Large scale bi-layer graphene by suppression of nucleation from a solid precursor
Mohsin Ahmed*,
Naoki Kishi and
Tetsuo Soga
Department of Frontier Materials, Nagoya Institute of Technology, Nagoya 466-8555, Japan. E-mail: mohsin94ee@yahoo.com
First published on 6th May 2015
Abstract
We report the synthesis of large-scale Bilayer Graphene (BLG) from the solid precursor, camphor using a Chemical Vapor Deposition (CVD) method at atmospheric pressure. Controlled insertion of the carbon source was obtained by a two-fold gas flow approach and found to have a profound impact to obtain continuous BLG. Suppression of nucleation by annealing the substrate for an extended period helped to obtain large scale BLG on Cooper foil. The impact of deposition temperature, deposition time, and flow of the carrier gas were examined for further optimization. The as grown BLG was transferred to an insulator substrate and the measured transmittance was found to be above 90%. This mechanism to obtain low cost BLG on a large scale may have profound implications in semiconductor industries.
1. Introduction
Graphene is a hexagonal lattice structured sp2 bonded network of carbon with extraordinary electron mobility. However, the promise and novelty of graphene is yet to be exploited to its highest level due to its zero band-gap property. The absence of a band gap limits the types of electronic devices that can be developed from graphene. Graphene-based transistors with very high operating frequencies requiring very low input power have the limitation of poor control over the carrier concentrations due to an inability to turn off the RF transistors.1–3 Therefore, opening the band gap in graphene has become very crucial in the field of graphene based electronic applications.4–6 For the band gap opening, several attempts have been envisaged by confining carriers physically, such as, patterning graphene into graphene nanoribbons (GNR)7,8 or unzipping carbon nanotubes (CNTs) into GNRs.9 Nevertheless, those methods are quite challenging as precise control of the width and edge roughness of nanometer-width GNRs or exact alignment of CNTs on the substrate is required.The bandgap opening has been done in recent years in Bernal (AB) stacked bilayer graphene by breaking symmetry of the electron wave functions.10–12 To introduce a gap between the Π and Π* states in graphene, the lattice symmetry in hexagonal structured graphene needs to be broken down. If two layers of graphene are stacked as in graphite and both graphene layers are yielded in-equivalent, an energy gap between low-energy bands is formed. It was demonstrated that, by controlling the carrier density in a bilayer graphene, the position of electronic states near EF and the magnitude of the gap between the VB and CB can be manipulated.13 The change in the charge state i.e. positioning of EF within the gap causes a semimetal-to-insulator transition. If this symmetry-breaking could be controlled externally, the electronic conductivity would change through this transition, suggesting that a switch with a thickness of two atomic layers could be constructed.14 Thus, the opening of bandgap and its tunable criteria provide immense opportunity for BLG to be used in electronic and photonic devices.15–17
Applications of BLG vastly depend on scalability and quality of the synthesized graphene.18 Up to now, mechanical exfoliation method was utilized mostly to fabricate AB stacked BLG.19–21 However, exfoliated graphenes are limited in size and not scalable. Recent advancement in CVD technology has paved the way to synthesis scalable graphene. This technique has proved to be effective in controlling number of graphene layers and stacking order and hence, spurred large-scale production of AB stacked bilayer graphene for numerous applications.22
Transition metals, such as Ni and Cu have been exploited mostly to synthesis graphene by CVD technique. It is quite challenging to produce BLG on Ni due to high carbon solubility and graphene grown on Ni lacking uniformity.23 On the other hand, exploitation of BLG on Cu is critical because of Cu's weak capability of decomposing hydrocarbons24 which makes this transition metal self-limiting. At the same time, self-limiting criteria of Cu is crucial to obtain continuous BLG which is difficult to achieve on Ni. In order to prepare BLG on Cu foils, the self-limiting growth process needs to be controlled precisely by adopting some methods, such as using Cu–Ni alloy, low-pressure synthesis and controlling the graphene nucleation on Cu. In recent times, BLG with high coverage has been prepared on engineered Cu–Ni alloy films.25 which requires a hectic process of controlling composition of Cu, Ni and appropriate condition for BLG growth. Z. Sun et al. showed that BLG could be grown by precisely tuning the pressure in the CVD chamber.26 BLG obtained by this approach still has some patches of tri-layer and tetralayer. In obtaining uniformity in single and bilayer graphene, purity of Cu substrate along with partial pressure of hydrocarbon was identified as the key factor by Wei Liu et al.22 In recent years, suppression of nucleation has been emphasized to achieve large-scale graphene on Cu substrate.27 However, most of the studies in this approach were based on obtaining single layer Graphene. Suppression of nucleation plays a pivotal role in the creation of uniform nucleation over the substrate and obtains maximum coverage of the single layer graphene.28 However, the impact of suppressing nucleation on forming BLG has not been reported up to now.
In this work, controlled nucleation for an extended period with low concentration of precursor was adopted to synthesize continuous BLG. Solid botanical derivative (C10H16O), a green and renewable carbon source was used as precursor to obtain low cost continuous BLG. We used two-way gas flow to the CVD chamber to obtain greater control over deposition. Using the solid carbon source, the effect of other growth parameters such as temperature and deposition time was investigated and optimized.
2. Experimental details
Polycrystalline Cu foil, purchased from Nilaco Corporation of 99% purity and 25 μm thickness was cut into 1 cm × 2 cm and 5 mm × 5 mm pieces. A long quartz tube 120 cm long and 75 mm in diameter was made for the CVD experiment. Besides, a precursor chamber made of glass was used to introduce carbon source and to facilitate its controlled insertion for reaction with substrate. Two separately controlled furnaces, one for the precursor chamber (furnace 1) and one for placing the substrate (furnace 2) were used at the same time. Argon and hydrogen with a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and purity of 99% were used to transport the camphor precursor to react with Cu foil placed in furnace 2. Flow of precursor to the substrate was tuned by two-way flow of carrier gas (Ar + H2); one way was carrier gas to the substrate through precursor chamber (F2) and another was through the quartz tube only (F1).
1 and purity of 99% were used to transport the camphor precursor to react with Cu foil placed in furnace 2. Flow of precursor to the substrate was tuned by two-way flow of carrier gas (Ar + H2); one way was carrier gas to the substrate through precursor chamber (F2) and another was through the quartz tube only (F1).
Cu substrates were cleaned by sonication for 5 minutes in acetone, then rinsed in methanol followed by isopropanol, and then loaded into quartz tube furnace. The substrate was annealed at 1000 °C for 60 minutes before deposition. A wide range of temperature ranging from 950 °C to 1050 °C was chosen in the current approach whereas, deposition time varied from 5 to 30 minutes. The precursor chamber was placed in the center of the furnace set at 85–110 °C. The flow rate of Ar + H2 was precisely controlled from 6–18 sccm inside the quartz tube (F1) and 2–6 sccm inside the precursor chamber (F2). Rapid cooling was performed29 immediately after the deposition. As-grown samples were characterized by Field Emission Scanning Electron Microscopy (FESEM, JEOL JSM -7001FF), Raman Spectroscopy (green laser with excitation wavelength of 532 nm, JASCO NRS-1500W) and Raman mapping by NRS-3300, Transmission Electron Microscopy (TEM) by JEM-2100F by JEOL and UV-visible spectrophotometer measured by V-570, JASCO.
3. Result and discussion
In our approach, we reduced the intensity of nucleation of carbon on the Cu substrate and obtained controlled growth by changing the carrier gas and carbon source insertion. This was accomplished by varying the temperature of furnace 1 in the range of 80–110 °C. Further control over the flow of precursor was maintained by controlling F2 and F1, shown in Fig. 1. | ||
| Fig. 1 Schematic diagram of two-furnace set side by side along with special arrangement of precursor chamber to control precursor flow. | ||
Raman spectroscopy and Raman mapping were carried out to characterize as grown deposition. Raman spectroscopy is a simple technique that uses the Raman scattering phenomena to evaluate the quality and justify the number of layers of graphene. The strongest Raman peaks in crystalline graphene are G (∼1584 cm−1) and 2D (∼2400–2800 cm−1) bands.30 Furthermore, the existence of disorder in the graphene lattice causes the appearance of so-called D bands (∼1200–1400 cm−1). The ratio of 2D and G peak (I2D/IG) and the Full Width Half Maximum (FWHM) of the 2D peak can be used to evaluate the number of graphene layers.31 The value of I2D/IG more than 1 and FWHM value less than 30 cm−1 indicates single layer graphene structure whereas for multilayer graphite, I2D/IG > 1 and FWHM ∼40 cm−1.32 Besides, the ratio of G to D peak (IG/ID) determines the quality and disorder in graphene.21,32
Raman spectroscopic information in Fig. 2(a) and (b) shows that the proportion of carrier gas flow to the precursor chamber (F2) and the quartz tube (F1) has significant impact on formation of bilayer graphene. These two flow rates were tuned while temperature, pressure and deposition time were kept constant at 1050 °C, 1 atmospheric pressure and 30 minutes respectively. Fig. 2(a) shows that while flow rate, F1 remained unchanged, changing flow rate, F2 impacts graphene deposition. When F2 and F1 are equal (F2, F1- 2 and 2 sccm respectively), a very low intensity G and 2D peak are observed. As the F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 is soared up to 2 (F2 and F1- 2 and 4 sccm respectively), intense G peak along with defective D and 2D band are observed (i.e. I2D/IG < 1) which indicates existence of few layer graphene with substantial defects. As F1 is increased further (F1 = 6 sccm), 2D and G peak with equal intensity are observed which justify BLG. Catalytic reaction of Cu with carbon vastly depends on carrier gas and precursor composition.24 It can be seen that for F2
F1 is soared up to 2 (F2 and F1- 2 and 4 sccm respectively), intense G peak along with defective D and 2D band are observed (i.e. I2D/IG < 1) which indicates existence of few layer graphene with substantial defects. As F1 is increased further (F1 = 6 sccm), 2D and G peak with equal intensity are observed which justify BLG. Catalytic reaction of Cu with carbon vastly depends on carrier gas and precursor composition.24 It can be seen that for F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 at minimum value ensures less amount of precursor to react with Cu per unit of time which contributes some small patches of carbon on Cu with less significant D, G and 2D peak. Constant precursor gas flow, F2 and further increase of carrier gas flow, F1 cause more carbon to react in a given time and hence gives few graphene layers with significant defectivity. As the F2
F1 at minimum value ensures less amount of precursor to react with Cu per unit of time which contributes some small patches of carbon on Cu with less significant D, G and 2D peak. Constant precursor gas flow, F2 and further increase of carrier gas flow, F1 cause more carbon to react in a given time and hence gives few graphene layers with significant defectivity. As the F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 is made 2
F1 is made 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, uniform catalytic reaction on Cu takes place which contributes to the formation of BLG. Furthermore, higher carrier gas flow, F1 may lead to high desorption rate and hence, reduce formation of multilayer graphene on Cu. An opposite attempt, increasing F2 and keeping F1 fixed is proven to be detrimental to obtain uniform BLG, shown in Fig. 2(b). For the flow ratio, F1
6, uniform catalytic reaction on Cu takes place which contributes to the formation of BLG. Furthermore, higher carrier gas flow, F1 may lead to high desorption rate and hence, reduce formation of multilayer graphene on Cu. An opposite attempt, increasing F2 and keeping F1 fixed is proven to be detrimental to obtain uniform BLG, shown in Fig. 2(b). For the flow ratio, F1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F2 = 6
F2 = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the G peak is seen to be slightly higher than 2D (I2D/IG < 1). As the proportion of F2 and F1 become 12
6, the G peak is seen to be slightly higher than 2D (I2D/IG < 1). As the proportion of F2 and F1 become 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, intensity of G peak becomes more intense, compared to 2D, which proves multilayer graphene. More intense G peak, almost twice of the 2D peak is observed while F2 and F1 become 18 sccm and 6 sccm respectively. Keeping carrier gas flow F1 at constant value and increasing precursor gas flow, F2 allows more adsorption than desorption and hence contributes formation of more layers. It is interesting to observe that at high F2
6, intensity of G peak becomes more intense, compared to 2D, which proves multilayer graphene. More intense G peak, almost twice of the 2D peak is observed while F2 and F1 become 18 sccm and 6 sccm respectively. Keeping carrier gas flow F1 at constant value and increasing precursor gas flow, F2 allows more adsorption than desorption and hence contributes formation of more layers. It is interesting to observe that at high F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1, D peak diminishes, shown in Fig. 2(b). As the deposition shows more graphitic pattern, the defect reduced significantly.33
F1, D peak diminishes, shown in Fig. 2(b). As the deposition shows more graphitic pattern, the defect reduced significantly.33
Deposition temperature has vast impact on obtaining larger flakes. In Fig. 2(c)–(e), FESEM images illustrate the impact of temperature on formation of larger flakes of graphene on Cu foil. A low influx of precursor was maintained by ensuring a low F2 (2 sccm) and moderate F1 (6 sccm) to the CVD furnace for 30 minutes deposition at three different temperatures (950 °C, 1000 °C and 1050 °C) and atmospheric pressure. It can be seen from Fig. 2(c), at deposition temperature of 950 °C, very few hexagon-shaped sites are observed indicated by white arrows. As the deposition temperature is raised to 1000 °C, more hexagonal shapes are visualized, shown in Fig. 2(d). At 1050 °C, the graphene structure spreads over the whole substrates, shown in Fig. 2(e). Thus, it is obvious that deposition at high temperature affects flakes size of graphene on Cu substrates.
According to the relation between diffusivity of carbon and temperature, it can be seen:
 | (1) |
In Fig. 3(a)–(f), it is observed from Raman mapping that deposition for an extended period helps to attain more BLG than deposition for small duration. The deposition temperature and pressure were kept at 1050 °C and 1 atmospheric pressure respectively with low insertion of precursor (F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 = 2
F1 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) by two way carrier gas flow. For the deposition time of 5 minutes, it is seen that I2D/IG varies from 0.2 to 1.5, shown in Fig. 3(a). For 15 minutes deposition (shown in Fig. 3(b)), I2D/IG is observed to be confined to the range of 0.4 to 1.2 which indicates existence of mostly BLG. As the deposition time is further increased to 30 minutes, 2D to G peak ratio becomes limited to the range of 0.9 to 1.2 (shown in Fig. 3(c)) which validates that extended period of deposition contributes more BLG sites. In Fig. 3(d)–(f), the graph of I2D/IG vs. FWHM was plotted, considering 100 points from the substrates (shown in Fig. 3(a)–(c)). Fig. 3(d) represents indiscriminate scattering of graphene/graphitic sites for the deposition of 5 minutes, where scattering of I2D/IG and FWHM ranges from 0.2 to 1.3 and 25 to 65 cm−1 respectively which indicates single to few layer graphene existence. Less dispersion is seen for the deposition of 15 minutes, illustrated in Fig. 3(e). It is visualized that the point of graphitization tends to locate near the range of 0.6–1.0 for I2D/IG and 40–50 cm−1 for FWHM which suggests more BLG for deposition for extended period. This spreading phenomenon is significantly reduced when the deposition time was extended for 30 minutes, shown in Fig. 3(f). For less deposition time, a small number of carbon particles are involved in diffusion with less nucleation sites. Therefore, single to few layer graphene is formed in those nucleated points. Deposition for extended time (15–30 minutes) contributes more nucleation sites which are distributed almost equally over the substrate and hence impart uniform diffusion of carbon. Formation of BLG initiates from the SLG flakes and largely depends on coverage of SLG on the substrate and the deposition time.37 As the precursor reacts with Cu and deposition of monolayer is yet to be completed, the deposition of bilayer starts from the middle of the monolayer flakes, shown in Fig. 4(a) and (b). Even, after the completion of full coverage of monolayer formation (shown in Fig. 4(c)) on substrate, bilayer formation goes on. Therefore, extended period of deposition helps to form continuous BLG on Cu substrate (Fig. 4(d)).
6) by two way carrier gas flow. For the deposition time of 5 minutes, it is seen that I2D/IG varies from 0.2 to 1.5, shown in Fig. 3(a). For 15 minutes deposition (shown in Fig. 3(b)), I2D/IG is observed to be confined to the range of 0.4 to 1.2 which indicates existence of mostly BLG. As the deposition time is further increased to 30 minutes, 2D to G peak ratio becomes limited to the range of 0.9 to 1.2 (shown in Fig. 3(c)) which validates that extended period of deposition contributes more BLG sites. In Fig. 3(d)–(f), the graph of I2D/IG vs. FWHM was plotted, considering 100 points from the substrates (shown in Fig. 3(a)–(c)). Fig. 3(d) represents indiscriminate scattering of graphene/graphitic sites for the deposition of 5 minutes, where scattering of I2D/IG and FWHM ranges from 0.2 to 1.3 and 25 to 65 cm−1 respectively which indicates single to few layer graphene existence. Less dispersion is seen for the deposition of 15 minutes, illustrated in Fig. 3(e). It is visualized that the point of graphitization tends to locate near the range of 0.6–1.0 for I2D/IG and 40–50 cm−1 for FWHM which suggests more BLG for deposition for extended period. This spreading phenomenon is significantly reduced when the deposition time was extended for 30 minutes, shown in Fig. 3(f). For less deposition time, a small number of carbon particles are involved in diffusion with less nucleation sites. Therefore, single to few layer graphene is formed in those nucleated points. Deposition for extended time (15–30 minutes) contributes more nucleation sites which are distributed almost equally over the substrate and hence impart uniform diffusion of carbon. Formation of BLG initiates from the SLG flakes and largely depends on coverage of SLG on the substrate and the deposition time.37 As the precursor reacts with Cu and deposition of monolayer is yet to be completed, the deposition of bilayer starts from the middle of the monolayer flakes, shown in Fig. 4(a) and (b). Even, after the completion of full coverage of monolayer formation (shown in Fig. 4(c)) on substrate, bilayer formation goes on. Therefore, extended period of deposition helps to form continuous BLG on Cu substrate (Fig. 4(d)).
It is also observed that deposition for extended period helps to reduce defects significantly in the suppression of nucleation approach. It is important to note that ratio of G to D depicts the defects, graphitization as well as crystallite dimension.38 Here, low insertion of precursor (F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 = 2
F1 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) was maintained while deposition temperature and pressure were kept at 1050 °C and 1 atmospheric pressure respectively. In Fig. 5(a)–(c), spatial representation of G to D ratio (IG/ID) shows that deposition with a short period contributes lower G to D ratio whereas, long-duration growth provides comparatively high G to D ratio. It can be seen from Fig. 5(a), the IG/ID ratio varies in the range of 0.7 to 3.3 for the deposition of 5 minutes which proves existence of substantial defects along with graphitization. Deposition for 15 minutes demonstrates more graphitization sites as IG/ID ranges from 1 to 7. For an extended period of 30 minutes, the peak intensity ratio of G to D varies from 3 to 7 which further proves more graphitization for the deposition of longer period. It is clear that higher deposition time is vital to obtain more graphitic carbon with the suppression of nucleation approach.
6) was maintained while deposition temperature and pressure were kept at 1050 °C and 1 atmospheric pressure respectively. In Fig. 5(a)–(c), spatial representation of G to D ratio (IG/ID) shows that deposition with a short period contributes lower G to D ratio whereas, long-duration growth provides comparatively high G to D ratio. It can be seen from Fig. 5(a), the IG/ID ratio varies in the range of 0.7 to 3.3 for the deposition of 5 minutes which proves existence of substantial defects along with graphitization. Deposition for 15 minutes demonstrates more graphitization sites as IG/ID ranges from 1 to 7. For an extended period of 30 minutes, the peak intensity ratio of G to D varies from 3 to 7 which further proves more graphitization for the deposition of longer period. It is clear that higher deposition time is vital to obtain more graphitic carbon with the suppression of nucleation approach.
It is well known that the IG/ID gives a glimpse of grain size of the flakes. According to the Tuinstra–Koenig39 relationship:
 | (2) |
To investigate the coverage of graphitization and continuity of BLG by suppression of nucleation, Raman mapping was performed in three different sites (27 μm × 27 μm each, shown in Fig. 5(d)) of samples annealed for 5, 15 and 30 minutes at 1050 °C with low insertion of precursor (F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F1 = 2
F1 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6). Extracted IG/ID, IG/ID deviation and deposition time were plotted, shown in Fig. 5(e). It can be seen from Fig. 5(e) that average IG/ID for the mapped three areas is much lower for 5 minutes deposition and increased significantly for the long period of deposition which is clearly in agreement with the previous claim of better graphitization for the deposition of extended period. In Fig. 5(f), it is observed that deposition for extended time is crucial to obtain continuous BLG. Extracted I2D/IG and deviation of I2D/IG from the three mapped areas are plotted against deposition time. It is observed that I2D/IG deviation is much higher for deposition of short period where as for extended time, the deviation reduced significantly along with escalation of I2D/IG value. Thus, it further proves that deposition for long time helps the growth of BLG in the current approach of suppression of nucleation.
6). Extracted IG/ID, IG/ID deviation and deposition time were plotted, shown in Fig. 5(e). It can be seen from Fig. 5(e) that average IG/ID for the mapped three areas is much lower for 5 minutes deposition and increased significantly for the long period of deposition which is clearly in agreement with the previous claim of better graphitization for the deposition of extended period. In Fig. 5(f), it is observed that deposition for extended time is crucial to obtain continuous BLG. Extracted I2D/IG and deviation of I2D/IG from the three mapped areas are plotted against deposition time. It is observed that I2D/IG deviation is much higher for deposition of short period where as for extended time, the deviation reduced significantly along with escalation of I2D/IG value. Thus, it further proves that deposition for long time helps the growth of BLG in the current approach of suppression of nucleation.
The stacking structure of graphene film was studied by TEM measurement, shown in Fig. 6(a) and (b) which further confirmed the existence of BLG. The film was transferred on TEM grid and existence of graphene was confirmed the by Raman spectroscopy. As shown in the Fig. 6(a), in the TEM grid, investigated portion of the film is shown by an arrow. A high-resolution TEM image is taken, shown in Fig. 6(b). The inset image shows the diffraction pattern of graphene displays hexagonal crystalline structure. The calculated interlayer spacing of the graphene is about 0.34 nm, shown in Fig. 6(b). In the TEM image, most of the layer observed are bi-layer providing clear evidence of bi-layer deposition.
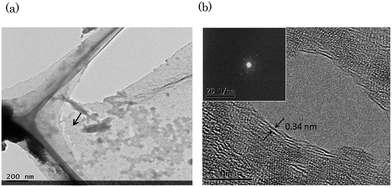 | ||
| Fig. 6 (a) Graphene in TEM grid and (b) as-grown Bi-layer graphene. Inset image shows diffraction pattern of BLG. | ||
Graphene with tunable properties and flexible criteria has numerous applications.40,41 In the current approach, the deposited graphene was transferred from Cu foil to an insulator substrate, silicone rubber (polymerized siloxanes), shown in Fig. 7(a)–(d). Silicone Rubber (SR) has transparent and flexible properties. At first, Graphene on Cu was hot pressed with SR at moderate temperature and pressure (shown in Fig. 7(a) and (b)). The Cu/BLG/SR structure was submerged into diluted HNO3 for 24 hours and the metal was etched (shown in Fig. 7(c) and (d)). Fig. 7(e) shows Raman spectroscopic information depicting quality of BLG before and after transfer process. As-grown BLG on Cu shows almost no D peak, whereas, after the transfer on SR, small D peak is observed. Chemical etching is supposed to impart small defects on BLG on SR. The measured transmittance of graphene on silicon rubber is ∼93% in the visible region (as shown in Fig. 7(f)), whereas inset image shows transferred graphene on SR which further validates our stance of synthesis of large scale BLG.
4. Conclusion
We synthesized large scale BLG from botanic precursor, camphor. Continuous bilayer was obtained by two-way carrier gas insertion, which has proven instrumental in obtaining much control over BLG growth. The deposition by suppressing the flow of carbon source to CVD chamber provided BLG with large flakes. It was also found that deposition of long duration and deposition temperature are crucial to reduce the defects in the film and increase the grain size too. Transferred bilayer in flexible and transparent silicones substrates provided high transmittance in the visible region. Our approach, continuous BLG from solid precursors is cost efficient and highly controllable and may have vast implications in electronic industries.References
- F. Schwierz, Graphene transistors, Nat. Nanotechnol., 2010, 5(7), 487–496 CrossRef CAS PubMed.
- C. Dimitrakopoulos, Y.-M. Lin, A. Grill, D. B. Farmer, M. Freitag and Y. Sun, et al., Wafer-scale epitaxial graphene growth on the Si-face of hexagonal SiC (0001) for high frequency transistors, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.–Process., Meas., Phenom., 2010, 28(5), 985–992 CrossRef CAS.
- M. Dragoman, D. Neculoiu, G. Deligeorgis, G. Konstantinidis, D. Dragoman and A. Cismaru, et al., Millimeter-wave generation via frequency multiplication in graphene, Appl. Phys. Lett., 2010, 97(9), 093101 CrossRef PubMed.
- M. Y. Han, B. Özyilmaz, Y. Zhang and P. Kim, Energy Band-Gap Engineering of Graphene Nanoribbons, Phys. Rev. Lett., 2007, 98(20), 206805 CrossRef.
- Y. Zhang, T.-T. Tang, C. Girit, Z. Hao, M. C. Martin and A. Zettl, et al., Direct observation of a widely tunable bandgap in bilayer graphene, Nature, 2009, 459(7248), 820–823 CrossRef CAS PubMed.
- X. Wang, Y. Ouyang, X. Li, H. Wang, J. Guo and H. Dai, Room-Temperature All-Semiconducting Sub-10-nm Graphene Nanoribbon Field-Effect Transistors, Phys. Rev. Lett., 2008, 100(20), 206803 CrossRef.
- M. Dvorak, W. Oswald and Z. Wu, Bandgap Opening by Patterning Graphene, Sci. Rep., 2013, 3, 2289 Search PubMed.
- R. Balog, B. Jorgensen, L. Nilsson, M. Andersen, E. Rienks and M. Bianchi, et al., Bandgap opening in graphene induced by patterned hydrogen adsorption, Nat. Mater., 2010, 9(4), 315–319 CrossRef CAS PubMed.
- L. Jiao, L. Zhang, X. Wang, G. Diankov and H. Dai, Narrow graphene nanoribbons from carbon nanotubes, Nature, 2009, 458(7240), 877–880 CrossRef CAS PubMed.
- J. Park, S. B. Jo, Y.-J. Yu, Y. Kim, J. W. Yang and W. H. Lee, et al., Single-Gate Bandgap Opening of Bilayer Graphene by Dual Molecular Doping, Adv. Mater., 2012, 24(3), 407–411 CrossRef CAS PubMed.
- T. H. Wang, Y. F. Zhu and Q. Jiang, Bandgap Opening of Bilayer Graphene by Dual Doping from Organic Molecule and Substrate, J. Phys. Chem. C, 2013, 117(24), 12873–12881 CAS.
- A. J. Samuels and J. D. Carey, Molecular Doping and Band-Gap Opening of Bilayer Graphene, ACS Nano, 2013, 7(3), 2790–2799 CrossRef CAS PubMed.
- Y. S. Shinichi Tanabe, H. Kageshima, M. Nagase and H. Hibino, Observation of Band Gap in Epitaxial Bilayer Graphene Field Effect Transistors, Jpn. J. Appl. Phys., 2011, 50, 04DN Search PubMed.
- T. B. A. Ohta, T. Seyller, K. Horn and E. Rotenberg, Controlling the electronic structure of bilayer graphene, Science, 2006, 313, 951 CrossRef CAS PubMed.
- P. Avouris, Graphene: Electronic and Photonic Properties and Devices, Nano Lett., 2010, 10(11), 4285–4294 CrossRef CAS PubMed.
- F. Xia, T. Mueller, Y.-M. Lin, A. Valdes-Garcia and P. Avouris, Ultrafast graphene photodetector, Nat. Nanotechnol., 2009, 4(12), 839–843 CrossRef CAS PubMed.
- Y. Chen, B. Zhang, G. Liu, X. Zhuang and E.-T. Kang, Graphene and its derivatives: switching ON and OFF, Chem. Soc. Rev., 2012, 41(13), 4688–4707 RSC.
- F. Schwierz, Graphene transistors, Nat. Nanotechnol., 2010, 5(7), 487–496 CrossRef CAS PubMed.
- W. Liu, S. Kraemer, D. Sarkar, H. Li, P. M. Ajayan and K. Banerjee, Controllable and Rapid Synthesis of High-Quality and Large-Area Bernal Stacked Bilayer Graphene Using Chemical Vapor Deposition, Chem. Mater., 2014, 26(2), 907–915 CrossRef CAS.
- W. Liu, H. Li, C. Xu, Y. Khatami and K. Banerjee, Synthesis of high-quality monolayer and bilayer graphene on copper using chemical vapor deposition, Carbon, 2011, 49(13), 4122–4130 CrossRef CAS PubMed.
- S. Lee, K. Lee and Z. Zhong, Wafer Scale Homogeneous Bilayer Graphene Films by Chemical Vapor Deposition, Nano Lett., 2010, 10(11), 4702–4707 CrossRef CAS PubMed.
- H. L. Wei Liu, C. Xu, Y. Khatami and K. Banerjee, Synthesis of high-quality monolayer and bilayer graphene on copper using chemical vapor deposition, Carbon, 2011, 49, 4122–4130 CrossRef PubMed.
- M. Ahmed, N. Kishi, R. Sugita, A. Fukaya, I. Khatri and J. Liang, et al., Graphene synthesis by thermal chemical vapor deposition using solid precursor, J. Mater. Sci.: Mater. Electron., 2013, 24, 2151–2155 CrossRef CAS PubMed.
- S. Bhaviripudi, X. Jia, M. S. Dresselhaus and J. Kong, Role of Kinetic Factors in Chemical Vapor Deposition Synthesis of Uniform Large Area Graphene Using Copper Catalyst, Nano Lett., 2010, 10(10), 4128–4133 CrossRef CAS PubMed.
- B. V. Cunning, M. Ahmed, N. Mishra, A. R. Kermany and F. Iacopi, Graphitized silicon carbide microbeams: wafer-level, self-aligned graphene on silicon wafers, Nanotechnology, 2014, 25, 325301 CrossRef PubMed.
- Z. Sun, A.-R. O. Raji, Y. Zhu, C. Xiang, Z. Yan and C. Kittrell, et al., Large-Area Bernal-Stacked Bi-, Tri-, and Tetralayer Graphene, ACS Nano, 2012, 6(11), 9790–9796 CrossRef CAS PubMed.
- D. Zhan, J. X. Yan, Z. H. Ni, L. Sun, L. F. Lai and L. Liu, et al., Bandgap-Opened Bilayer Graphene Approached by Asymmetrical Intercalation of Trilayer Graphene, Small, 2015, 11(9–10), 1177–1182 CrossRef CAS PubMed.
- T. N. Seiya Suzuki, Y. Matsuoka and M. Yoshimura, Threefold atmospheric-pressure annealing for suppressing graphene nucleation on copper in chemical vapor deposition, Jpn. J. Appl. Phys., 2014, 53, 095101 CrossRef.
- X. Li, C. W. Magnuson, A. Venugopal, R. M. Tromp, J. B. Hannon and E. M. Vogel, et al., Large-Area Graphene Single Crystals Grown by Low-Pressure Chemical Vapor Deposition of Methane on Copper, J. Am. Chem. Soc., 2011, 133(9), 2816–2819 CrossRef CAS PubMed.
- A. C. Ferrari and D. M. Basko, Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat. Nanotechnol., 2013, 8(4), 235–246 CrossRef CAS PubMed.
- S. S. Ivan Vlassiouk, I. Ivanov, P. F. Fulvio, S. Dai, M. C. Harry Meyer, D. Hensley, P. Datskos and N. V. Lavrik, Electrical and thermal conductivity of low temperature CVD graphene: the effect of disorder, Nanotechnology, 2011, 22, 275716 CrossRef PubMed.
- K. Yan, H. Peng, Y. Zhou, H. Li and Z. Liu, Formation of Bilayer Bernal Graphene: Layer-by-Layer Epitaxy via Chemical Vapor Deposition, Nano Lett., 2011, 11(3), 1106–1110 CrossRef CAS PubMed.
- A. Jorio, E. H. M. Ferreira, M. V. O. Moutinho, F. Stavale, C. A. Achete and R. B. Capaz, Measuring disorder in graphene with the G and D bands, Phys. Status Solidi B, 2010, 247(11–12), 2980–2982 CrossRef CAS PubMed.
- H. Kim, C. Mattevi, M. R. Calvo, J. C. Oberg, L. Artiglia and S. Agnoli, et al., Activation Energy Paths for Graphene Nucleation and Growth on Cu, ACS Nano, 2012, 6(4), 3614–3623 CrossRef CAS PubMed.
- M. Ajmal, S. Lee, Y. C. Cho, S. J. Kim, S. E. Park and C. R. Cho, et al., Fabrication of the best conductor from single-crystal copper and the contribution of grain boundaries to the Debye temperature, CrystEngComm, 2012, 14(4), 1463–1467 RSC.
- L. Fan, K. Wang, J. Wei, M. Zhong, D. Wu and H. Zhu, Correlation between nanoparticle location and graphene nucleation in chemical vapour deposition of graphene, J. Mater. Chem. A, 2014, 2(32), 13123–13128 CAS.
- W. Fang, A. L. Hsu, Y. Song, A. G. Birdwell, M. Amani and M. Dubey, et al., Asymmetric Growth of Bilayer Graphene on Copper Enclosures Using Low-Pressure Chemical Vapor Deposition, ACS Nano, 2014, 8(6), 6491–6499 CrossRef CAS PubMed.
- M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cançado and R. Saito, Studying disorder in graphite-based systems by Raman spectroscopy, Phys. Chem. Chem. Phys., 2007, 9, 1276–1291 RSC.
- F. Tuinstra and J. L. Koenig, Raman Spectrum of Graphite, J. Chem. Phys., 1970, 53(3), 1126–1130 CrossRef CAS PubMed.
- K. L. Seunghyun Lee, C.-H. Liu and Z. Zhong, Homogeneous bilayer graphene film based flexible transparent conductor, Nanoscale, 2012, 4, 639 RSC.
- L. G. P. Martins, Y. Song, T. Zeng, M. S. Dresselhaus, J. Kong and P. T. Araujo, Direct transfer of graphene onto flexible substrates, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(44), 17762–17767 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |





