Modulating the mixed micellization of CTAB and an ionic liquid 1-hexadecyl-3-methylimidazollium bromide via varying physical states of ionic liquid†
Chanda Chadhaa,
Gurbir Singhb,
Gurpreet Singhb,
Harsh Kumar*a and
Tejwant Singh Kang*b
aDepartment of Chemistry, Dr B. R. Ambedkar National Institute of Technology, Jalandhar, 144011, India. E-mail: h.786.man@gmail.com
bDepartment of Chemistry, UGC-centre for Advance Studies – II, Guru Nanak Dev University, Amritsar, 143005, India. E-mail: tejwantsinghkang@gmail.com; Tel: +91-183-2258802 ext. 207
First published on 12th April 2016
Abstract
The mixed micellization behavior in aqueous systems of CTAB and an ionic liquid (IL), 1-hexadecyl-3-mthylimidazolium bromide, [C16mim][Br], has been investigated at different fixed concentrations of IL i.e. 1, 2 and 5 mmol L−1. The mixed micellization phenomenon has been investigated using conductivity and steady-state fluorescence measurements to get the critical micelle concentration (cmc) and aggregation number (Nagg) of formed mixed micelles. The insight into thermodynamic forces behind the mixed micellization has been obtained from isothermal titration calorimetry (ITC). The size and shape of the formed micelles has been investigated using dynamic light scattering (DLS) and transmission electron microscopy (TEM) measurements. The presence of IL in varying physical forms i.e. predominantly in monomeric form at 1 mmol L−1, in the micellar form at 2 mmol L−1 and rich in micelles at 5 mmol L−1 of IL is expected to modulate the formation of mixed micelles in aqueous medium. At different concentrations of IL, varying forms of self-assembled structures such as IL rich CTAB–IL mixed vesicles (1 mmol L−1) and IL rich CTAB–IL mixed micelles (2 and 5 mmol L−1) have been observed. 1H NMR measurements along with 1H spin–lattice relaxation time have shed light on the relative content of IL and CTAB in the formed mixed structures.
1. Introduction
Ionic liquids (ILs) are salts which remain in the molten state below 100 °C and are gaining wide attention from the scientific community around the globe for their use in various applications owing to their unusual and interesting physicochemical properties.1–5 The uniqueness of the ILs lies in the fine tune ability of their physicochemical properties by choosing a variety of constituent ions differing in structure and property. In this regard, an increase in alkyl chain length of cation or anion beyond 6 number of carbon atoms with oppositely charged hydrophilic counterions has led to induction of amphiphilic behaviour in many of the ILs. Owing to their inherent amphiphilic nature, many of the ILs have been investigated for their self-assembling behavior in aqueous,6–18 non-aqueous19–21 or even in IL media.22 Among the investigated ILs, the most widely investigated ILs are based on 1-alkyl-3-methylimidazolium cation ([Cnmim]+), where n is the number of carbon atoms of the alkyl chain. These ILs appended with appropriate length of alkyl chain have been found to exhibit surfactant like behavior, which induces the amphiphilicity leading to the micelle formation.15,23–25 In this regard, Wang et al. have investigated the aggregation behavior of aqueous solutions of 1-n-alkyl-3-methylimidazolium bromide based ILs, [Cnmim][Br] (n = 4, 6, 8, 10, 12) by NMR measurements at 298.2 K.12 Similarly, aggregation behavior of ILs having 1-alkyl-3-methylimidazolium cation, [Cnmim]+, with different alkyl chain lengths, CnH2n+1 (1/2n = 1–7), and different counterions, namely [Cnmim][Cl] (n = 2–14), [Cnmim][PF6] (n = 4 or 10), and [C10mim][NTf2] were examined by interfacial tension, fluorescence, and 1H NMR techniques by the research group of Rebelo et al.13 Our group has also investigated the aggregation behavior of [Cnmim]+ based ILs with different alkyl chain length and different counterions.11 It has been established that even the ILs having shorter alkyl chain length (n = 4) self-assembles into relatively weaker micelle like aggregates.7,11 Exploiting the functionalization of alkyl chain of ILs, Garcia et al. synthesized ester-functionalized imidazolium- and pyridinium-based ILs and investigated their antimicrobial activity and micellization behavior in aqueous medium.6 On similar lines, our group has also reported the aggregation behavior of amide- and ester-functionalized morpholinium based ILs,18,26 which exhibits better surface active behavior as compared to their non-functionalized counterparts.On the other hand, depending on the nature of constituent ions of ILs and the content of the ILs, ILs have been found to behave like an electrolyte or an ionic co-surfactant in modifying the micellization behavior of conventional surfactants in aqueous medium.27–33 The phenomenon of modulation of micellization behavior of a variety of anionic and cationic surfactants by short chain ILs has been exploited by the research group of S. Pandey. In this regard, Pandey et al. studied the changes in the micellization behavior of sodium dodecyl sulfate (SDS) in aqueous medium in the presence of hydrophobic ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate, [Bmim][PF6] having limited solubility in aqueous medium.28 It has been observed that with the addition of IL up to ≈0.10 wt%, a decrease in the critical micelle concentration (cmc) of SDS accompanied by micellar growth takes place. It has been observed that the presence of strong electrostatic attraction between bmim+ and anionic micellar surface was the most dominant reason for micellar growth. Considering its amphiphilic nature, medium chain IL, 1-hexyl-3-methylimidazolium bromide, [C6mim][Br] have been tested to modify the micellization behavior of an industrially important cationic surfactant cetyltrimethylammonium bromide, CTAB, in aqueous medium and the results were compared with homologous conventional co-surfactant hexyltrimethylammonium bromide, HeTAB.33 It has been observed that both [C6mim][Br] and HeTAB show electrolytic as well as co-surfactant type behavior in modifying the behavior of aqueous CTAB when present at low concentrations. On the other hand, [C6mim][Br] and HeTAB behave differently when present in relatively higher concentration, where HeTAB still acts as a co-surfactant forming mixed micelles with CTAB, however, [C6mim][Br] behaves partly as a co-solvent toward altering the physicochemical properties of aqueous CTAB. Javadian et al. have investigated the aggregation behavior of CTAB in aqueous solutions of three imidazolium-based ILs, N-butyl imidazolium chloride [N-BIm][Cl], 1-butyl-3-methyl imidazolium chloride [C4mim][Cl] and 1-hexyl-3-methyl imidazolium bromide [C6mim][Br] by a variety of techniques.31 Very surprisingly, the presence of ILs has been found to increase the cmc of CTAB, contrary to the normal observation. It was shown that [C6mim][Br] can be incorporated in CTAB micelles and form mixed micelles, but [N-BIm][Cl] behaves as a co-solvent toward alerting the physicochemical properties of CTAB. In general, it has been observed that the types of anion and alkyl substitutions on the imidazolium ring affect the aggregation behavior of CTAB. The choice of CTAB is justified over other conventional cationic surfactants owing to its industrial importance where it is commonly used in chemicals, pharmaceuticals, bio-chemical industries and has wide applications in different fields.34 As the micellization behavior of CTAB in the presence of relatively short chain ILs has already been investigated providing some contrasting results, it is supposed that it would be quite interesting to investigate the mixed micellization behavior of CTAB and a micelle forming homologous IL, 3-methyl-1-hexadecylimidazolium bromide, [C16mim][Br], in aqueous media at different fixed concentrations of IL. In the aqueous solutions of IL, different types of structures of [C16mim][Br] ranging from monomeric solution to micellar solution is expected depending on its concentration, which is supposed to affect the micellization of CTAB in different ways. The novelty of the present work lies in the fact that there exists no report involving the effect of micelle forming ILs such as [C16mim][Br] on micellization of CTAB at different fixed concentrations of IL in aqueous media, although reports pertaining to alteration in micellization behavior of CTAB by short chain ILs are present in literature as cited above. It is anticipated that the work would prompt other researchers to follow such studies considering the amphiphilic nature of ILs and the utilization of colloidal suspensions involving CTAB in pharmaceutical and many other applications.35
In the present work, we have investigated the effect of long chain imidazolium based IL, 1-hexadecyl-3-methylimidazolium bromide, [C16mim][Br], by varying its physical nature to modify the mixed micellization behavior of a cationic surfactant, hexadecyltrimethylammonium bromide (CTAB) in aqueous medium at different fixed concentrations of IL i.e. 1, 2 and 5 mmol L−1. The varying concentrations of IL has been chosen to have knowledge about the varying effect of IL mainly in monomeric form (1 mmol L−1), in micellar form (2 mmol L−1) and in solution rich in micelles at higher concentration of IL (5 mmol L−1). For the purpose, a variety of techniques such as conductivity, fluorescence, dynamic light scattering (DLS), isothermal titration calorimetry (ITC), transmission electron microscopy (TEM) and 1H NMR have been exploited to get detailed information about the phenomenon. The characteristic properties of the micellization, such as critical micelle concentration (cmc), degree of counter ion binding (β), thermodynamic parameters of micellization i.e. free energy (ΔGom), enthalpy (ΔHom), and entropy (TΔSom) of micellization at 298.15 K has been obtained from conductivity and isothermal calorimetry measurements, respectively. The aggregation number (Nagg) obtained from pyrene fluorescence quenching measurements is analyzed in combination with hydrodynamic diameter (Dh) of micelle obtained from dynamic light scattering technique. 1H NMR measurements have been found to be very useful in supporting the nature of formed micelles as IL rich or CTAB rich mixed micelles at different concentrations of IL, which is supported by relative change in 1H spin–lattice relaxation time in different mixed systems as compared to that of either of the constituents i.e. IL or CTAB only in aqueous media. It is observed that the content of long chain imidazolium based IL, [C16mim][Br], in mixed systems with CTAB strongly influences the micellization behavior.
2. Materials and methods
2.1 Materials
N-Methylimidazole (>99%), 1-bromohexadecane (>97%) were purchased from Sigma-Aldrich and used without further purification. Hexane and diethyl ether (AR grade) were purchased from SD Fine-Chem Ltd., Mumbai, India. Pyrene was purchased from Sigma Aldrich and used after recrystallization from ethanol. Cetyltrimethylammonium bromide (CTAB) (purity > 99.0%) was purchased from Sigma Aldrich. The ionic liquid (IL), 1-hexadecyl-3-methylimidazolium bromide was synthesized according to the procedure reported by Dupont et al.36 1-Methylimidazole and excess molar amount of appropriate alkyl bromide were dissolved in dichloromethane, and the mixture was stirred at 80 °C for 48 h. The dichloromethane was then removed using rotary evaporator under reduced pressure. The product was purified by repeating recrystallization from ethyl acetate, and then dried under vacuum for one day. The purity of the product was ascertained by 1H NMR spectrum in CDCl3.2.2. Methods
3. Results and discussion
3.1. Conductivity measurements
Micellization behavior in aqueous solutions of hexadecyltrimethylammonium bromide (CTAB) and micelle forming ionic liquid (IL), 1-hexadecyl-3-methylimidazolium bromide, [C16mim][Br] has been investigated by electrical conductivity measurements at fixed concentrations of IL i.e. 1, 2, and 5 mmol L−1. It is important to mention that the IL under investigation is also a surfactant having critical micelle concentration ≈ 0.65 mmol L−1.18 Therefore, the motto of choosing the above said concentrations for investigation was to probe the effect of presence of [C16mim]+ predominantly in monomeric form (1 mmol L−1) or in the presence of varying amount of micelles of IL (2 and 5 mmol L−1) on the mixed micellization of CTAB and IL. Further, the interaction between micelles of IL (2 and 5 mmol L−1) and added CTAB as a function of concentration was thought to shed light on the phenomenon that whether the expectedly formed mixed micelle structure is rich in IL or CTAB or the CTAB is just altering the properties of already present micelles of IL (2 and 5 mmol L−1) in aqueous solutions. Fig. 1(A)–(C) shows the variation of specific conductivity in different aqueous solutions of [C16mim][Br] as function of concentration of CTAB. | ||
| Fig. 1 Variation of specific conductance (κ) as a function of concentration of CTAB in aqueous solutions of IL [C16mim][Br] as (A) 1 mmol L−1; (B) 2 mmol L−1; and (C) 5 mmol L−1 at 298.15 K. | ||
The critical micelle concentration (cmc) values were obtained from the intersection of the two straight lines obtained by fitting the conductivity data in lower (below cmc) and higher concentration region (above cmc) of CTAB. The obtained values of cmc are summarized in Table 1. It can be seen from Table 1 that the cmc do not follow the linear trend with variation in content of IL in aqueous media. It is always expected that the cmc values decreases with increase in concentration of added electrolyte or surfactant in the solvent. In general, the cmc in investigated mixed systems is lower as compared that of cmc of CTAB in aqueous medium.37
| S. no. | Components | cmc/mmol L−1 | βa | ΔGom a | ΔGom b | ΔHom b | TΔSom b | Naggc | I1/I3c | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cond. | Flr | ITC | Ave | |||||||||
| a Conductivity.b Isothermal titration calorimetry.c Steady-state fluorescence measurements. The units of ΔGom, ΔHom and TΔSom are kJ mol−1. | ||||||||||||
| 1 | Only CTAB | 0.95 (ref. 37) | 0.90 (ref. 37) | — | — | 0.29 (ref. 37) | −46.5 (ref. 37) | 64 (ref. 37) | 1.05 (ref. 47) | |||
| 2 | Only [C16mim][Br] | 0.65 (ref. 18) 0.64 (ref. 14) | 0.84 (ref. 25) | 0.77 (ref. 18) | −50 (ref. 18) | −56.4 (ref. 14) | −4.4 (ref. 14) | 51.9 (ref. 14) | 64 (ref. 25) | 1.24 (ref. 25) | ||
| 3 | 1 mmol L−1 | 0.49 | 0.67 | 0.42 | 0.53 | 0.36 | −39.2 | −39.7 | −0.64 | 40.3 | 51 | 1.149 |
| 4 | 2 mmol L−1 | 0.83 | 0.60 | 0.61 | 0.68 | 0.20 | −33.2 | −35.7 | −1.05 | 34.6 | 42 | 1.158 |
| 5 | 5 mmol L−1 | 0.22 | 0.29 | 0.21 | 0.24 | 0.41 | −43.6 | −45.8 | −4.30 | 41.5 | 38 | 1.164 |
As can be seen from Table 1, contrary to the expectation, the cmc of CTAB (0.83 mmol L−1) in 2 mmol L−1 IL [C16mim][Br] has been found to be more than the cmc of CTAB in 1 mmol L−1 (0.49 mmol L−1) and 5 mmol L−1 (0.22 mmol L−1) of [C16mim][Br]. The presence of IL in different forms such as monomers (1 mmol L−1) or micelles (2 and 5 mmol L−1) along with change in water structure could be the reason. The observed behavior can be analyzed in view of the following points: (i) as per the law of mass action, the concentration of IL in the form of micelles are believed to be C-cmc and the cmc corresponds to the number of monomers in solution, C being the total concentration of surfactant in its aqueous solution above cmc.38 Therefore, it is expected that the maximum amount of IL remains in the form of [C16mim]+ ions in aqueous solutions at a concentration of 1 mmol L−1 providing the extended opportunity for added CTA+ to interact with [C16mim]+ via hydrophobic interactions. Further, it would always be easy for coming CTA+ to interact with hydrophobically hydrated [C16mim]+ as compared to that present in micellar form having relatively stronger hydrophobic and electrostatic interactions. Such stronger hydrophobic interactions between [C16mim]+ and CTA+ owing to same alkyl chain length could lead to lowering of cmc of CTAB in aqueous solutions of IL at a concentration of 1 mmol L−1; (ii) it is quite possible that while going from 1 mmol L−1 to 2 mmol L−1 of IL, there could be change in size and shape of micelle of IL similar to that of conventional ionic surfactants.39,40 There are reports that show that there exists π–π interactions between imidazolium ring present in micelle depending upon the nature of counterion.41 Therefore, it is speculated that there exists stronger π–π interactions between imidazolium rings present in micelles of IL already present in aqueous solution in case of 2 mmol L−1 IL; (iii) an increase in concentration of IL leads to increase in content of positively charged micelles of cationic [C16mim][Br] which could shield the incorporation of incoming positively charged CTA+ via electrostatic repulsions. This hinders the incorporation of added CTA+ into the micelles and delays the cmc in mixed solutions. There might be the partial insertion of CTA+ into the micelles, which increases the repulsions between the imidazolium head groups of IL, which is also supported by the relatively smaller degree of counterion binding in case of 2 mmol L−1 as seen from Table 1. Further, at a concentration of 2 mmol L−1 of IL, both the micelles as well as monomers of [C16mim]+ are present in solution, where there could a competition for the access of incoming [CTA]+ delaying its cmc. Therefore in case of 1 mmol L−1 of IL, it is proposed that the formed mixed micelles are rich in [C16mim]+, whereas in case of 2 mmol L−1 of IL, there is only insertion of CTA+ into the already present micelles of IL. As can be seen from Fig. 1(C), there exist two transitions, namely, C1 (0.11 mmol L−1) and cmc (0.23 mmol L−1) in the presence of IL at a concentration of 5 mmol L−1. Up to C1, the conductivity increases with a relatively lower slope, and is assigned to the hindered diffusion of added CTA+ and Br− in the presence of large number of charged micelles of IL present in solution. At the same time the slight insertion of CTA+ into the micelles of IL cannot be ruled out at this point. At C1, the micelles of IL seems to be saturated for incoming CTA+ as incorporation of larger amount of CTA+ into the IL micelles could destabilize the micelle owing to non-planar nature of CTA+ between the planer imidazolium head groups. Thereafter, the conductivity rises with relatively higher slope with the addition of CTAB up to cmc after which, it again increases by a relatively lower slope. The increase in slope after C1 is presumed to be due to increase in number of free CTA+ and Br− in the solution along with change in structure of solvent, which self-assembles in the form of CTA+ rich micelles at cmc. It is important to discuss the lowering of cmc of CTAB from 0.92 mmol L−1 to 0.22 mmol L−1 in the presence of IL (5 mmol L−1). In the aqueous solution of IL at this concentration, the presence of large number of micelles of IL as well as appropriate amount of counterions could lead to change in water structure. This change in water structure along with the high charge density owing to presence of large amount of micelles of IL–CTAB could lead to such lowering of cmc.42 From 1H NMR and 1H spin–lattice relaxation time (T1) measurements (discussed later), it is observed that there exists negligible insertion of added CTA+ into the already present micelles of IL, where electrostatic repulsion between incoming CTA+ and positively charged micelles seems to play an important role. Therefore, it is proposed that at 5 mmol L−1 of IL, at C1, the mixed micelles formed are rich in IL phase whereas at cmc, both IL rich and CTAB rich micelles coexist together. The counterion binding parameter (β) gives the average number of counterions per surfactant ion in the micelle and is estimated from the ratio of the slopes (S2/S1) of conductivity change after cmc (S2) and before cmc (S1) as 1 − S2/S1.43 The obtained values of β have been summarized in Table 1. The values of β for the investigated systems follow the order: 5 mmol L−1 > 1 mmol L−1 > 2 mmol L−1, which is in line with that of obtained cmc values. The higher value of β in aqueous solution of IL at a concentration of 5 mmol L−1 helps in shielding the electrostatic repulsions between the ionic head groups of [C16mim]+ and [CTA]+ effectively and thus decreases the cmc of the mixed micelles of IL–CTAB. The phenomenon of lowering of cmc in mixed systems of IL and CTAB is quite similar to that of lowering of cmc of CTAB in the presence of different co-surfactants such as short chain amphiphilic surfactant, hexyltrimethylammonium bromide or amphiphilic IL, 1-hexyl-3-methylimidazolium bromide at different concentrations.33 The cmc first decreases up to IL content of 1 mmol L−1 followed by further increase up to an IL concentration of 2 mmol L−1, whereas it again decreases dramatically in the presence of higher IL content of 5 mmol L−1. The presence of [C16mim]+ ions in majority in monomeric form at a concentration of 1 mmol L−1 forces them to act as co-surfactant similar to that observed in the presence of HeTAB or [C6mim][Br] at lower concentrations, whereas at higher concentration (5 mmol L−1), the varying charge density and change in solvent structures seems to be responsible for lowering of cmc in the mixed systems. The proposed mechanism for formation of different types of mixed micelles is depicted in Scheme 1.
 | ||
| Scheme 1 Pictorial representation showing the mechanism of interactions between IL and CTAB and the formation of different IL–CTAB mixed micelles in different aqueous solutions of IL. | ||
The Gibbs free energy of the micellization (ΔGom) was calculated using the obtained values of β using the following equation:44
ΔGom = (1 + β)RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xcmc Xcmc
| (1) |
3.2. Steady-state fluorescence measurements
The steady-state fluorescence exploiting the pyrene as fluorescent probe has been employed to investigate the micellization in mixed systems under investigation. This method is based on the changes in the intensity of the vibrational bands of pyrene solubilized in micellar medium. The ratio of intensity of first vibrational band (I1 at 373 nm) to that of third vibrational band (I3 at 384 nm) i.e., I1/I3, is sensitive to the polarity of the surrounding microenvironment45 and this ratio decreases with decreasing solvent polarity.45–47 Owing to the hydrophobic nature of pyrene, upon micellization, the movement of pyrene from water to the hydrophobic regions of micelle leads to abrupt change in I1/I3 values and indicates the cmc. Fig. 2(A) shows the variation of I1/I3 of pyrene as a function of concentration of CTAB in aqueous solutions of IL at different concentrations i.e., 1, 2 and 5 mmol L−1.The lower values of I1/I3 in aqueous solutions of ILs in the absence of CTAB as compared to that in water25 only suggests that the pyrene is solubilized in the hydrophobic regions of micelle formed by IL, and it is relatively lower in case of IL at a concentration of 5 mmol L−1. Contrary to that observed from conductivity measurements, a decrease in cmc with increase content of IL in aqueous solutions has been observed. Further, we could only observe a faint transitions corresponding to C1 in the aqueous IL solutions having IL content of 5 mmol L−1 even after repeated measurements. On the other hand, two transitions in the variation of I1/I3 have been observed in case of aqueous solutions of IL at a concentration of 1 mmol L−1. This is assigned to the fact that different analytical techniques sense different stages of micellization and can be more or less sensitive towards particular changes upon micellization. In conductivity measurements, the ionic diffusion is measured and using pyrene as fluorescent probe, we detect the changes in polarity of pyrene upon micellization. Therefore, it is inferred that there is no detectable abrupt change in polarity of cybotactic region of pyrene in two concentration range between C1 and cmc as discussed in conductivity measurements in case of aqueous IL solutions at 5 mmol L−1. In case of aqueous IL solutions at 1 mmol L−1 of concentration, two transitions in I1/I3 has been observed. Initially an abrupt decrease in I1/I3 from the beginning up to C1 is observed, which then increases marginally up to C2 in a defined concentration regime after which, again a sudden decrease is observed before reaching a plateau region upon micellization at cmc. As discussed in the conductivity section, at this concentration of IL, the number of [C16mim]+ in monomeric form is more as compared to that of number of micelles formed by IL. Therefore, it is expected that added CTA+ would interact primarily with the [C16mim]+. Therefore, the transition assigned as C1 occurs at very low concentration of CTAB and is assigned to the formation of mixed CTAB–IL premicelles as such structures could possess lower polarity as compared to that of solvent. The marginal increase in I1/I3 value at C2 is due to water penetration, which makes the microenvironment of pyrene relatively polar. The incorporation of more of the CTA+ into the micelles of IL could lead to such changes as this may provide some space for water to penetrate into micelle owing to asymmetric packing at micelle–water interface. It is important to mention that the results shown here are for an average environment of pyrene present in different types of formed micelle however the results can be compared for understanding the phenomenon. On comparing the I1/I3 values upon completion of mixed micelle formation, it is observed that it varies in the order: 1 mmol L−1 < 2 mmol L−1 < 5 mmol L−1. This indicates that the IL rich CTAB–IL mixed micelles formed in the presence of 1 mmol L−1 of IL are more compact in nature, whereas the CTAB rich CTAB–IL mixed micelles formed at higher content of IL are relatively looser one. The increased values of I1/I3 for solutions having mixed micelles with increasing IL content can be analyzed in terms of water penetration into micelles.48,49 It has been suggested that water can enter the micelles and extend up to four carbons from the head group.50,51 The polarity index depends on the surfactant head group type and should not depend on the surfactant chain length, concentration, counter ion and added electrolyte.45 It is expected that there exists stronger interaction between the π-electron cloud of pyrene and the delocalized positive charge of imidazolium group as compared to that with the quaternary ammonium headgroup having localized positive charge. Such interactions may draw pyrene closer to the micelle surface when concentration of IL is high supporting the fact that I1/I3 is more for higher concentrations of IL. It is important to keep in mind the change in the solvent structure and the microenvironment of pyrene which is an averaged of all types of micelles present in the system while analyzing such systems. The I1/I3 values in hydrocarbon solvent vary in the range of 0.57–0.61 and in polar solvents the value is 1.25–2.00.45 For various micellar systems involving conventional surfactants, I1/I3 ratio remains in the range of 1.1–1.5.45 Upon comparison with the results obtained here (I1/I3 = 1.15–1.17), it is observed that the polarity of the cybotactic region of pyrene is between those of aromatic solvents and hydrocarbon solvents, which is much lower than that of micelles of IL (1.24) in the absence of CTAB.52 This indicates that pyrene is solubilized relatively deeper in palisade layer of micelle in all the investigated systems.
Steady state fluorescence quenching measurements have been used to determine the aggregation number (Nagg) of micelles in different aqueous IL solutions using pyrene as the fluorescent probe and cetylpyridinium chloride as the quencher using the following equation:53
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = ln I = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I0 − Cq/Cm = ln I0 − Cq/Cm = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I0 − NaggCq/(Ct − cmc) I0 − NaggCq/(Ct − cmc)
| (2) |
3.3. Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) is a useful technique to determine the cmc values and enthalpy of micellization (ΔHomΔH) for surfactants. Fig. 3(A)–(C) shows the variation of differential enthalpy (dH) as function of the CTAB concentration in presence of different amount of [C16mim][Br] as 1, 2, and 5 mmol L−1 in aqueous solutions.With the addition of stock solution of CTAB in sample cell reaching final concentration of surfactant below cmc, dissociation of micelles takes place to form CTA+, which subsequently gets hydrated. As the total concentration of surfactant in sample cell exceeds the cmc, the micelles are formed, after which only dilution of micelles takes place with further addition of surfactant.54 The shape and enthalpy outcomes from the enthalpograms depends on different factors such as surfactant concentration, aggregation number (Nagg), degree of counterion binding, solvation–desolvation, temperature, molar enthalpy of monomer and micelle etc.55–57 In case of aqueous IL solutions at a concentration of 1 mmol L−1, a gradual decay in dH from endothermic region towards relatively less endothermic region leading to an overall exothermic effect is observed, which becomes almost constant after formation of mixed micelles. The main contribution to the enthalpy of micellization is associated with the transfer of the hydrocarbon chain of the surfactant monomer from the aqueous environment to the micelle thereby enhancing hydrophobic interactions between the alkyl chains by releasing solvating water molecules, which gives rise to exothermic effect.58,59 Electrostatic interactions involve repulsion between the similarly charged ions contributes exothermically towards total enthalpy change, whereas an attraction between the head-group and the counter-ion contributes endothermically towards total enthalpy change of micellization. It is thought that with the addition of stock solution of CTAB, demicellization followed by hydrophobic interactions with the alkyl chain of the [C16mim]+ takes place forming premicelles like aggregates, which remains highly hydrated giving to endothermic change in enthalpy. With the increase in concentration of CTAB, the hydrophobic interactions get stronger leading to dehydration of alkyl chains near to cmc resulting in a net exothermic change. Therefore it is speculated that the events such as transfer of alkyl chain from aqueous to hydrophobic environment involving release of water molecules along with electrostatic repulsions between similarly charged head groups of CTA+ and [C16mim]+ dominates over the electrostatic interactions between surfactant head group and counterions leading to a decrease in magnitude of endothermic enthalpy changes towards formation of mixed micelle. At higher concentration (2 and 5 mmol L−1) of IL [C16mim][Br], the dH values remains almost constant at very low concentration of CTAB followed by a rise with relatively larger slope before cmc as compared to that at concentrations after cmc, representing an almost sigmoidal form. From the enthalpogram, it is clear that before cmc, an exothermic change has been observed which turns to endothermic after cmc at 2 mmol L−1 concentration of IL, whereas similar change much small in magnitude is observed at higher concentration of IL, i.e. (5 mmol L−1) for the higher concentration of IL (2 and 5 mmol L−1), it is expected that the insertion of CTA+ into the already present micelles is difficult due to strong electrostatic repulsions between them as compared to that its interactions with IL monomers present in case of 1 mmol L−1 of IL. Therefore, it is speculated that the presence of IL micelles could affect the demicellization process (endothermic), whereas it provides an environment for CTA+ to get inside the IL micelles via releasing hydrating water which give rise to exothermic effect below cmc. After cmc, other factors such as Nagg, β and inter-micellar interactions or repulsions are expected to play their role. In case of system having IL content of 5 mmol L−1, there can be some additional factors affecting the outcome. One of the important factor is the presence of large amount of micelles which decreases the intermicellar distance and causes greater repulsion between the adjacent micelles (exothermic). Therefore to minimize the charge repulsion between adjacent micelles, it induces the rapid uptake of counterions, as established by larger β values, for stabilization of mixed micelle at higher concentration (endothermic).
The values of cmc and related change in ΔHom were obtained using the standard procedures55 and their values are reported in Table 1. The values of ΔGom has been obtained using the degree of counterion binding (β) from the conductivity method and the cmc obtained from ITC measurements based on the standard protocol.56 The standard entropy of micellization TΔSom has been obtained using Gibbs–Helmolthz equation. The cmc values obtained from ITC measurements in different concentrations of [C16mim][Br] (1, 2 and 5 mmol L−1) are in line with those obtained from conductivity measurements. As can be seen from Table 1, The ΔGom obtained from ITC measurements follow similar trend and are similar in magnitude to that obtained from conductivity measurements. ΔHom for the formation of mixed micelles is negative for all the concentrations of [C16mim][Br] and follows the order: 1 mmol L−1 (−0.64 kJ mol−1) < 2 mmol L−1 (−1.05 kJ mol−1) < 5 mmol L−1 (−4.30 kJ mol−1). The enthalpic contribution towards ΔGom has been found to increase with increase in concentration of IL, where it contributes around 2%, 3% and 9% for mixed micellization of CTAB-[C16mim][Br] at IL concentrations of 1, 2 and 5 mmol L−1, respectively. The factors contributing towards entropy of micellization, TΔSom are (i) modification in head-group hydration consistent with surface charge density, (ii) releasing hydrating water molecules by surfactant molecules, (iii) counter-ion condensation and (iv) increase in the degrees of freedom of the surfactant molecules in the micelle.60,61 The contribution of all these processes results in an increase in the entropy of the whole system, i.e. positive entropy change. The entropic contribution to ΔGom follows the order: (2 mmol L−1) < (1 mmol L−1) < (5 mmol L−1), which is of the same order as exhibited by ΔGom. On comparing the values of ΔHom and TΔSom, it is inferred that the mixed micellization at all the concentrations is an entropy driven phenomenon and there is much less contribution of the enthalpy change.
3.4 Dynamic light scattering and transmission electron microscopy measurements
Dynamic light scattering (DLS) is used to obtain the average size of the mixed micelles formed by CTAB and IL, [C16mim][Br], in different aqueous solutions of IL. Fig. 4 shows the intensity weighed size distribution profile for CTAB in aqueous IL solutions with three different concentrations i.e. 1, 2, and 5 mmol L−1 along with DLS profiles for aqueous IL solutions in the absence of CTAB.The intensity weighed size distribution profile shows the presence of two size distributions corresponding to different hydrodynamic diameter (Dh) for both in the absence and in the presence of CTAB. The scattering peak in the range of 1.2–4.5 nm corresponds to the scattering from micelles, whereas the scattering peak corresponding to Dh of 65–300 nm is assigned to larger aggregates present in the system. As the intensity of the scattered light is directly proportional to 6th power of the radius of a particle, therefore it is considered that larger particles scatter much more light than smaller ones. It has been established that a particle twice the size of the small particle scatters 64 times more light.62 Therefore, the relative intensity of scattered light along with the nature of correlograms shown in Fig. S1 (ESI†) obtained from DLS was considered while analyzing the DLS data. It is important to discuss the DLS data for [C16mim][Br] micellar solutions at different concentrations in the absence of CTAB for better understanding. In case of 1 mmol L−1 IL only, (Fig. 4, solid black line), the intensity of scattered light for scattering peaks corresponding to smaller and larger Dh have almost same intensity and the corresponding correlation function (Fig. S1, ESI†) does not show any remarkable signature for presence of larger micelles. Therefore, only the presence of smaller micelles is considered. In case of 2 mmol L−1 of IL (Fig. 4, solid black line), the intensity of scattered light for scattering peaks corresponding to larger Dh is higher as compared to that for peak corresponding to smaller Dh along with presence of a weak signature of two relaxation regimes in the correlogram showing the presence of two types of micelles where smaller micelles are assumed to exist in majority considering the facts described above. However, in case of 5 mmol L−1 of IL (Fig. 4, solid black line), there exist clear evidence in the correlogram (Fig. S1, ESI†) for the presence of two types of micellar structures along with relatively higher intensity of the peak corresponding to larger particles. In this system, the presence of peak corresponding to larger particles is assigned to the agglomeration of smaller micelles as observed earlier. Considering the above described phenomenon, the presence of larger aggregates is only considered in case of systems comprising 5 mmol L−1 of IL. As can be seen from Fig. 4, the Dh decreases with increase in concentration of IL. Further, the interesting point is that in all the investigated systems, the size of micelle decreases in the presence of CTAB as compared to that of micelles of IL. This shows that the presence of CTAB in mixed micelles destabilized the micelles of IL. This could be due to the fact that there is a mismatch between [C16mim]+ and [CTA]+ in terms of charge localization, size, symmetry as well as polarity. This mismatch is considered to be a main factor leading to such destabilization. Although, the content of IL has a say in this phenomenon of mixed micelle formation, however, it is quite clear that the mixed micelles formed by CTAB and IL are relatively less stable as indicated by lower degree of counterion binding in the presence of IL as compared to that of neat CTAB (Table 1). On comparison, it is observed that the largest size of 3.6 nm in mixed micelle is exhibited in the presence of 1 mmol L−1 of IL, whereas with increase in content of IL to 5 mmol L−1, the corresponding size decreases to ≈1.2 nm. The observation is also supported by variation in Nagg, which decreases with increase in content of IL in aqueous solution.
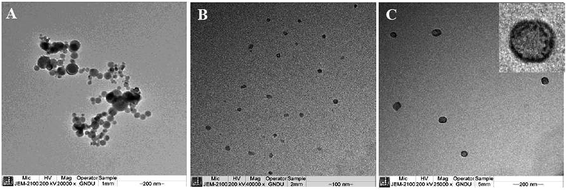
The morphology of the formed micelles has been investigated by transmission electron microscopy (TEM) measurements. Fig. 5(A)–(C) shows the TEM images of the formed mixed micelles at different concentrations of ILs. As can be observed from Fig. 5(A), the mixed structures of CTAB and IL formed in the presence of 1 mmol L−1 of IL are highly spherical vesicles having a size in the range of 20 to 150 nm which is in line with the Dh obtained from DLS measurements.
As discussed earlier, the presence of the IL in monomeric form interacts strongly with similarly charged homologous CTA+ at IL concentration of 1 mmol L−1. The presence of stronger hydrophobic interactions between the two cations is expected to be a cause behind the formation of vesicles, where the longer alkyl chains provide enough hydrophobicity along with alteration in head group area at the interface to change the curvature of forming aggregates leading to formation of vesicles. At a concentration of 2 mmol L−1 of IL, relatively smaller sized mixed micelles of size 15–25 nm has been observed as shown in Fig. 5(B). It is supposed that these mixed micelles are composed of relatively smaller micelles which have also been detected from DLS measurements (Fig. 4). At an IL content of 5 mmol L−1, the formation of CTAB rich CTAB–IL mixed micelles are expected based on observations made from other techniques. As per the expectation, the presence of large micelles in the range of 50–150 nm has been observed in corroboration with DLS results. A careful analysis of TEM image shows that these large aggregates are composed of a large number of smaller aggregates as shown in inset of Fig. 5(C). The observations made from TEM have shed light on mechanistic aspects of formation of mixed micelle as can be seen from Scheme 1.
3.5 1H NMR and 1H spin–lattice relaxation time measurements
1H NMR spectra recorded below and above the cmc observed in different mixed systems of the CTAB and [C16mim][Br] proved to be useful in predicting the type of micelle formed and the relative placement of the two cations having same chain length in the mixed micelles. The 1H NMR spectra of CTAB–IL mixtures in the absence and presence of CTAB is provided in Fig. 6(A)–(C).A downfield shift for the protons at terminal methyl group (Ha) and protons of the aliphatic chain (Hb) of CTAB is observed for the lower IL concentrations (1 and 2 mmol L−1). Interestingly, the broadening of resonance peak corresponding to Hb in the presence of 1 mmol L−1 of IL supports the formation of vesicles at this composition of systems as observed from TEM measurements.63 The change in chemical shift values Δδ(δabove cmc − δbelow cmc) for all the protons is shown in the Fig. 7 for all the investigated concentrations of IL.
 | ||
| Fig. 7 Plot showing the magnitude of variation in Δδ = δabove cmc − δbelow cmc for different protons of CTAB and [C16mim][Br] in the aqueous solutions of [C16mim][Br] at the investigated concentrations (proton numbering is according to Fig. 6). | ||
As can be seen from Fig. 7, the extent of downfield shift for the protons Ha and Hb in the presence of different content of IL follows the order: 1 mmol L−1 > 2 mmol L−1 > 5 mmol L−1 indicating the greater effect of mixed micelle formation at lower content of IL. The transition from gauche to trans conformation in the alkyl chain as a consequence decreased packing density due to the formation of mixed micelles is responsible for this downfield.64 The transfer of alkyl chain from aqueous environment to hydrophobic interior of micelle on the formation of IL–CTAB mixed micelles also leads to such downfield shift. On the other hand, negligible downfield shift observed for protons (Ha and Hb) at higher concentration of [C16mim][Br] (5 mmol L−1) as compared to that at lower content of IL i.e. 1 and 2 mmol L−1 clearly indicates the partial insertion of CTA+ into the already present aggregates of [C16mim][Br]. The presence of large number of micelles along with a change in solvent structure could lead to such behavior. A similar downfield shift is observed for the protons of the alkyl chain present in vicinity of the imidazolium head group (Hd and Hh), which follows the same order of downfield shift as followed by Ha and Hb. However, the magnitude of downfield shift in case of Hd and Hh is more as compared to Ha and Hb. Such behavior is ascribed to a decrease in the hydrophobic environment near these protons because of more water penetration as a consequence of formation of mixed micelles. The tetrahedral geometry of the ammonium head group of CTAB create enough free space for the water to penetrate via electrostatic and hydrophobic interactions between the π stacked closely spaced imidazolium head groups of previously present micelles of IL.
Similar downfield shift trend is observed for the protons (Hc and Hf) of the CTA+ present near the ammonium head group of CTAB. As shown in Fig. 7, the imidazolium head group protons (Hi, Hj and Hk) experiences the downfield shift in the order: 1 mmol L−1 > 2 mmol L−1 > 5 mmol L−1 supporting the fact that the extent of formation of mixed micelles of CTAB and [C16mim][Br], which disrupts the π stacking that is present between the imidazolium rings of pure micelle of [C16mim][Br] decreases with increase in content of IL. Further the splitting pattern of resonance peak below and above cmc supports the above observations. In case of protons Hi and Hj of imidazolium ring, the singlet resonance peak below cmc splits into the doublet at above cmc in case of 1 and 2 mmol L−1 of IL, however remains singlet in case of 5 mmol L−1 concentration of IL. The splitting of the signal is due to the presence two protons (Hi and Hj) in different chemical environments, which could have aroused due the presence of ammonium head groups of CTA+ between the imidazolium head groups of IL in micelle. This observation clearly supports the formation of mixed micelles of IL and CTAB at lower IL concentration (1 and 2 mmol L−1) as compared to the relative higher concentration of IL (5 mmol L−1), where CTA+ could not penetrate into the micelles of IL. From above facts, the notion that can be generalized for these three investigated systems is that the extent of downfield shift and splitting in the signals of various protons is directly proportional to the extent of mixed micelle formation. The above observations fully support the mechanism of interactions between IL and CTAB and the formation of different IL–CTAB mixed micelles in different aqueous solutions of IL as shown in Scheme 1.
To support the formation of different types of micellar aggregates in different solutions of IL with varying concentrations, we have measured 1H spin–lattice relaxation time (T1) for mixed systems comprising IL and CTAB above the observed cmc and compared with that of aqueous systems of IL (1, 2 and 5 mmol L−1) or CTAB (below its cmc in water i.e. at 0.2 mmol L−1 or above its cmc in water i.e. at 2 mmol L−1). The relative magnitude T1 provides information about steric environment of different protons,12 which has been used to confirm the formation of different types of micellar structures in various systems at different concentrations as shown in Scheme 1. We have analyzed mainly the 1H spin–lattice relaxation time for imidazolium ring protons numbered Hi, Hj and Hk (Fig. 6) of IL and head group protons of CTAB numbered He (Fig. 6) as these protons are present at the micelle–water interface, thus provides maximum information. The obtained 1H spin–lattice relaxation time (T1) is provided in Table S1 (ESI†). In general, upon micellization, the motion of surfactant monomers gets restricted due to incorporation in micelle leading to decrease in T1.12 A similar decrease in T1 for both CTAB as well as IL in aqueous solutions has been observed in the present study as can be seen from Table S1 (ESI†).
In case of CTAB-[C16mim][Br], T1 for head group protons of imidazolium ring i.e. Hi, Hj and Hk in their aqueous solutions in the absence of CTAB decreases upon formation of mixed micellization in case of 1 and 2 mmol L−1 whereas it remains almost unchanged in case of 5 mmol L−1 of IL as can be seen from Table S1 (ESI†). The relative change in the absence and presence of CTAB upon formation of mixed micelles follows the order: 1 mmol L−1 > 2 mmol L−1 > 5 mmol L−1. This shows that the interaction of CTA+ is predominantly with monomers of IL in case of 1 mmol L−1 of IL, where incorporation of [C16mim]+ in formed mixed vesicles leads to large decrease in T1 as the surfactant ions remains in more constrained environment in vesicles as compared to that in micelles. This is also supported from TEM imaging. The relatively smaller decrease in T1 in CTAB-[C16mim][Br] (2 mmol L−1) system as compared to [C16mim][Br] (2 mmol L−1) in water indicates relatively lesser change in microenvironment of these ring protons upon mixed micellization which can be only true in case of formation of IL rich mixed micelles. The constancy of T1 in CTAB-[C16mim][Br] (5 mmol L−1) system even after cmc indicates almost negligible change in microenvironment of aromatic protons resulting from partial insertion of CTA+ into the already present micelles of IL as also indicated by 1H NMR measurements and shown in Scheme 1. The above discussed results are corroborated by comparing the variation in T1 of head group protons of CTAB i.e. He in CTAB-[C16mim][Br] mixed systems to that of CTAB only at concentrations below and above its cmc in water. As can be seen from Table S1 (ESI†), T1 for He is lower in mixed CTAB-[C16mim][Br] systems as compared to its micellar solutions. This indicates a more restricted environment for CTA+ in the mixed micelle systems. Further, T1 for He in CTAB-[C16mim][Br] systems increases with increase in content of IL where as it much lower in case of 1 mmol L−1 of IL as compared to other systems confirming the tightest engagement of CTA+ in mixed vesicles as discussed above. T1 for He for CTAB-[C16mim][Br] (5 mmol L−1) is quite close to that observed for micelles of CTAB in the absence of water, which suggest the formation of CTAB rich micelles as also indicated by different measurements. Although, there is an overlap of resonance peaks for protons of alkyl chain of IL and CTAB, however, in general, the T1 for alkyl chain protons (Ha and Hb) also supports the above findings.
4. Conclusion
The effect of varying physical nature of a surfactant like ionic liquid (IL), 1-hexadecyl-3-methylimidazolium bromide, [C16mim][Br] on mixed micellization with cetyltrimethylammonium bromide, [CTAB] has been investigated at different fixed concentrations of IL i.e. 1, 2 and 5 mmol L−1. The physical nature of IL i.e. predominantly as monomers (1 mmol L−1) or micelles (2 and 5 mmol L−1) has been found to exert great influence on critical micelle concentration (cmc) along with various characteristic properties of micellization such as degree of counterion binding (β), thermodynamic parameters and nature of the mixed micelle. The cmc and β values in mixed systems were found to be relatively higher and lower, respectively, in middle concentration range (2 mmol L−1) as compared to either low (1 mmol L−1) or high (5 mmol L−1) content of IL. The variation in compactness of micelle as a function of content of IL in the mixed system is corroborated by aggregation number (Nagg) measurements, where an increase in content of IL leads to formation of relatively less compact micelles with lower Nagg. The formation of IL rich CTAB–IL mixed vesicles is observed at lower content of IL (1 mmol L−1), whereas at higher content (2 and 5 mmol L−1) of IL, the added CTA+ penetrates into the already present micelles of IL at this concentration with decreasing extent of penetration with increase in concentration of IL. Such behavior was manifested by formation of mixed CTAB–IL vesicles at lower content of IL (1 mmol L−1) as compared to the micelles of varying size at higher content of IL (2 and 5 mmol L−1). In nutshell, the physical nature of the IL along with change in solvent structure is found to exert influence on cmc, shape and size of the formed mixed micelles of IL and CTAB.Acknowledgements
The authors are thankful to DST, Government of India, for financial assistance to carry out this work wide Project Scheme No. SB/FT/CS-057/2013. Chanda Chadha is thankful to DST, Government of India, for providing DST Inspire Fellowship (IF120453) wide sanction order no. DST/INSPIRE FELLOWSHIP/2012/428. We are also thankful to the UGC, India, for UGC-CAS (Centre for Advanced Studies) program and UPE program for creating infrastructure and research facilities at Guru Nanak Dev University, Amritsar. Authors are thankful to Mr Ravinder Singh for TEM measurements. Authors are thankful to reviewers for their suggestions.References
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2nd edn, 2007 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- R. Sheldon, Chem. Commun., 2001, 23, 2399–2407 RSC.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- P. Wang, S. M. Zakeeruddin, M. Shaik, R. Humphry Baker and M. Gratzel, Chem. Mater., 2004, 16, 2694–2696 CrossRef CAS.
- A. Cornellas, L. Perez, F. Comelles, I. Ribosa, A. Manresa and M. T. Garcia, J. Colloid Interface Sci., 2011, 355, 164–171 CrossRef CAS PubMed.
- J. Bowers, C. P. Butts, P. J. Martin, M. C. Vergara Gutierrez and R. K. Heenan, Langmuir, 2004, 20, 2191–2198 CrossRef CAS PubMed.
- B. Dong, N. Li, L. Q. Zheng, L. Yu and T. Inoue, Langmuir, 2007, 23, 4178–4182 CrossRef CAS PubMed.
- C. Jungnickel, J. Luczak, J. Ranke, J. F. Fernandez, A. Muller and J. Thoming, Colloids Surf., A, 2008, 316, 278–284 CrossRef CAS.
- Z. Miskolczy, K. Sebok Nagy, L. Biczok and S. Goktutk, Chem. Phys. Lett., 2004, 400, 296–300 CrossRef CAS.
- T. Singh and A. Kumar, J. Phys. Chem. B, 2007, 111, 7843–7851 CrossRef CAS PubMed.
- Y. Zhao, S. J. Gao, J. J. Wang and J. M. Tang, J. Phys. Chem. B, 2008, 112, 2031–2039 CrossRef CAS PubMed.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Green Chem., 2007, 9, 481–490 RSC.
- F. Geng, J. Liu, L. Zheng, L. Yu, Z. Li, G. Li and C. Tung, J. Chem. Eng. Data, 2010, 55, 147–151 CrossRef CAS.
- H. Y. Wang, J. J. Wang, S. B. Zhang and X. P. Xuan, J. Phys. Chem. B, 2008, 112, 16682–16689 CrossRef CAS PubMed.
- J. J. Wang, H. Y. Wang, S. B. Zhang, H. C. Zhang and Y. Zhao, J. Phys. Chem. B, 2007, 111, 6181–6188 CrossRef CAS PubMed.
- R. Kamboj, P. Bharmoria, V. Chauhan, S. Singh, A. Kumar, V. S. Mithu and T. S. Kang, Langmuir, 2014, 30, 9920–9930 CrossRef CAS PubMed.
- T. Inoue, H. Ebina, B. Dong and L. Zheng, J. Colloid Interface Sci., 2007, 314, 236–241 CrossRef CAS PubMed.
- T. Singh and A. Kumar, J. Phys. Chem. B, 2008, 112, 4079–4086 CrossRef CAS PubMed.
- T. Singh, K. S. Rao and A. Kumar, ChemPhysChem, 2011, 12, 836–845 CrossRef CAS PubMed.
- J. Wang, L. Zhang, H. Wang and C. Wu, J. Phys. Chem. B, 2011, 115, 4955–4962 CrossRef CAS PubMed.
- N. Li, S. Zhang, L. Zheng, B. Dong, X. Li and L. Yu, Phys. Chem. Chem. Phys., 2008, 10, 4375–4377 RSC.
- G. Y. Bai, A. Lopes and M. Bastos, J. Chem. Thermodyn., 2008, 40, 1509–1516 CrossRef CAS.
- I. Goodchild, L. Collier, S. L. Millar, I. Prokes, J. C. D. Lord, C. P. Butts, J. Bowers, J. R. P. Webster and R. K. Heenan, J. Colloid Interface Sci., 2007, 307, 455–468 CrossRef CAS PubMed.
- B. Dong, X. Zhao, L. Zheng, J. Zhang, N. Li and T. Inoue, Colloids Surf., A, 2008, 317, 666–672 CrossRef CAS.
- V. Chauhan, R. Kamboj, S. P. S. Rana, T. Kaur, G. Kaur, S. Singh and T. S. Kang, J. Colloid Interface Sci., 2015, 446, 263–271 CrossRef CAS PubMed.
- Y. Shang, T. Wang, X. Han, C. Peng and H. Liu, Ind. Eng. Chem. Res., 2010, 49, 8852–8857 CrossRef CAS.
- K. Behera and S. Pandey, J. Colloid Interface Sci., 2007, 316, 803–814 CrossRef CAS PubMed.
- K. Behera, P. Dahiya and S. Pandey, J. Colloid Interface Sci., 2007, 307, 235–245 CrossRef CAS PubMed.
- K. Behera, V. Kumar and S. Pandey, ChemPhysChem, 2010, 11, 1044–1052 CrossRef CAS PubMed.
- S. Javadian, V. Ruhi, A. Heydari, A. A. Shahir, A. Yousefi and J. Akbari, Ind. Eng. Chem. Res., 2013, 52, 4517–4526 CrossRef CAS.
- K. Behera, M. D. Pandey, M. Porel and S. Pandey, J. Chem. Phys., 2007, 127, 184501–184510 CrossRef PubMed.
- K. Behera, H. Om and S. Pandey, J. Phys. Chem. B, 2009, 113, 786–793 CrossRef CAS PubMed.
- L. L. Schramm, E. N. Stasiuk and D. G. Marangoni, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2003, 99, 3–48 RSC.
- L. S. Walekar, U. R. Kondekar, A. H. Gore, S. P. Pawar, V. Sudarsan, P. V. Anbhule, S. R. Patil and G. B. Kolekar, RSC Adv., 2014, 4, 58481–58488 RSC.
- J. Dupont, C. S. Consorti, P. A. Suarez and R. F. Souza, Org. Synth., 2002, 79, 236–241 CrossRef CAS.
- P. Bharmoria, Vaneet, P. M. Banipal, A. Kumar and T. S. Kang, J. Solution Chem., 2015, 44, 16–33 CrossRef CAS.
- A. I. Rusanov, Adv. Colloid Interface Sci., 1993, 45, 1–78 CrossRef CAS.
- S. Hayashi and S. Ikeda, J. Phys. Chem., 1980, 84, 744–751 CrossRef CAS.
- C. Tanford, J. Phys. Chem., 1972, 76, 3020–3024 CrossRef CAS.
- P. S. Gehlot, K. S. Rao, P. Bharmoria, K. Damarla, H. Gupta, M. Drechsler and A. Kumar, J. Phys. Chem. B, 2015, 119, 15300–15309 CrossRef CAS PubMed.
- S. Javadian, V. Ruhi, A. A. Shahir, A. Heydari and J. Akbari, Ind. Eng. Chem. Res., 2013, 52, 15838–15846 CrossRef CAS.
- H. Hoffmann and W. Ulbricht, Z. Phys. Chem., 1977, 106, 167–184 CrossRef CAS.
- M. J. Rosen, Surfactants and Interfacial Phenomena, Wiley, New York, 2nd edn, 1989, vol. 269 Search PubMed.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039–2044 CrossRef CAS.
- Surfactant Solutions. New Methods of Investigation, ed. R. Zana, Surfactant Science Series, Marcel Dekker, New York, 1987, vol. 22, p. 30 Search PubMed.
- J. Aguiar, P. Carpena, J. A. Molina-Bolivar and C. J. Carnero Ruiz, J. Colloid Interface Sci., 2003, 258, 116–122 CrossRef CAS.
- L. Zhai, X. Lu, W. Chen, C. Hu and L. Zheng, Colloids Surf., A, 2004, 236, 1–5 CrossRef CAS.
- F. M. Menger, Acc. Chem. Res., 1979, 12, 111–117 CrossRef CAS.
- F. M. Menger, J. M. Jerkumica and J. C. Jhonston, J. Am. Chem. Soc., 1978, 100, 4676–4678 CrossRef CAS.
- J. H. Fendler and E. J. Fendier, Catalysis in Micellar and Macromolecular Systems, Academic Press, New York, NY, USA, 1975 Search PubMed.
- N. Muller, Reaction Kinetics in Micelles, ed. E. A. Cordes, Plenum Press, New York, NY, 1973, pp. 1–24 Search PubMed.
- N. J. Turro and A. Yekta, J. Am. Chem. Soc., 1978, 100, 5951–5952 CrossRef CAS.
- G. Wang and G. Olofsson, J. Phys. Chem., 1995, 99, 5588–5596 CrossRef CAS.
- K. Bijma, J. B. F. N. Engberts, M. J. Blandamer, P. M. Cullis, P. M. Last, K. D. Irlam and L. Giorgio, J. Chem. Soc., Faraday Trans., 1997, 93, 1579–1584 RSC.
- J. Lah, C. Pohar and G. Vesnaver, J. Phys. Chem. B, 2000, 104, 2522–2526 CrossRef CAS.
- K. Bouchemal, F. Agnely, A. Koffi and G. A. Ponchel, J. Colloid Interface Sci., 2009, 338, 169–176 CrossRef CAS PubMed.
- M. T. Bashford and E. M. Woolley, J. Phys. Chem., 1985, 89, 3173–3179 CrossRef CAS.
- B. Faucompre, M. Bouzerda, M. Lindheimer, J. M. Douillard and S. Partyka, J. Therm. Anal., 1994, 41, 1325–1333 CrossRef CAS.
- P. D. Galgano and O. A. E. Seoud, J. Colloid Interface Sci., 2010, 345, 1–11 CrossRef CAS PubMed.
- P. D. Galgano and O. A. E. Seoud, J. Colloid Interface Sci., 2011, 361, 186–194 CrossRef CAS PubMed.
- C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, John Wiley, New York, 1983, p. 530 Search PubMed.
- T. Nagamura, S. Mihara, Y. Okahata, T. Kunitake and T. Matsuo, Ber. Bunsen-Ges. Phys. Chem., 1978, 82, 1093 CrossRef CAS.
- T. Singh, M. Drechsler, A. H. E. Muller, I. Mukhopadhyay and A. Kumar, Phys. Chem. Chem. Phys., 2010, 12, 11728–11735 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05330k |
| This journal is © The Royal Society of Chemistry 2016 |