale, 2010, 2, 1684–1689 RSC.
- C. Yi, Y. Yang, B. Liu, J. He and Z. Nie, Chem. Soc. Rev., 2020, 49, 465–508 RSC.
- Q. Wang, X. Zhao, Y.-I. Lee and H.-G. Liu, RSC Adv., 2015, 5, 86564–86571 RSC.
- C. Yi, S. Zhang, K. T. Webb and Z. Nie, Acc. Chem. Res., 2017, 50, 12–21 CrossRef CAS.
- Y. Liu, Y. Liu, J.-J. Yin and Z. Nie, Macromol. Rapid Commun., 2015, 36, 711–725 CrossRef CAS PubMed.
- J. He, X. Huang, Y.-C. Li, Y. Liu, T. Babu, M. A. Aronova, S. Wang, Z. Lu, X. Chen and Z. Nie, J. Am. Chem. Soc., 2013, 135, 7974–7984 CrossRef CAS.
- T. Bian, L. Shang, H. Yu, M. T. Perez, L.-Z. Wu, C.-H. Tung, Z. Nie, Z. Tang and T. Zhang, Adv. Mater., 2014, 26, 5613–5618 CrossRef CAS.
- J. Song, J. Zhou and H. Duan, J. Am. Chem. Soc., 2012, 134, 13458–13469 CrossRef CAS.
- J. Song, L. Pu, J. Zhou, B. Duan and H. Duan, ACS Nano, 2013, 7, 9947–9960 CrossRef CAS PubMed.
- W.-M. Wan, C.-Y. Hong and C.-Y. Pan, Chem. Commun., 2009, 5883–5885 RSC.
- M. Chen, J.-W. Li, W.-J. Zhang, C.-Y. Hong and C.-Y. Pan, Macromolecules, 2019, 52, 1140–1149 CrossRef.
- W.-D. He, X.-L. Sun, W.-M. Wan and C.-Y. Pan, Macromolecules, 2011, 44, 3358–3365 CrossRef CAS.
- J. Kadirkhanov, C.-L. Yang, Z.-X. Chang, R.-M. Zhu, C.-Y. Pan, Y.-Z. You, W.-J. Zhang and C.-Y. Hong, Polym. Chem., 2021, 12, 1768–1775 RSC.
- Y. Li and S. P. Armes, Angew. Chem., Int. Ed., 2010, 49, 4042–4046 CrossRef CAS PubMed.
- A. Blanazs, R. Verber, O. O. Mykhaylyk, A. J. Ryan, J. Z. Heath, C. W. I. Douglas and S. P. Armes, J. Am. Chem. Soc., 2012, 134, 9741–9748 CrossRef CAS PubMed.
- C. Gonzato, M. Semsarilar, E. R. Jones, F. Li, G. J. P. Krooshof, P. Wyman, O. O. Mykhaylyk, R. Tuinier and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 11100–11106 CrossRef CAS PubMed.
- L. A. Fielding, J. A. Lane, M. J. Derry, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 5790–5798 CrossRef CAS PubMed.
- A. Czajka and S. P. Armes, J. Am. Chem. Soc., 2021, 143, 1474–1484 CrossRef CAS PubMed.
- C. J. Mable, L. A. Fielding, M. J. Derry, O. O. Mykhaylyk, P. Chambon and S. P. Armes, Chem. Sci., 2018, 9, 1454–1463 RSC.
- W. Zhou, Q. Qu, Y. Xu and Z. An, ACS Macro Lett., 2015, 4, 495–499 CrossRef CAS.
- Y. Li, Z. Ye, L. Shen, Y. Xu, A. Zhu, P. Wu and Z. An, Macromolecules, 2016, 49, 3038–3048 CrossRef CAS.
- X. Wang, C. A. Figg, X. Lv, Y. Yang, B. S. Sumerlin and Z. An, ACS Macro Lett., 2017, 6, 337–342 CrossRef CAS.
- F. Lv, Z. An and P. Wu, Nat. Commun., 2019, 10, 1397 CrossRef PubMed.
- B. Zhang, X. Lv and Z. An, ACS Macro Lett., 2017, 6, 224–228 CrossRef CAS.
- M. Cao, H. Nie, Y. Hou, G. Han and W. Zhang, Polym. Chem., 2019, 10, 403–411 RSC.
- C. Gao, J. Wu, H. Zhou, Y. Qu, B. Li and W. Zhang, Macromolecules, 2016, 49, 4490–4500 CrossRef CAS.
- S. Li, X. He, Q. Li, P. Shi and W. Zhang, ACS Macro Lett., 2014, 3, 916–921 CrossRef CAS.
- S. Qu, K. Wang, H. Khan, W. Xiong and W. Zhang, Polym. Chem., 2019, 10, 1150–1157 RSC.
- P. Shi, Y. Qu, C. Liu, H. Khan, P. Sun and W. Zhang, ACS Macro Lett., 2016, 5, 88–93 CrossRef CAS.
- Y. Ding, M. Cai, Z. Cui, L. Huang, L. Wang, X. Lu and Y. Cai, Angew. Chem., Int. Ed., 2018, 57, 1053–1056 CrossRef CAS PubMed.
- M. Cai, Y. Ding, L. Wang, L. Huang, X. Lu and Y. Cai, ACS Macro Lett., 2018, 7, 208–212 CrossRef CAS.
- L. Huang, Y. Ding, Y. Ma, L. Wang, Q. Liu, X. Lu and Y. Cai, Macromolecules, 2019, 52, 4703–4712 CrossRef CAS.
- L. Wang, Y. Ding, Q. Liu, Q. Zhao, X. Dai, X. Lu and Y. Cai, ACS Macro Lett., 2019, 8, 623–628 CrossRef CAS.
- X. Chen, L. Liu, M. Huo, M. Zeng, L. Peng, A. Feng, X. Wang and J. Yuan, Angew. Chem., Int. Ed., 2017, 56, 16541–16545 CrossRef CAS PubMed.
- M. Huo, Q. Ye, H. Che, X. Wang, Y. Wei and J. Yuan, Macromolecules, 2017, 50, 1126–1133 CrossRef CAS.
- M. Huo, M. Zeng, D. Li, L. Liu, Y. Wei and J. Yuan, Macromolecules, 2017, 50, 8212–8220 CrossRef CAS.
- S. Guan and A. Chen, Macromolecules, 2020, 53, 6235–6245 CrossRef CAS.
- L. D. Blackman, K. E. B. Doncom, M. I. Gibson and R. K. O'Reilly, Polym. Chem., 2017, 8, 2860–2871 RSC.
- S. Varlas, J. C. Foster, P. G. Georgiou, R. Keogh, J. T. Husband, D. S. Williams and R. K. O'Reilly, Nanoscale, 2019, 11, 12643–12654 RSC.
- L. D. Blackman, S. Varlas, M. C. Arno, Z. H. Houston, N. L. Fletcher, K. J. Thurecht, M. Hasan, M. I. Gibson and R. K. O'Reilly, ACS Cent. Sci., 2018, 4, 718–723 CrossRef CAS PubMed.
- S. Varlas, R. Keogh, Y. Xie, S. L. Horswell, J. C. Foster and R. K. O'Reilly, J. Am. Chem. Soc., 2019, 141, 20234–20248 CrossRef CAS PubMed.
- J. Yeow, J. Xu and C. Boyer, ACS Macro Lett., 2015, 4, 984–990 CrossRef CAS.
- J. Yeow, O. R. Sugita and C. Boyer, ACS Macro Lett., 2016, 5, 558–564 CrossRef CAS.
- S. Xu, G. Ng, J. Xu, R. P. Kuchel, J. Yeow and C. Boyer, ACS Macro Lett., 2017, 6, 1237–1244 CrossRef CAS.
- N. Zaquen, J. Yeow, T. Junkers, C. Boyer and P. B. Zetterlund, Macromolecules, 2018, 51, 5165–5172 CrossRef CAS.
- S. Dong, W. Zhao, F. P. Lucien, S. Perrier and P. B. Zetterlund, Polym. Chem., 2015, 6, 2249–2254 RSC.
- D. Zhou, R. P. Kuchel and P. B. Zetterlund, Polym. Chem., 2017, 8, 4177–4181 RSC.
- G. K. K. Clothier, T. R. Guimarães, M. Khan, G. Moad, S. Perrier and P. B. Zetterlund, ACS Macro Lett., 2019, 8, 989–995 CrossRef CAS.
- T. R. Guimarães, M. Khan, R. P. Kuchel, I. C. Morrow, H. Minami, G. Moad, S. Perrier and P. B. Zetterlund, Macromolecules, 2019, 52, 2965–2974 CrossRef.
- M. A. Touve, C. A. Figg, D. B. Wright, C. Park, J. Cantlon, B. S. Sumerlin and N. C. Gianneschi, ACS Cent. Sci., 2018, 4, 543–547 CrossRef CAS PubMed.
- J. Y. Rho, G. M. Scheutz, S. Häkkinen, J. B. Garrison, Q. Song, J. Yang, R. Richardson, S. Perrier and B. S. Sumerlin, Polym. Chem., 2021, 12, 3947–3952 RSC.
- C. A. Figg, A. Simula, K. A. Gebre, B. S. Tucker, D. M. Haddleton and B. S. Sumerlin, Chem. Sci., 2015, 6, 1230–1236 RSC.
- J. Cao, Y. Tan, X. Dai, Y. Chen, L. Zhang and J. Tan, Polymer, 2021, 230, 124095 CrossRef CAS.
- X. Luo, S. Zhao, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2021, 54, 2948–2959 CrossRef CAS.
- S. Han, J. Wu, Y. Zhang, J. Lai, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2021, 54, 4669–4681 CrossRef CAS.
- J. Cao, Y. Tan, Y. Chen, L. Zhang and J. Tan, Macromol. Rapid Commun., 2021, 42, 2100333 CrossRef CAS PubMed.
- J. He, D. Lin, Y. Chen, L. Zhang and J. Tan, Macromol. Rapid Commun., 2021, 42, 2100201 CrossRef CAS PubMed.
- R. Zeng, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2020, 53, 1557–1566 CrossRef CAS.
- Q. Zhang, R. Zeng, Y. Zhang, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2020, 53, 8982–8991 CrossRef CAS.
- D. Liu, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2020, 53, 9725–9735 CrossRef CAS.
- D. Liu, W. Cai, L. Zhang, C. Boyer and J. Tan, Macromolecules, 2020, 53, 1212–1223 CrossRef CAS.
- C. J. Ferguson, R. J. Hughes, B. T. T. Pham, B. S. Hawkett, R. G. Gilbert, A. K. Serelis and C. H. Such, Macromolecules, 2002, 35, 9243–9245 CrossRef CAS.
- G. Wang, M. Schmitt, Z. Wang, B. Lee, X. Pan, L. Fu, J. Yan, S. Li, G. Xie, M. R. Bockstaller and K. Matyjaszewski, Macromolecules, 2016, 49, 8605–8615 CrossRef CAS.
- Y. Wang, G. Han, W. Duan and W. Zhang, Macromol. Rapid Commun., 2019, 40, 1800140 CrossRef PubMed.
- C. Dire, S. Magnet, L. Couvreur and B. Charleux, Macromolecules, 2009, 42, 95–103 CrossRef CAS.
- G. Delaittre, C. Dire, J. Rieger, J.-L. Putaux and B. Charleux, Chem. Commun., 2009, 2887–2889 RSC.
- D. B. Wright, M. A. Touve, L. Adamiak and N. C. Gianneschi, ACS Macro Lett., 2017, 6, 925–929 CrossRef CAS.
- S. Varlas, J. C. Foster, L. A. Arkinstall, J. R. Jones, R. Keogh, R. T. Mathers and R. K. O'Reilly, ACS Macro Lett., 2019, 8, 466–472 CrossRef CAS PubMed.
- J. Jiang, X. Zhang, Z. Fan and J. Du, ACS Macro Lett., 2019, 8, 1216–1221 CrossRef CAS.
- C. Grazon, P. Salas-Ambrosio, E. Ibarboure, A. Buol, E. Garanger, M. W. Grinstaff, S. Lecommandoux and C. Bonduelle, Angew. Chem., Int. Ed., 2020, 59, 622–626 CrossRef CAS PubMed.
- J. Wang, M. Cao, P. Zhou and G. Wang, Macromolecules, 2020, 53, 3157–3165 CrossRef CAS.
- Y. Kitayama, A. Chaiyasat, H. Minami and M. Okubo, Macromolecules, 2010, 43, 7465–7471 CrossRef CAS.
- W.-J. Zhang, C.-Y. Hong and C.-Y. Pan, Macromol. Rapid Commun., 2019, 40, 1800279 CrossRef PubMed.
- J.-T. Sun, C.-Y. Hong and C.-Y. Pan, Polym. Chem., 2013, 4, 873–881 RSC.
- B. Charleux, G. Delaittre, J. Rieger and F. D'Agosto, Macromolecules, 2012, 45, 6753–6765 CrossRef CAS.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS PubMed.
- S. L. Canning, G. N. Smith and S. P. Armes, Macromolecules, 2016, 49, 1985–2001 CrossRef CAS PubMed.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS.
- M. J. Derry, L. A. Fielding and S. P. Armes, Prog. Polym. Sci., 2016, 52, 1–18 CrossRef CAS.
- X. Wang, L. Shen and Z. An, Prog. Polym. Sci., 2018, 83, 1–27 CrossRef CAS.
- D. Liu, J. He, L. Zhang and J. Tan, ACS Macro Lett., 2019, 8, 1660–1669 CrossRef CAS.
- J. Cao, Y. Tan, Y. Chen, L. Zhang and J. Tan, Macromol. Rapid Commun., 2021, 42, 2100498 CrossRef CAS PubMed.
- F. D'Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef PubMed.
- G. Cheng and J. Pérez-Mercader, Macromol. Rapid Commun., 2019, 40, 1800513 CrossRef PubMed.
- S. Y. Khor, J. F. Quinn, M. R. Whittaker, N. P. Truong and T. P. Davis, Macromol. Rapid Commun., 2019, 40, 1800438 CrossRef PubMed.
- D. Le, D. Keller and G. Delaittre, Macromol. Rapid Commun., 2019, 40, 1800551 CrossRef PubMed.
- J. Huang, Y. Guo, S. Gu, G. Han, W. Duan, C. Gao and W. Zhang, Polym. Chem., 2019, 10, 3426–3435 RSC.
- P. B. Zetterlund, S. C. Thickett, S. Perrier, E. Bourgeat-Lami and M. Lansalot, Chem. Rev., 2015, 115, 9745–9800 CrossRef CAS PubMed.
- F. Jasinski, P. B. Zetterlund, A. M. Braun and A. Chemtob, Prog. Polym. Sci., 2018, 84, 47–88 CrossRef CAS.
- J. Yeow and C. Boyer, Adv. Sci., 2017, 4, 1700137 CrossRef PubMed.
- N. An, X. Chen and J. Yuan, Polym. Chem., 2021, 12, 3220–3232 RSC.
- S. C. Thickett and G. H. Teo, Polym. Chem., 2019, 10, 2906–2924 RSC.
- C. Liu, C.-Y. Hong and C.-Y. Pan, Polym. Chem., 2020, 11, 3673–3689 RSC.
- Y. Ning and S. P. Armes, Acc. Chem. Res., 2020, 53, 1176–1186 CrossRef CAS PubMed.
- W. Zhou, Q. Qu, W. Yu and Z. An, ACS Macro Lett., 2014, 3, 1220–1224 CrossRef CAS.
- J. Huang, D. Liu, Y. Chen, L. Zhang and J. Tan, Macromol. Rapid Commun., 2021, 42, 2000720 CrossRef CAS PubMed.
- M. Semsarilar, E. R. Jones, A. Blanazs and S. P. Armes, Adv. Mater., 2012, 24, 3378–3382 CrossRef CAS PubMed.
- B. Karagoz, J. Yeow, L. Esser, S. M. Prakash, R. P. Kuchel, T. P. Davis and C. Boyer, Langmuir, 2014, 30, 10493–10502 CrossRef CAS PubMed.
- L. Yu, Y. Zhang, X. Dai, L. Zhang and J. Tan, Chem. Commun., 2019, 55, 7848–7851 RSC.
- M. Tan, Y. Shi, Z. Fu and W. Yang, Polym. Chem., 2018, 9, 1082–1094 RSC.
- R. Bleach, B. Karagoz, S. M. Prakash, T. P. Davis and C. Boyer, ACS Macro Lett., 2014, 3, 591–596 CrossRef CAS.
- W.-J. Zhang, C.-Y. Hong and C.-Y. Pan, J. Mater. Chem. A, 2014, 2, 7819–7828 RSC.
- Y. Zhang, Z. Wang, K. Matyjaszewski and J. Pietrasik, Eur. Polym. J., 2019, 110, 49–55 CrossRef CAS.
- A. Rubio, G. Desnos and M. Semsarilar, Macromol. Chem. Phys., 2018, 219, 1800351 CrossRef.
- L. Yu, Y. Zhang, X. Dai, Q. Xu, L. Zhang and J. Tan, Chem. Commun., 2019, 55, 11920–11923 RSC.
- J. Huang, D. Li, H. Liang and J. Lu, Macromol. Rapid Commun., 2017, 38, 1700202 CrossRef PubMed.
- J. Wan, B. Fan and S. H. Thang, Nanoscale Adv., 2021, 3, 3306–3315 RSC.
- B. Fan, Y. Liu, J. Wan, S. Crawford and S. H. Thang, ACS Mater. Lett., 2020, 2, 492–498 CrossRef CAS.
- P. Shi, C. Gao, X. He, P. Sun and W. Zhang, Macromolecules, 2015, 48, 1380–1389 CrossRef CAS.
- C. Huang, J. Tan, Q. Xu, J. He, X. Li, D. Liu and L. Zhang, RSC Adv., 2017, 7, 46069–46081 RSC.
- J. Tan, D. Liu, C. Huang, X. Li, J. He, Q. Xu and L. Zhang, Macromol. Rapid Commun., 2017, 38, 1700195 CrossRef PubMed.
- B. Shi, H. Zhang, Y. Liu, J. Wang, P. Zhou, M. Cao and G. Wang, Macromol. Rapid Commun., 2019, 40, 1900547 CrossRef CAS PubMed.
- G. H. Teo, R. P. Kuchel, P. B. Zetterlund and S. C. Thickett, Polym. Chem., 2016, 7, 6575–6585 RSC.
- J. Tan, X. Li, R. Zeng, D. Liu, Q. Xu, J. He, Y. Zhang, X. Dai, L. Yu, Z. Zeng and L. Zhang, ACS Macro Lett., 2018, 7, 255–262 CrossRef CAS.
- X. Luo, K. Zhang, R. Zeng, Y. Chen, L. Zhang and J. Tan, Macromolecules, 2022, 55, 65–77 CrossRef CAS.
- S. Berger, O. Ornatsky, V. Baranov, M. A. Winnik and A. Pich, J. Mater. Chem., 2010, 20, 5141–5150 RSC.
- H. Kong and J. Jang, Biomacromolecules, 2008, 9, 2677–2681 CrossRef CAS PubMed.
- Q. Xu, Y. Zhang, X. Li, J. He, J. Tan and L. Zhang, Polym. Chem., 2018, 9, 4908–4916 RSC.
- C. Song, Y. Sun, J. Li, C. Dong, J. Zhang, X. Jiang and L. Wang, Nanoscale, 2019, 11, 18881–18893 RSC.
- X. Wang, C. Wang, L. Cheng, S.-T. Lee and Z. Liu, J. Am. Chem. Soc., 2012, 134, 7414–7422 CrossRef CAS PubMed.
- B. B. Sahoo, N. Kumar, H. S. Panda, B. Panigrahy, N. K. Sahoo, A. Soam, B. S. Mahanto and P. K. Sahoo, J. Energy Storage, 2021, 43, 103157 CrossRef.
- C. Gao, Q. Zhang, Z. Lu and Y. Yin, J. Am. Chem. Soc., 2011, 133, 19706–19709 CrossRef CAS PubMed.
- Z. Shu, Y. Chen, J. Zhou, T. Li, Z. Sheng, C. Tao and Y. Wang, Appl. Clay Sci., 2016, 132–133, 114–121 CrossRef CAS.
- N. Zhang, Y. Qiu, H. Sun, J. Hao, J. Chen, J. Xi, J. Liu, B. He and Z.-W. Bai, ACS Appl. Nano Mater., 2021, 4, 5854–5863 CrossRef CAS.
- X. Chen, R. Klingeler, M. Kath, A. A. El Gendy, K. Cendrowski, R. J. Kalenczuk and E. Borowiak-Palen, ACS Appl. Mater. Interfaces, 2012, 4, 2303–2309 CrossRef CAS PubMed.
- B. Ebeling and P. Vana, Macromolecules, 2013, 46, 4862–4871 CrossRef CAS.
- W. Peng, C. Rossner, V. Roddatis and P. Vana, ACS Macro Lett., 2016, 5, 1227–1231 CrossRef CAS.
- Y. Zhu, B. Yang, S. Chen and J. Du, Prog. Polym. Sci., 2017, 64, 1–22 CrossRef CAS.
- J. He, J. Cao, Y. Chen, L. Zhang and J. Tan, ACS Macro Lett., 2020, 9, 533–539 CrossRef CAS.
- H. Che and J. C. M. van Hest, J. Mater. Chem. B, 2016, 4, 4632–4647 RSC.
- J. Tan, D. Liu, Y. Bai, C. Huang, X. Li, J. He, Q. Xu, X. Zhang and L. Zhang, Polym. Chem., 2017, 8, 1315–1327 RSC.
- A. Blanazs, A. J. Ryan and S. P. Armes, Macromolecules, 2012, 45, 5099–5107 CrossRef CAS.
- J. Tan, D. Liu, Y. Bai, C. Huang, X. Li, J. He, Q. Xu and L. Zhang, Macromolecules, 2017, 50, 5798–5806 CrossRef CAS.
- A. Blanazs, J. Madsen, G. Battaglia, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2011, 133, 16581–16587 CrossRef CAS PubMed.
- J. Tan, H. Sun, M. Yu, B. S. Sumerlin and L. Zhang, ACS Macro Lett., 2015, 4, 1249–1253 CrossRef CAS.
- J. Tan, D. Liu, X. Zhang, C. Huang, J. He, Q. Xu, X. Li and L. Zhang, RSC Adv., 2017, 7, 23114–23121 RSC.
- J. Tan, Q. Xu, X. Li, J. He, Y. Zhang, X. Dai, L. Yu, R. Zeng and L. Zhang, Macromol. Rapid Commun., 2018, 39, 1700871 CrossRef PubMed.
- C. J. Mable, R. R. Gibson, S. Prevost, B. E. McKenzie, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2015, 137, 16098–16108 CrossRef CAS PubMed.
- Z. Ding, M. Ding, C. Gao, C. Boyer and W. Zhang, Macromolecules, 2017, 50, 7593–7602 CrossRef CAS.
- S. Rose, A. Prevoteau, P. Elzière, D. Hourdet, A. Marcellan and L. Leibler, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- C. J. Mable, M. J. Derry, K. L. Thompson, L. A. Fielding, O. O. Mykhaylyk and S. P. Armes, Macromolecules, 2017, 50, 4465–4473 CrossRef CAS PubMed.
- R. Deng, M. J. Derry, C. J. Mable, Y. Ning and S. P. Armes, J. Am. Chem. Soc., 2017, 139, 7616–7623 CrossRef CAS PubMed.
- E. E. Brotherton, F. L. Hatton, A. A. Cockram, M. J. Derry, A. Czajka, E. J. Cornel, P. D. Topham, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2019, 141, 13664–13675 CrossRef CAS PubMed.
- X. Dai, L. Yu, Y. Zhang, L. Zhang and J. Tan, Macromolecules, 2019, 52, 7468–7476 CrossRef CAS.
- J. Tan, X. Dai, Y. Zhang, L. Yu, H. Sun and L. Zhang, ACS Macro Lett., 2019, 8, 205–212 CrossRef CAS.
- D. Nguyen, V. Huynh, M. Lam, A. Serelis, T. Davey, O. Paravagna, C. Such and B. Hawkett, Macromol. Rapid Commun., 2021, 42, 2170036 CrossRef CAS.
- Y. Ning, L. Han, M. J. Derry, F. C. Meldrum and S. P. Armes, J. Am. Chem. Soc., 2019, 141, 2557–2567 CrossRef CAS PubMed.
- J. Tan, C. Huang, D. Liu, X. Li, J. He, Q. Xu and L. Zhang, ACS Macro Lett., 2017, 6, 298–303 CrossRef CAS.
- B. Yuan, X. He, Y. Qu, C. Gao, E. Eiser and W. Zhang, Polym. Chem., 2017, 8, 2173–2181 RSC.
- A. Zhu, X. Lv, L. Shen, B. Zhang and Z. An, ACS Macro Lett., 2017, 6, 304–309 CrossRef CAS.
- J. Tan, Q. Xu, Y. Zhang, C. Huang, X. Li, J. He and L. Zhang, Macromolecules, 2018, 51, 7396–7406 CrossRef CAS.
- Y. Zheng, Y. Huang, Z. M. Abbas and B. C. Benicewicz, Polym. Chem., 2016, 7, 5347–5350 RSC.
- Y. Zheng, Y. Huang, Z. M. Abbas and B. C. Benicewicz, Polym. Chem., 2017, 8, 370–374 RSC.
- J. Wang, B. Zhu, Y. Wang, Y. Hao, J. Zhang and Z. Li, Soft Matter, 2021, 18, 97–106 RSC.
- W. Hou, H. Wang, Y. Cui, Y. Liu, X. Ma and H. Zhao, Macromolecules, 2019, 52, 8404–8414 CrossRef CAS.
- W. Hou, W. Zhong and H. Zhao, Macromolecules, 2021, 54, 2617–2626 CrossRef CAS.
- X. G. Qiao, P.-Y. Dugas, B. Charleux, M. Lansalot and E. Bourgeat-Lami, Macromolecules, 2015, 48, 545–556 CrossRef CAS.
- X. G. Qiao, O. Lambert, J.-C. Taveau, P.-Y. Dugas, B. Charleux, M. Lansalot and E. Bourgeat-Lami, Macromolecules, 2017, 50, 3796–3806 CrossRef CAS.
- E. Bourgeat-Lami, A. J. P. G. França, T. C. Chaparro, R. D. Silva, P.-Y. Dugas, G. M. Alves and A. M. Santos, Macromolecules, 2016, 49, 4431–4440 CrossRef CAS.
- L. Upadhyaya, C. Egbosimba, X. Qian, R. Wickramasinghe, R. Fernández-Pacheco, I. M. Coelhoso, C. A. M. Portugal, J. G. Crespo, D. Quemener and M. Semsarilar, Macromol. Rapid Commun., 2019, 40, 1800333 CrossRef PubMed.
- Z. Liu, C. Wu, Y. Fu, X. Xu, J. Ying, J. Sheng, Y. Huang, C. Ma and T. Chen, Nanoscale Adv., 2021, 3, 347–352 RSC.
- A. M. Oliver, J. Gwyther, C. E. Boott, S. Davis, S. Pearce and I. Manners, J. Am. Chem. Soc., 2018, 140, 18104–18114 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |
