Bio-degradation of polyethylene waste by simultaneous use of two bacteria: Bacillus licheniformis for production of bio-surfactant and Lysinibacillus fusiformis for bio-degradation†
Shritama Mukherjeea,
Uttam Roy Chowdhurib and
Patit P. Kundu*a
aAdvanced Polymer Laboratory, Department of Polymer Science and Technology, University of Calcutta, 92 APC Road, Kolkata-9, West Bengal, India. E-mail: ppk923@yahoo.com; Tel: +91 3323525106
bChemical Technology, University of Calcutta, 92 APC Road, Kolkata-9, West Bengal, India
First published on 23rd December 2015
Abstract
A unique method of biodegradation of commercial polyethylene by using simultaneously a bio-surfactant produced by Bacillus licheniformis and Lysinibacillus bacterium in various combinations was investigated in this study. Bio-surfactant produced by B. licheniformis did not have any anti-adhesive property for bio-film formation unlike other types of bio-surfactant produced by other strains of the same bacterium. It was also observed that the lower the surface tension, the higher is the level of oxidation of polyethylene. Lysinibacillus was able to form a bio-film on the control polyethylene without any pre-oxidation step and simultaneously oxidize the polyethylene. Polyethylene samples which were treated with Lysinibacillus along with bio-surfactant showed weight loss. A maximum weight loss of 2.97 ± 0.05% was achieved in the case of polyethylene treated with Lysinibacillus for 1 month, then treated with bio-surfactant for 1 month, followed by treatment with Lysinibacillus for another 1 month. Polyethylene is biodegraded via conversion of carbonyl groups into unsaturated hydrocarbon by both bio-surfactant and Lysinibacillus bacterium. In GC-MS analysis, partial oxidation of anti-oxidant used in commercial polyethylene was also observed. So, our present method of biodegradation by simultaneous use of two bacteria is very environmentally friendly as well as very efficient.
1. Introduction
The need for an environmentally friendly and efficient degradation process has risen with the drastic increase in the usage of polyethylene and its subsequent accumulation in the environment in the last three decades. Additional inertness of commercial polyethylene towards oxidation and bio-degradation can be due to the presence of anti-oxidants. Anti-oxidants are mixed with commercial polyethylene (less than 1%) as polyethylene can undergo oxidation during high temperature processing; this results in the loss of physical properties of polyethylene, leading to its premature failure.1 Several studies have been reported that the microbial degradation or bio-degradation can be used as an environmental friendly method for degradation of polyethylene. Bio-degradation of polyethylene mainly consists of two steps. The primary step is the oxidation of polyethylene and the secondary step is the bacterial incubation of oxidised polyethylene for bio-degradation. Microbes cannot utilise polyethylene due to its highly hydrophobic nature and large molecular weight. It is reported that the pre-oxidation step is used to increase hydrophilic nature of polyethylene by introducing polar groups like carbonyl groups into the carbon-hydrogen backbone of polyethylene. Increased hydrophilic nature and carbonyl groups introduced into the polymer backbone can enhance utilisation of polyethylene by microbes and thereby increasing the bio-degradation.2 Photooxidation by irradiation with U.V. light at temperature above 50 °C, thermal and chemical oxidation are the examples of abiotic oxidation generally used in studies for oxidation of polyethylene.3 Polyethylene mixed with U.V. photosensitizer (pro-oxidant) has been photooxidised by irradiation with U.V. at 70 °C for 60 hour before subjecting it to the biodegradation by Rhodococcus ruber and Brevibacillus borstelensis.4,5 Pre-oxidation of polyethylene, prior to biodegradation by fungi, has been done by two methods which are by accelerated aging at 70 °C under U.V. light for 29 days and by thermal treatment at 105 °C and 150 °C for 120 hour.6 Prior to biodegradation, polyethylene has also been photooxidised by natural weathering for 93 days, followed by thermal treatment at temperature ranging from 45 °C to 65 °C for 200 days. In this case, polyethylene mixed with pro-oxidant has been used.1 Although, oxidation level of polyethylene mixed with pro-oxidant is high, when oxidised by abiotic method; but, these methods have inherent disadvantages. Use of high temperature, U.V. light and chemicals is not cost effective. A more economical way can be the biotic method i.e. using microbial sp. In our previous study, commercial polyethylene is oxidised by the bio-surfactant produced by Bacillus sp. (ATCC-39307).7 Oxidation of polyethylene by bio-surfactant cause similar effect in the chemical structure of polyethylene as reported in case of abiotically oxidised polyethylene like an increase in the carbonyl index. Surfactant is an amphiphilic molecule. Hydrophobic part of the bio-surfactant remains attached with the hydrophobic surface of the polyethylene while hydrophilic part remains protruding towards the aqueous solution. This method increases polyethylene's availability to dissolved oxygen which leads to oxidation of polyethylene. So, even after proper washing and drying, surfactant can remain attached with the surface of the polyethylene. On other hand, bio-surfactant isolated from other variants of Bacillus licheniformis bacterium like B. licheniformis strain 603, B. licheniformis BAS50, B. licheniformis V9714 have shown anti-microbial and anti-adhesive property for bio-film formation against different microbial sp.8–11 So, further investigation is required to confirm whether microbes can form bio-film on the biotically oxidised polyethylene. Due to higher metabolic activity of bio-film forming microbial population than that of the suspended bacteria, formation of bio-film on polyethylene surface is important for bio-degradation. Another advantage is that the carbon availability is much greater where solid surface serves as the support and substrate for the bio-film formation.4 Several bacterial sp. have been reported for their ability to form bio-film on the polyethylene surface. Rhodococcus ruber, Brevibacillus borstelensis are such bacteria which have the ability to form bio-film on the polyethylene surface.4,5 One bacterial sp., identified as Lysinibacillus fusiformis has been isolated from Kolkata municipal wastewater and it is previously reported that this bacterium is able to form bio-film on di-(2-ethylhexyl) phthalate which has short –CH2 chain.12 Two bacterial sp., one for oxidation of polyethylene (Bacillus licheniformis) and another for degradation (Lysinibacillus fusiformis) of polyethylene has never been used simultaneously for biodegradation of commercial polyethylene waste bags.In this study, polyethylene was treated with Bacillus licheniformis and Lysinibacillus fusiformis in different combinations for 3 months when the time allowed for each treatment was 1 month to obtain maximum weight loss due to bio-degradation. B. licheniformis was used for biotic oxidation of polyethylene by the formation of bio-surfactant. Lysinibacillus sp. was used for its ability to form bio-film on the oxidized and un-oxidized polyethylene samples.
2. Methods and materials
2.1. Test materials
Daily used 0.01 mm thick, transparent colourless polyethylene bags were collected from the waste bins of Kolkata Municipal Corporation. Rectangular pieces (5 mm × 5 mm) of the polyethylene bags were vigorously washed with soap water and distilled water. Rectangular pieces were dried at 60 °C for overnight and were used as control polyethylene in this study.2.2. Microbial culture
Bacillus licheniformis JF2 (ATCC no. 39307, MTCC no. 2454) was used for bio-treatment study. This microbial culture was obtained from Institute of Microbial Technology, Chandigarh, India. Microbial culture was maintained in nutrient broth (Himedia). One bacterial strain was isolated from the waste water collected from the Bangur area of Kolkata Municipal Corporation through serial dilution method. After purification, isolated strain was maintained in nutrient media (Himedia) at 37 °C. The isolated strain was identified as Lysinibacillus fusiformis and partial nucleotide sequence of 16s rDNA was submitted in NCBI database with accession number: HE648060.Mediums used for treatment of polyethylene by B. licheniformis were YPD media containing yeast extract 10 g, glucose 20 g, peptone 20 g and sodium chloride (NaCl) 10 g in 1 litre of double distilled water and mineral media containing glucose 10 g, sodium chloride 10 g, NH4NO3 4 g, Na2HPO4 1.5 g, MgSO4 0.4 g, CaCl2 0.1 g in 1 litre of double distilled water. Medium used for treatment of polyethylene by Lysinibacillus sp. contained glucose 10 g, NH4NO3 3 g, KH2PO4 0.4 g, K2HPO4 0.5 g, MgSO4 0.2 g per litre of double distilled water. Liquid culture mediums were sterilised at 120 °C for 15 minutes. Control polyethylene samples were treated as described in Table 1 with B. licheniformis and Lysinibacillus sp. For environmental aging, polyethylene samples were kept under sun continuously for 1 month during day and night in an enclosed glass beaker with a white paper to prevent polyethylene samples from dust and other air polluting agent. Each bacterial treatment was carried out in triplicate at 37 °C for 1 month. Control polyethylene (commercial unoxidized) was incubated with Bacillus licheniformis in YPD medium (PE 1.1), in mineral media (PE 6.1) and with Lysinibacillus bacterium in mineral media (PE 4.1) for 1 month. Then PE 1.1 was aged under sun light for 1 month (PE 1.2) and PE 1.2 was incubated with B. licheniformis in YPD medium for 1 month (PE 1.3). Control polyethylene was incubated with B. licheniformis in YPD medium for 2 months (PE 2.2) and PE 2.2 was further incubated with Lysinibacillus for 1 month (PE 2.3). PE 1.1 was incubated with Lysinibacillus. Samples were collected after 1 month (PE 3.2) and 2 months (PE 3.3). PE 4.1 was subjected to natural aging under sun light for 1 month (PE 4.2) and PE 4.2 was further incubated with Lysinibacillus for 1 month (PE 4.3). PE 4.1 was incubated with B. licheniformis in YPD medium for 1 month (PE 5.2) and PE 5.2 was further incubated with B. licheniformis for 1 month (PE 5.3). PE 6.1 was treated similarly as PE 3.2 (PE 6.2) and PE 6.2 was treated similarly as PE 3.3 (PE 6.3). PE 6.1 was treated similarly as PE 1.2 (PE 7.2) and PE 7.2 was treated similarly as PE 1.3, but using mineral medium instead of YPD medium (PE 7.3). Control polyethylene was incubated with B. licheniformis for 2 months in mineral media (PE 8.2) and PE 8.2 was incubated with Lysinibacillus for 1 month (PE 8.3) (Table 1). Polyethylene kept in three different media without any bacterial sp. was kept as negative control. After each treatment, polyethylene samples were carefully washed and dried at 60 °C. These treated polyethylene samples were then characterized by FTIR, SEM, and XRD. Initial weight and final weight of each treated polyethylene samples was noted.
| Sample name | Starting material | Types of treatment | Name of the bacteria | Types of medium | Duration |
|---|---|---|---|---|---|
| PE 1.1 | Commercial polyethylene | Bio-surfactant treatment | Bacillus licheniformis | YPD medium | 1 month |
| PE 1.2 | PE 1.1 | Natural aging under sunlight | — | — | 1 month |
| PE 1.3 | PE 1.2 | Bio-surfactant treatment | Bacillus licheniformis | YPD medium | 1 month |
| PE 2.2 | PE 1.1 | Bio-surfactant treatment | Bacillus licheniformis | YPD medium | 1 month |
| PE 2.3 | PE 2.2 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 3.2 | PE 1.1 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 3.3 | PE 3.2 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 4.1 | Commercial polyethylene | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 4.2 | PE 4.1 | Natural aging under sunlight | — | — | 1 month |
| PE 4.3 | PE 4.2 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 5.2 | PE 4.1 | Bio-surfactant treatment | Bacillus licheniformis | YPD medium | 1 month |
| PE 5.3 | PE 5.2 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 6.1 | Commercial polyethylene | Bio-surfactant treatment | Bacillus licheniformis | Mineral medium | 1 month |
| PE 6.2 | PE 6.1 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 6.3 | PE 6.2 | Bacterial treatment | Lysinibacillus fusiformis | Mineral medium | 1 month |
| PE 7.2 | PE 6.1 | Natural aging under sunlight | — | — | 1 month |
| PE 7.3 | PE 7.2 | Bio-surfactant treatment | Bacillus licheniformis | Mineral medium | 1 month |
| PE 8.2 | PE 6.1 | Bio-surfactant treatment | Bacillus licheniformis | Mineral medium | 1 month |
| PE 8.3 | PE 8.2 | Bio-surfactant treatment | Bacillus licheniformis | Mineral medium | 1 month |
2.3. Characterization
Surface tension (σ) of culture media incubated with B. licheniformis was measured by stalagmometer at 25 °C at day zero and at different intervals of time (days).13 Reduction of surface tension is an indirect measure of bio-surfactant production by B. licheniformis. Surface tension was calculated by following formula.Fourier transform infrared spectra (FTIR) analysis was carried out with ATR-FTIR (model alpha, Bruker, Germany) spectrometer, scanning from 4000 cm−1 to 500 cm−1 at room temperature. The resolution was set at 4 cm−1 with 42 scans per spectrum. Carbonyl index (C.I.) and double bond index (D.B.I.) were calculated using the ratio of absorbance frequency of the carbonyl peak (1740 cm−1) and double bond peak (1650 cm−1) to that of the CH2 group bending frequency (1465 cm−1) respectively.
All polyethylene samples were sputter coated with gold layer by a Hitachi sputter coater (model-E1010 Ion Sputter), Japan. Photomicrographs were observed under scanning electron microscope (EVO 18, Carl Zeiss, Germany).
X-Ray diffraction study of all types of polyethylene samples were recorded with an X-ray diffractometer (PANalytical, Netherlands) at an angle of 2θ from 3° to 50° and fixed scan rate of 1° min−1. Percentage (%) of crystallinity was calculated by using following formula.
‘BATH’ (bacterial adhesion to hydrocarbon) is a test to measure bacterial hydrophobicity.4 Affinity of bacterial cells towards hydrocarbon increases with bacterial hydrophobicity. Due to this property, bacterial cells with higher affinity for hydrocarbon [hexadecane (C16H34) in the present case], transfer from aqueous suspension to organic phase, leading to the reduction in the turbidity of the culture. ‘BATH’ method was carried out to check hydrophobicity of Lysinibacillus sp. as described by Gilan et al.4 E. coli was used as negative control.
Extraction of the degraded part of polyethylene samples in chloroform was performed as per procedure reported by Roy et al.14 Unoxidized commercial polyethylene was used as control. The presence of different compounds in commercial polyethylene and in the oxidation products and degradation products of commercial polyethylene was identified by GC-MS (Thermo Scientific TSQ 8000) analysis. The oven temperature was programmed at 40 °C for 3 min, then rose to 280 °C at a rate of 10 °C min−1, and then held for 4 min at 280 °C. Helium was used as carrier gas. The identification of degradation products was established by comparison of their mass spectra with NST database.
3. Result and discussion
3.1. Bacterial hydrophobicity
BATH assay is the measure of bacterial hydrophobicity. The graph depicted from BATH assay of Lysinibacillus sp. and E. coli is represented in Fig. 1. From the BATH assay, affinity of Lysinibacillus towards hydrocarbon is evident. More than 30% reduction in cell turbidity is observed after addition of 0.6 ml of hexadecane. After this concentration, reduction of cell turbidity becomes stabilized. Reduction of turbidity occurs as hydrophobic bacterial cells get attached with the hydrocarbon, due to which the transfer of bacterial cells from aqueous phase to organic phase occurs. Similar result has been observed in case of another bacterium, Rhodococcus rubber. These bacteria have been able to form bio-film on the surface of polyethylene, leading to the biodegradation of polyethylene.4 So, Lysinibacillus sp. can be used for bio-film formation on the surface of the polyethylene.E. coli used as the negative control, does not show any change of cell turbidity during addition of different concentration of hexadecane.
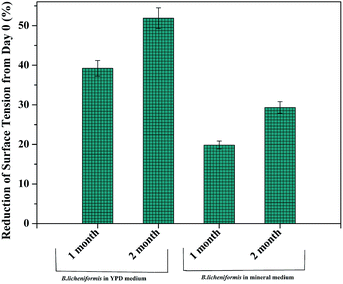
3.2. Surface tension
Surface tension reduction by B. licheniformis incubated in two different mediums is represented in Fig. 2. From this observation, it is clear that the maximum surface tension reduction i.e. 51.9% is achieved in case of the B. licheniformis grown in YPD medium for 2 months. Surface tension reduction in case of B. licheniformis grown in mineral media for 2 months is 29% which is much less than the earlier case. Higher amount of bio-surfactant produced by B. licheniformis in YPD medium is due to the abundant presence of carbon and nitrogen sources in the YPD medium. In the case of B. licheniformis grown in the mineral medium, surfactant production is much less due to the presence of limited amount of nitrogen and carbon sources. As B. licheniformis is able to produce bio-surfactant in both YPD and mineral media, both media has been used for the treatment of polyethylene to study the effect of surface tension on polyethylene oxidation.3.3. Bio-surfactant induced oxidation followed by natural aging under sunlight and biodegradation treatment
Polyethylene sample oxidised by bio-surfactant produced by B. licheniformis grown in YPD medium for 1 month was subjected to natural aging under sunlight for 1 month to improve oxidation level. In the next step of treatment, this oxidized polyethylene incubated with Lysinibacillus for 1 and 2 months. It was observed that Lysinibacillus was able to form a bio-film on the oxidised polyethylene sample.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O containing oxidation product is formed. Mainly ketones (1740 cm−1), aldehydes (1733 cm−1) and unsaturated hydrocarbons (1650 cm−1) are formed as oxidation product.15,16 After further oxidation by natural aging under sunlight (PE 1.2), another absorbance peak appears at 1715 cm−1. This peak is due to formation of acids as the oxidation product of polyethylene.17 Polyethylene sample oxidised in the presence of bio-surfactant and natural aging, again treated with bio-surfactant for 1 month (PE 1.3). In this case of PE 1.3, drastic change in the absorbance can be observed in the 1500–1800 cm−1 region in the FTIR spectra. Absorbance peak at 1650 cm−1 increases rapidly, indicating formation of higher amount of unsaturated hydrocarbons. In our previous study, similar observation of conversion of carbonyl groups into unsaturated hydrocarbons during oxidation by bio-surfactant has been observed.7
O containing oxidation product is formed. Mainly ketones (1740 cm−1), aldehydes (1733 cm−1) and unsaturated hydrocarbons (1650 cm−1) are formed as oxidation product.15,16 After further oxidation by natural aging under sunlight (PE 1.2), another absorbance peak appears at 1715 cm−1. This peak is due to formation of acids as the oxidation product of polyethylene.17 Polyethylene sample oxidised in the presence of bio-surfactant and natural aging, again treated with bio-surfactant for 1 month (PE 1.3). In this case of PE 1.3, drastic change in the absorbance can be observed in the 1500–1800 cm−1 region in the FTIR spectra. Absorbance peak at 1650 cm−1 increases rapidly, indicating formation of higher amount of unsaturated hydrocarbons. In our previous study, similar observation of conversion of carbonyl groups into unsaturated hydrocarbons during oxidation by bio-surfactant has been observed.7Absorbance peak at 1650 cm−1 in the FTIR spectra of PE 2.2 increases and this is due to the formation of unsaturated hydrocarbons as oxidation product (ESI Fig. S2†). This oxidised polyethylene was incubated with Lysinibacillus sp. for 1 month for bio-film formation (PE 2.3). After 1 month, absorbance peak at 1740 cm−1 almost disappears and absorbance peak at 1650 cm−1 increases. This may be due to the utilization of oxidation product by Lysinibacillus sp. and also conversion of carbonyl groups into unsaturated hydrocarbon by the same bacterium. Similar phenomenon exhibited by other polyethylene degrading bacteria is also reported.18
After oxidation of polyethylene in the presence of bio-surfactant for 1 month, it was incubated with Lysinibacillus for 2 months (PE 3.3). After 2 months incubation with Lysinibacillus, absorbance peak at 1500–1800 cm−1 region increases further than that of the PE 1.1; this can be due to formation of ketones, aldehydes and unsaturated hydrocarbons (ESI Fig. S3†).
Carbonyl index (C.I.) and double bond index (D.B.I.) of all samples is represented in Fig. 3. C.I. of P.E 1.2 is found to be higher than PE 1.1. It is reported that the presence of carbonyl groups in polyethylene can act as the initiator of photo-oxidation during natural aging under sunlight15 due to which, oxidation level of PE 1.2 is much higher than that of the PE 1.1. But in case of PE 1.3, C.I. decreases and D.B.I. increases drastically. In this case, oxidation product of polyethylene gets solubilised into the aqueous media. During oxidation by bio-surfactant, initially, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O containing oxidation product i.e. ketones, aldehydes along with unsaturated hydrocarbons are formed. During later stage of this oxidation, solubilisation of oxidation products and conversion of carbonyl groups into unsaturated hydrocarbon is reported in the previous study.7 This phenomenon can be resulted due to hydrocarbon solubilisation ability of bio-surfactant which is also reported by other studies.19 In case of PE 2.3, its C.I. decreases, whereas its D.B.I. increases in comparison to that of the PE 2.2. In this case, Lysinibacillus is able to utilize the oxidation product for bio-film formation and is also able to convert the carbonyl groups into unsaturated hydrocarbons. In case of PE 3.2 and PE 3.3, both C.I. and D.B.I. increases. Lysinibacillus bacterium may be able oxidize polyethylene further, due to which, more carbonyl groups and unsaturated hydrocarbons are formed. But in case of PE 2.3, such oxidation is not observed. Lysinibacillus only utilizes that oxidized part of polyethylene for forming the bio-film due to the presence of higher amount of the oxidation product in PE 2.2. But in case of PE 1.1, the amount of oxidation product is comparatively less, enabling Lysinibacillus to oxidise the polyethylene sample further as well as to utilize oxidation product.
O containing oxidation product i.e. ketones, aldehydes along with unsaturated hydrocarbons are formed. During later stage of this oxidation, solubilisation of oxidation products and conversion of carbonyl groups into unsaturated hydrocarbon is reported in the previous study.7 This phenomenon can be resulted due to hydrocarbon solubilisation ability of bio-surfactant which is also reported by other studies.19 In case of PE 2.3, its C.I. decreases, whereas its D.B.I. increases in comparison to that of the PE 2.2. In this case, Lysinibacillus is able to utilize the oxidation product for bio-film formation and is also able to convert the carbonyl groups into unsaturated hydrocarbons. In case of PE 3.2 and PE 3.3, both C.I. and D.B.I. increases. Lysinibacillus bacterium may be able oxidize polyethylene further, due to which, more carbonyl groups and unsaturated hydrocarbons are formed. But in case of PE 2.3, such oxidation is not observed. Lysinibacillus only utilizes that oxidized part of polyethylene for forming the bio-film due to the presence of higher amount of the oxidation product in PE 2.2. But in case of PE 1.1, the amount of oxidation product is comparatively less, enabling Lysinibacillus to oxidise the polyethylene sample further as well as to utilize oxidation product.
3.4. Bio-film formation and oxidation of control polyethylene by Lysinibacillus bacterium
Additional oxidation ability of Lysinibacillus along with its ability to form bio-film on polyethylene is observed during the first part of this study. In this part, control (unoxidized) polyethylene was incubated with Lysinibacillus. Natural aging under sunlight and bio-surfactant induced oxidation were also used to enhance oxidation level (Table 1). Bio-film formation was observed on the control polyethylene within 7 days of incubation with Lysinibacillus.After incubating polyethylene with Lysinibacillus, polyethylene was incubated in the presence of bio-surfactant for 1 month (PE 5.2). In the FTIR spectra of PE 5.2, absorbance peak at 1740 cm−1 decreases, while the absorbance peak at 1650 cm−1 increases (ESI Fig. S5†). This is due to the solubilisation of oxidation products into aqueous media and subsequent conversion of carbonyl groups into unsaturated hydrocarbons in the presence of bio-surfactant. This bio-surfactant treatment of polyethylene is followed by 1 month incubation with Lysinibacillus for biodegradation (PE 5.3). In the FTIR spectra of PE 5.3, a drastic increase in the absorbance peak at 1650 cm−1 is observed; this is due to the utilization of oxidized part by Lysinibacillus and subsequent conversion of carbonyl groups into unsaturated hydrocarbons.
After 1 month incubation of control polyethylene with Lysinibacillus, significant rise in the values of both C.I. and D.B.I can be observed (Fig. 5). Carbonyl groups present in the polyethylene act as the initiator of photo-oxidation, due to which higher oxidation level is resulted in PE 4.2. Slight decrease in both of C.I. and D.B.I. of PE 4.3 is due to the utilization of oxidation products by Lysinibacillus. In case of PE 5.2, the decrease in C.I. and D.B.I. is possibly due to the solubilisation of oxidation product into the aqueous media in the presence of bio-surfactant. In case of PE 5.3, drastic increase in D.B.I. and comparatively less increase in C.I. are apparent. So, Lysinibacillus bacterium utilizes carbonyl groups and converts it into unsaturated hydrocarbon. Slight increase in the C.I. in case of PE 5.3 is due to the oxidation of polyethylene by extracellular enzymes of Lysinibacillus and by the dissolved oxygen present in the bacterial media.
3.5. Oxidation of polyethylene by bio-surfactant produced by B. licheniformis in mineral media and subsequent biodegradation
Similar type of treatment of polyethylene as first part of this study was done in this section (Table 1). Use of mineral media as growth media for B. licheniformis instead of YPD media was the only difference in this case. Higher surface tension was observed in case of B. licheniformis grown in mineral media than B. licheniformis grown in YPD media. Treatment used for PE 6.1 and PE 1.1 were the same except the difference in the growth media used for bio-surfactant production. Similarly, treatment used for PE 6.2–6.3, PE 7.2–7.3, and PE 8.2–8.3 were the same for PE 3.2–3.3, PE 1.2–1.3, PE 2.2–2.3, respectively (Table 1).C.I. of PE 6.1 is comparatively less than the C.I. of PE 1.1 though both of the polyethylene samples are oxidised by bio-surfactant (Fig. 7). Higher surface tension is recorded in case of B. licheniformis grown in mineral media; this can be a reason for lower level of oxidation in case of PE 6.1. Characteristic higher D.B.I and lower C.I. of PE 3.2 and 3.3 is also observed in case of PE 6.2 and 6.3. But, the value of both C.I. and D.B.I. of PE 6.2 and 6.3 is comparatively less than that of the PE 3.2 and 3.3. This is also due to the lower oxidation level of polyethylene. In case of PE 7.2–7.3 and PE 8.2–8.3, changing pattern of C.I. and D.B.I. value is identical to that of the PE 1.2–1.3 and PE 2.2–2.3 respectively. But, the value of C.I. and D.B.I. of PE 7.2–7.3 and PE 8.2–8.3 are comparatively low. From this observation, this is apparent that the lower the surface tension of the culture media, the higher is the oxidation level of polyethylene by bio-surfactant and vice versa.
3.6. Morphological analysis
SEM images of the control polyethylene and other treated polyethylene samples are represented in Fig. 9. Rough surface is observed in all eight treated polyethylene samples by different methods. Solubilisation of oxidation product and release of volatile oxidation products may give rise to such cracks on the polyethylene surface. In case of the polyethylene samples incubated with Lysinibacillus at the last stage of treatment, cracks and rough surface are formed due to bio-degradation and bio-deterioration of oxidised polyethylene surface by this bacterium. Such cracks and rough surface has also been observed in other studies after oxidation and biodegradation of polyethylene.14,18 These cracks are the weak points for the bacteria for bio-film formation on the polyethylene surface. Such bio-film formation around the cracks on the bio-degraded polyethylene sample can be observed in case of PE 6.3 and 8.3.24 Comparatively smoothed surface is resulted from this kind of bio-film formation around cracks due to the bio-degradation of the oxidized polyethylene. Surface of PE 2.3 and 3.3 are comparatively smoother than the surface of PE 7.3, 8.3 and 6.3 respectively. Comparatively smoothed surface is resulted due to higher rate of bio-degradation by Lysinibacillus as the oxidation level in both the samples PE 2.3 and PE 3.3 is high.3.7. Gravimetric analysis
Out of eight treated polyethylene samples, six shows weight-loss after treatment for 3 months (Fig. 10). No weight loss is observed after 3 months of treatment in case of PE 1.3 and PE 7.3 which were also not treated with Lysinibacillus bacterium. Weight-loss is observed in other six treated polyethylene samples i.e. PE 2.3, PE 3.3, PE 4.3, PE 5.3, PE 6.3 and PE 8.3 which were treated with Lysinibacillus during last stage of the treatment (Table 1). So, it is apparent that biodegradation of polyethylene is only caused by Lysinibacillus bacterium. Maximum weight loss is achieved in case of PE 5.3 which is 2.97 ± 0.5%. In case of PE 5.3, during first month of treatment, control polyethylene is simultaneously oxidised and that oxidised part is utilised by Lysinibacillus bacterium (PE 4.1). Then during second month, that polyethylene is oxidised and the oxidised part of polyethylene is solubilised by bio-surfactant produced by B. licheniformis (PE 5.2). During third month of treatment, that polyethylene is again oxidised and biodegraded by Lysinibacillus simultaneously (PE 5.3) (Table 1). In case of PE 4.1, 5.2 and 5.3, these changes can be observed in FTIR analysis by the respective increase and decrease in the value of C.I. and D.B.I. (Fig. 5). This way, polyethylene is biodegraded. In a recent study, less than 1% weight loss is achieved during the biodegradation of thermally treated polyethylene by Bacillus cereus and Bacillus sphaericus after 3 months.16 In another study, rate of polyethylene degradation ranging from 3.5% to 8.4% in 10 years has been reported.25 After 15 years of treatment in soil, 16% weight-loss of polyethylene has been achieved in another study.26 The achieved rate of degradation in this study is higher than the rate of degradation of polyethylene as reported in the previous studies. | ||
| Fig. 10 The bio-degradation of polyethylene. Dry weight loss of polyethylene after 90 days or 3 months of treatment. | ||
3.8. GC-MS analysis
Summarized list of all products identified in GCMS analysis of treated polyethylene is represented in Table 2. Con_PE is the control polyethylene. Con_lyn is the control polyethylene incubated with Lysinibacillus for 2 months. Con_lich is the control polyethylene incubated with bio-surfactant produced by B. licheniformis for 1 month using YPD medium. Acids, alcohols and ether are formed during oxidation of polyethylene by Lysinibacillus and bio-surfactant.14,22 But only one type of ketones and esters formed after oxidation. As observed in Fig. 3 and 5, the value of C.I. and D.B.I. of PE 3.3 is comparatively higher than that of the PE 5.3. Presence of comparatively higher variety of alcohols, acids and unsaturated hydrocarbons in PE 3.3 compared to PE 5.3 is in correspondence with the observed value of C.I. and D.B.I (Fig. 3 and 5). In case of PE 5.3, biodegradation of treated polyethylene is apparent from the presence of small molecule of hydrocarbons i.e. decane, dodecane. But less variety of oxidation products is identified in case of PE 5.3 which is due to utilization of oxidation product by Lysinibacillus. From GC-MS analysis, it is also apparent that polyethylene is bio-degraded via conversion of carbonyl group into unsaturated hydrocarbons by the two bacteria.| Compound name | Con_PE | Con_lyn | Con_lich | PE 3.3 | PE 5.3 | |
|---|---|---|---|---|---|---|
| Antioxidants | ||||||
| 1 | Phenol, 2,4-bis(1,1-dimethylethyl)- | Y | Y | Y | Y | Y |
| Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | Y | Y | Y | Y | Y | |
| Phenol, 2,6-bis(1,1-dimethylethyl)- | Y | Y | Y | Y | Y | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| CH2 group | ||||||
| 2 | Tetracosane, 11-decyl-(24C) | Y | N | N | N | N |
| 3 | Heneicosane (21C) | N | Y | Y | N | N |
| 4 | Eicosane, 7-hexyl-(20C) | Y | N | N | N | N |
| Eicosane | Y | Y | Y | N | N | |
| Eicosane, 2-methyl- | N | N | N | N | N | |
| Cycloeicosane | N | N | N | N | Y | |
| Eicosane, 10-methyl- | N | N | Y | N | N | |
| 5 | Nonadecane | N | N | Y | N | N |
| Nonadecane, 9-methyl-(19C) | N | Y | N | N | N | |
| 6 | Octadecane, 3-ethyl-5-(2-ethylbutyl)-(18C) | N | Y | N | N | N |
| Octadecane | N | Y | N | N | N | |
| 7 | Heptadecane, 9-hexyl-(17C) | Y | Y | N | N | N |
| Heptadecane, 7-methyl | N | N | N | N | N | |
| Heptadecane | Y | N | Y | N | N | |
| 8 | Hexadecane, 2,6,11,15-tetramethyl-(16C) | N | Y | N | N | N |
| Hexadecane, 2,6,10,14-tetramethyl- | N | N | N | N | N | |
| Cyclohexadecane | N | Y | N | N | ||
| Hexadecane | Y | Y | Y | N | N | |
| 9 | Pentadecane (15C) | N | Y | N | N | N |
| Pentadecane, 7-methyl | N | Y | N | N | N | |
| Pentadecane, 3-methyl- | N | N | N | N | N | |
| 10 | Tetradecane, 2,6,10-trimethyl-(14C) | N | N | Y | N | N |
| Tetradecane, 4-methyl- | N | N | N | N | N | |
| Tetradecane, 2-methyl- | N | N | N | N | N | |
| Cyclotetradecane | N | N | N | Y | Y | |
| Tetradecane | Y | Y | N | Y | N | |
| Tridecane | N | N | Y | N | N | |
| 11 | Dodecane, 5,8-diethyl-(12C) | N | Y | N | N | N |
| Dodecane, 2,5-dimethyl- | N | N | N | N | N | |
| Dodecane, 2,6,10-trimethyl | Y | N | N | Y | Y | |
| Dodecane | Y | Y | Y | Y | Y | |
| 12 | Undecane (11C) | N | Y | N | N | N |
| Undecane, 2,6-dimethyl- | N | N | N | Y | N | |
| 13 | Decane, 3,6-dimethyl-(10C) | N | Y | Y | N | N |
| Decane | N | N | N | Y | Y | |
| Decane, 2-methyl- | N | N | N | N | ||
| 14 | Octane, 3,5-dimethyl- | N | N | N | Y | N |
| 15 | Benzene, 1,3-bis(1,1-dimethylethyl)-(6C) | Y | Y | N | N | N |
| Benzene, 1,4-bis(1,1-dimethylethyl)- | Y | Y | N | N | N | |
| 16 | m-Cymene, 5-tert-butyl- | Y | Y | N | N | N |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Oxidation product | ||||||
| Acids | ||||||
| 17 | 9-Hexadecenoic acid | N | Y | Y | N | N |
| Pentadecanoic acid | N | N | Y | N | N | |
| Oleic acid, 3-(octadecyloxy)propyl ester | N | Y | Y | N | N | |
| Octadecanoic acid | N | N | Y | N | N | |
| Docosahexaenoic acid, 1,2,3-propanetriyl ester | N | Y | N | N | N | |
| Oleic acid, eicosyl ester | N | N | Y | N | N | |
| 9-Octadecenoic acid (Z)-, tetradecyl ester | N | N | Y | N | N | |
| Erucic acid | N | N | Y | N | N | |
| 22-Tricosenoic acid | N | N | Y | N | N | |
| cis-13-Octadecenoic acid | N | N | Y | N | N | |
| cis-Vaccenic acid | N | N | Y | N | N | |
| Nonahexacontanoic acid | N | N | Y | N | N | |
| Cyclopropaneoctanoic acid, 2-[(2-pentylcyclopropyl)methyl]-, methyl ester, trans,trans- | N | N | Y | N | N | |
| Ketones | ||||||
| 18 | 2-Pentanone, 4-hydroxy-4-methyl- | N | Y | Y | N | N |
| Alcohol | ||||||
| N19 | 1-Heptatriacotanol | N | N | Y | N | N |
| 2-Hexanol, 2-methyl- | N | Y | Y | N | N | |
| 2-Pentanol, 2,4-dimethyl- | N | N | Y | N | N | |
| 1-Octanol, 2-butyl- | N | N | Y | N | N | |
| 1-Undecanol | N | N | N | N | Y | |
| Ethanol, 2-(9-octadecenyloxy)-, (Z)-/2-(tetradecyloxy)- | N | N | Y | N | N | |
| E,E,Z-1,3,12-Nonadecatriene-5,14-diol | N | N | Y | N | N | |
| Esters | ||||||
| 20 | E-8-Methyl-9-tetradecen-1-ol acetate | N | N | Y | N | N |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Other oxidation product | ||||||
| 21 | 3,8,12-Tri-O-acetoxy-7-desoxyingol-7-one | N | Y | N | N | N |
| 9-Octadecene, 1-[2-(octadecyloxy)ethoxy]- | N | Y | N | N | N | |
| Dodecane, 1-methoxy- | N | N | Y | N | N | |
| 1-Tetradecanol, methyl ether | N | N | Y | N | N | |
| 1-Docosanol, methyl ether | N | N | N | Y | N | |
| Citral | N | N | N | Y | N | |
| 2,6-Octadienal, 3,7-dimethyl-, (Z)- | N | N | N | Y | N | |
| Benzaldehyde, 2,5-dimethyl- | N | N | N | Y | Y | |
| Benzaldehyde, 2,4-dimethyl- | N | N | N | Y | Y | |
| Benzaldehyde, 2-ethyl- | N | N | N | Y | Y | |
| Benzaldehyde, 2-hydroxy-/3-hydroxy/4-hydroxy | N | N | Y | N | N | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Unsaturated hydrocarbon | ||||||
| 22 | 3-Tetradecene, (Z)- | N | N | N | Y | N |
| trans-3-Decene | N | N | N | Y | N | |
| 1-Dodecene | N | N | N | Y | N | |
| 10-Heneicosene (c,t) | N | N | N | Y | Y | |
| 6-Dodecene, (Z)-/(E) | N | N | N | Y | Y | |
| 9-Nonadecene | N | N | N | Y | Y | |
| 1-Octadecene | N | N | N | N | Y | |
Different varieties of nitrogenous compounds are identified in the case of control polyethylene incubated with Lysinibacillus and PE 3.3, PE 5.3, which are parts of bio-film of bacterium formed on the polyethylene surface. Benzaldehyde present in oxidized polyethylene is formed due to partial oxidation of anti-oxidant i.e. phenol, 2,4-bis(1,1-dimethylethyl) present in the commercial polyethylene.27
Two negative control polyethylenes kept in mineral medium and YPD medium without any bacterium do not exhibit any chemical, structural and morphological changes during 3 months of incubation.
4. Conclusion
From the above observation, it is apparent that Lysinibacillus is a unique bacterium with the ability of oxidation and bio-degradation of commercial polyethylene waste bags. Mainly oxidation of polyethylene by Lysinibacillus is observed when used polyethylene is either unoxidized or oxidation level is very low. But, when the oxidation level of polyethylene is very high, then the conversion of the carbonyl groups into unsaturated hydrocarbons is observed rather than formation of more carbonyl groups. So, polyethylene is oxidised by Lysinibacillus for the formation of oxidation product from polyethylene which can later be utilized by the same bacterium for bio-film formation. Bio-surfactant produced by B. licheniformis JF2 (ATCC-39307) does not have any anti-adhesive property. Lysinibacillus has been able to form bio-film on the surface of the polyethylene, oxidised by bio-surfactant as well as on the surface of the control polyethylene. Out of the eight treated polyethylene samples, six samples have been incubated with Lysinibacillus. Weight-loss is observed in these six polyethylene samples after treatment of 3 months. Oxidation level of other two polyethylene samples i.e. PE 1.3 and 7.3, treated with bio-surfactant and aged under sun-light is comparatively higher, though no weight-loss is observed for these two polyethylene samples. So, Lysinibacillus is mainly responsible for bio-degradation process of polyethylene and this biodegradation process of polyethylene mainly advanced through the conversion of carbonyl group into unsaturated hydrocarbons. In this study, biodegradation is carried out using two bacteria. One bacterium B. licheniformis is used for the production of bio-surfactant and oxidation of polyethylene followed by solubilisation of oxidation product by that bio-surfactant. Another bacterium Lysinibacillus is used for biodegradation as well as oxidation of commercial polyethylene. Using these two bacteria simultaneously, one can lead to the biodegradation and bio-deterioration of commercial polyethylene. This method is also very environmental friendly.Acknowledgements
Shritama Mukherjee is grateful to Council for Scientific and Industrial Research (CSIR), New Delhi, for providing senior research fellowship for conducting this research. Thanks to the Central Instrumentation Laboratory, Panjab University, India for providing GC-MS analysis of the polyethylene sample.References
- A. Corti, S. Muniyasamy, M. Vitali, S. H. Imam and E. Chiellini, Polym. Degrad. Stab., 2010, 95, 1106–1114 CrossRef CAS.
- A.-C. Albertsson, S. O. Andersson and S. Karlsson, Polym. Degrad. Stab., 1987, 18, 73–87 CrossRef CAS.
- K. Yamada-Onodera, H. Mukumoto, Y. Katsuyaya, A. Saiganji and Y. Tani, Polym. Degrad. Stab., 2001, 72, 323–327 CrossRef CAS.
- I. G. (Orr), Y. Hadar and A. Sivan, Appl. Microbiol. Biotechnol., 2004, 65, 97–104 CrossRef PubMed.
- D. Hadad, S. Geresh and A. Sivan, J. Appl. Microbiol., 2005, 98, 1093–1100 CrossRef CAS PubMed.
- A. Manzur, M. Limon-Gonzalez and E. Favela-Torres, J. Appl. Polym. Sci., 2004, 92, 265–271 CrossRef CAS.
- S. Mukherjee, U. R. Chaudhuri and P. P. Kundu, RSC Adv., 2015, 5, 75089–75097 RSC.
- K. Jenny, O. Käppeli and A. Fiechter, Appl. Microbiol. Biotechnol., 1991, 36, 5–13 CrossRef CAS PubMed.
- F. Rivardo, R. J. Turner, G. Allegrone, H. Ceri and M. G. Martinotti, Appl. Microbiol. Biotechnol., 2009, 83, 541–553 CrossRef CAS PubMed.
- S. G. Batrakov, T. A. Rodionova, S. E. Esipov, N. B. Polyakov, V. I. Sheichenko, N. V. Shekhovtsova, S. M. Lukin, N. S. Panikov and Y. A. Nikolaev, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2003, 1634, 107–115 CrossRef CAS.
- M. M. Yakimov, K. N. Timmis, V. Wray and H. L. Fredrickson, Appl. Environ. Microbiol., 1995, 61, 1706–1713 CAS.
- I. Latorre, S. Hwang and R. Montalvo-Rodriguez, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2012, 47, 2254–2262 CrossRef CAS PubMed.
- D. Konz, S. Doekel and M. A. Marahiel, J. Bacteriol., 1999, 181, 133–140 CAS.
- P. K. Roy, S. Titus, P. Surekha, E. Tulsi, C. Deshmukh and C. Rajagopal, Polym. Degrad. Stab., 2008, 93, 1917–1922 CrossRef CAS.
- P. Roy, P. Surekha, C. Rajagopal, S. Chatterjee and V. Choudhary, Polym. Degrad. Stab., 2007, 92, 1151–1160 CrossRef CAS.
- M. Sudhakar, M. Doble, P. S. Murthy and R. Venkatesan, Int. Biodeterior. Biodegrad., 2008, 61, 203–213 CrossRef CAS.
- C. Naddeo, L. Guadagno and V. Vittoria, Polym. Degrad. Stab., 2004, 85, 1009–1013 CrossRef CAS.
- A. Esmaeili, A. A. Pourbabaee, H. A. Alikhani, F. Shabani and E. Esmaeili, PLoS One, 2013, 8, 71720 Search PubMed.
- A. K. Singh and S. S. Cameotra, Environ. Sci. Pollut. Res., 2013, 20, 7367–7376 CrossRef CAS PubMed.
- T. Ojeda, A. Freitas, K. Birck, E. Dalmolin, R. Jacques, F. Bento and F. Camargo, Polym. Degrad. Stab., 2011, 96, 703–707 CrossRef CAS.
- Y.-C. Hsu, M. P. Weir, R. W. Truss, C. J. Garvey, T. M. Nicholson and P. J. Halley, Polymer, 2012, 53, 2385–2393 CrossRef CAS.
- F. Khabbaz, A. Albertsson and S. Karlsson, Polym. Degrad. Stab., 1999, 63, 127–138 CrossRef CAS.
- A. Esmaeili, A. A. Pourbabaee, H. A. Alikhani, F. Shabani and L. Kumar, Biorem. J., 2014, 18, 213–226 CrossRef CAS.
- S. Bonhomme, A. Cuer, A.-M. Delort, J. Lemaire, M. Sancelme and G. Scott, Polym. Degrad. Stab., 2003, 81, 441–452 CrossRef CAS.
- A.-C. Albertsson and S. Karlsson, Prog. Polym. Sci., 1990, 15, 177–192 CrossRef CAS.
- S. Karlsson and A. Albertsson, Polym. Eng. Sci., 1998, 38, 1251–1253 CAS.
- M. S. Dopico-García, J. M. López-Vilariñó and M. V. González-Rodríguez, J. Agric. Food Chem., 2007, 55, 3225–3231 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S8. See DOI: 10.1039/c5ra25128a |
| This journal is © The Royal Society of Chemistry 2016 |