Remediation of DDTr contaminated soil by the combination of solvent extraction and catalytic hydrodechlorination
Xuanxuan Maab,
Yongsheng Luanc,
Sujing Liuab,
Ying Liu*a and
Chuanhai Xia*a
aKey Laboratory of Coastal Biology and Biological Resources Utilization, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China. E-mail: yliu@yic.ac.cn; chxia@yic.ac.cn; Fax: +86 535 2109000; Tel: +86 535 2109173
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cYantai Environmental Monitoring Centre, Yantai, 264010, China
First published on 6th May 2015
Abstract
A combination technique for remediation of DDT and its metabolites (DDTr) contaminated soil based on successive steps of solvent extraction, followed by catalytic hydrodechlorination (HDC) was studied. Firstly, solvent extraction was applied to extract DDTr contaminated soil at ambient temperature and pressure. According to GC-MS analysis, the extracts from DDTr contaminated soil are mainly composed of p,p′-DDT, o,p′DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP. Subsequently, catalytic HDH over a Pd/C catalyst was introduced to treat the extract from DDTr contaminated soil, and the HDC process of DDTr was surveyed by monitoring the GC-MS analysis. These results demonstrate that the combined technique of solvent extraction and catalytic HDC can effectively remediate DDTr contaminated soil and reduce its toxicity.
1. Introduction
DDT (1,1,1-trichloro-2,2-bi(p-chlorophenyl)ethane) is a persistent organic pollutant that has been widely used as a broad-spectrum pesticide against forest and agricultural pests, malaria, and other mosquito borne diseases.1–3 Because of its negative impact on wildlife and ill effects on human life via the food chain, the use of DDT has recently been prohibited in most countries.4,5 The US Environmental Protection Agency (EPA) has classified DDT and its metabolites, DDD (1,1-dichloro-2,2-bis(p-chlorophenyl)ethane) and DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene), commonly known as DDTr, as priority pollutants.3 However, DDT is still used for essential public health purposes in some tropical countries due to its effectiveness and low cost in controlling mosquito-borne malaria.6 Although the use of DDT has declined, DDTr continue to be detected in environmental media and human tissues as they are persistent, lipophilic, and liable to bioaccumulation and biomagnification.7–11 Even after decades, the environmental risk of DDTr contaminated soil due to chemical factories has long been a significant problem in most countries.12 Therefore, it is of great urgency to develop a practical and efficient remediation method for DDTr contaminated soil.At present, several remediation methods for DDTr have been developed, including physical treatment,13–15 incineration,16 advanced oxidation processes (AOPs),17–19 bioremediation,20–22 zero-valent metal (ZVM) reduction,23–25 and catalytic hydrodechlorination (HDC).26–28 In general, physical treatment just transfers DDTr from one medium to another medium with no contaminants destroyed, so further treatment is necessary to reduce the toxicity of target compounds.29 Currently, the most common destructive technique incineration requires extremely high temperature and can lead to the formation of highly noxious by-products (such as polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-furans) due to incomplete combustion.30 AOPs including Fenton oxidation, wet oxidation, supercritical water oxidation, and photochemical processes have been widely investigated as effective technology but also have some drawbacks such as need of relatively high temperature and/or pressure, large amount of reagents, and/or complex equipment.31–34 Bioremediation is a potential option for the degradation of DDTr in soil, but is rather slow and affected by DDTr toxicity being limited to fairly low concentration.35,36 Catalytic HDC is considered to be a promising detoxifying technology for its potential economic and environmental advantages and wide application for reducing dramatically the toxicity of chlorinated organic compounds (COCs) under mild/ambient condition.37–39 In fact, complete removal of COCs such as DDTr from soil is very difficult by a single technique due to the high cost or limitation. Thus, hybrid methods become imperative for the abatement of COCs.
In the last few years, various hybrid methods have received increasing attention for the remediation of COCs. For the abatement of COCs contaminated water, many researches focus on the combined AOPs-biological degradation, HDC-biological degradation, and HDC–AOPs.40–45 However, few studies focus on the remediation of COCs contaminated soil with combined treatment system.46 For the remediation of COCs contaminated soil, solvent extraction is an ex situ separation and concentration process in which a non-aqueous liquid is used to transfer contaminants from soil.46 In order to reduce the toxicity of the contaminants, the solvent extraction method needs to be integrated with complementary technology suitable for the specific contaminants. Among the approaches mentioned above, catalytic HDC can reduce dramatically the toxicity of COCs under mild/ambient condition and recover some valuable raw materials without production of more hazardous byproducts.47
On the basis of these observations, it was thought of developing combined technique of solvent extraction and catalytic HDC for the remediation of DDTr contaminated soil. We designed the experimental apparatus according to previous experimental experience (Scheme 1). Solvent extraction was applied to extract DDTr contaminated soil firstly. As the DDTr extracted from the soil samples were transferred into the liquid extract, their toxicity needed to be reduced. Then catalytic HDH was introduced to treat the extract from DDTr contaminated soil. The qualitative and quantitative analyses of DDTr and their dechlorination products were performed by GC-MS and GC-FID, respectively. The combination technique designed in this way was expected to remediate the DDTr contaminated soil and reduce its toxicity effectively.
 | ||
| Scheme 1 Experimental apparatus for combination of solvent extraction and catalytic HDC for DDTr contaminated soil. | ||
2. Materials and methods
2.1. Chemicals
5% Pd/C catalyst used in this study was purchased from C&P Chemical Co., China. The catalyst was not pre-treated before all the experiments and only kept in a desiccator. DDTr contaminated soil selected for this investigation was surface loam samples (0 to 5.0 cm) and obtained from a former DDT manufacturing plant in Jiangsu, China. The soil was naturally dried in air and then grinded. After that, the soil was sieved at 0.25 mm mesh and homogenized to obtain laboratory soil sample. The standards of 1,1,1-trichloro-2,2-bi(p-chlorophenyl)ethane (p,p′-DDT), 1-chloro-4-[2,2,2-trichloro-1-(o-chlorophenyl)ethyl]benzene (o,p′-DDT), 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p′-DDE), 1-chloro-4-[2,2-dichloro-1-(o-chlorophenyl)ethenyl]benzene (o,p′-DDE), 1-chloro-2-[2,2-dichloro-1-(p-chlorophenyl)ethyl]benzene (o,p′-DDD), and p,p′-dichlorobenzophenone (DCBP) were bought from Beijing InnoChem Sciences & Technology Co., Ltd. The other reagents, such as acetone, n-hexane, and NaOH, are analytical grade and are supplied by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Deionized water was used in the reaction. The purity of hydrogen and nitrogen used in the experiments is more than 99.99%.2.2. Extraction and catalytic procedure
The experimental apparatus for combination of solvent extraction and catalytic HDC for DDTr contaminated soil is shown in Scheme 1. The solvent extraction procedure was adopted from USEPA Method 3540 for extracting DDTr from soil samples.48 The soil samples (20.0 g, accurately weighed) were extracted under reflux with 150 mL of organic solvent (acetone, n-hexane, or mixed solvent). The liquid extract was quantitatively transferred to a cooling system. The composition of the extract from DDTr contaminated soil was determined using GC-MS.The liquid-phase HDC reaction was carried out subsequently. At the beginning of each experiment, 40 mL solution was added into the flask, containing DDTr and NaOH. After the air in catalytic HDC reactor was completely replaced by nitrogen, 5% Pd/C catalyst was added and agitation was started.
2.3. Analytical methods
The identification of DDTr and their dechlorination products in the HDC was determined by GC-MS (Thermo Fisher ITQ-900) with a column of DB-5 (30 m in length, 0.25 mm ID, 0.25 μm film thickness). The quantification of DDTr and their dechlorination products was analyzed by GC-FID (Agilent-7890A) with a column of HP-5 (30 m in length, 0.32 mm ID, 0.25 μm film thickness). And the concentration (%) of DDTr refers to the percentage of DDTr quantified by GC-FID. It is well-established that DDT could be broken down during GC injection, a careful analytical method was chosen for the HDC of DDT during GC-MS measurement.49 The temperature program used for analysis was as follows: the initial temperature of column was 50 °C, held for 2.0 min, and the rate of temperature increase was 10 °C min−1 up to 180 °C, and held for 1.0 min. Then, the increase rate was changed to 5 °C min−1 up to 260 °C, with a final hold time of 6 min. The injection port and detector temperature were set at 220 °C and 260 °C, respectively.According to our experiments, about 0.5 g extract was obtained from 20.0 g DDTr contaminated soil, and the average extraction quantity of 20.0 g soil samples was 0.5019 g DDTr within 4 hours or more extraction time. After complete extraction from DDTr contaminated soil, n-hexane, acetone, and n-hexane–acetone were used to extract the soil subsequently. However, no DDTr was detected with the analysis of GC-MS. Hence, extraction yield is defined as
Measurements of DDTr detected before and after the HDC reaction were divided into 9 groups on the basis of numbers of chlorine atoms in a molecular nucleus. The average chlorine atom number (ACN) is obtained as follows:
3. Results and discussion
Firstly, solvent extraction was used to extract DDTr from soil samples. The extract containing DDTr was subjected to liquid-phase HDC under catalytic condition that allowed to greatly reduce the harmfulness of the products. After the HDC over Pd/C catalyst, the extract was converted into 1,1-diphenylethane (DPE) which was easily biodegraded.48 The two sections will be discussed separately.3.1. Solvent extraction of DDTr contaminated soil
Solvent extraction was introduced to extract DDTr from soil samples. In general, extraction solvent plays an important role on the yield of solvent extraction from DDTr contaminated soil.50 Hence, the influence of solvent on extraction of DDTr contaminated soil was investigated firstly. Fitzpatrick et al. reported that hexane and acetone exhibited good extraction yield for extracting DDT, DDE, and DDD from contaminated soil.13 Thus, n-hexane and acetone were selected as solvent for the investigation. As shown in Fig. 1, the extraction yield of DDTr for n-hexane from soil samples reaches 76.9% within 8 hours, while the extraction yield of DDTr for acetone from soil samples reaches 100% within 6 hours. However, the liquid extract from DDTr contaminated soil using acetone as solvent is clay-coloured, which is due to humic substances and might cause the lower reaction rate in liquid-phase HDC.51,52 | ||
| Fig. 1 Solvent extraction yield of DDTr contaminated soil with reaction time. Reaction condition: soil sample (20 g), extraction solvent (150 mL). | ||
Considering that azeotropic point of n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) is as low as 35.0 °C,53 the energy consumption of solvent extraction could be efficiently reduced when n-hexane–acetone (49
51, v/v) is as low as 35.0 °C,53 the energy consumption of solvent extraction could be efficiently reduced when n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) was used as solvent. In addition, US EPA pointed out that n-hexane–acetone (1
51, v/v) was used as solvent. In addition, US EPA pointed out that n-hexane–acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent system has lower disposal cost and lower toxicity.54 Thus, n-hexane–acetone (49
1, v/v) solvent system has lower disposal cost and lower toxicity.54 Thus, n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) was applied to solvent extraction to extract DDTr from the soil sample. It can be seen that n-hexane–acetone (49
51, v/v) was applied to solvent extraction to extract DDTr from the soil sample. It can be seen that n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) as an extraction solvent exhibits higher efficiency than n-hexane and acetone. Moreover, complete extraction of DDTr for n-hexane–acetone (49
51, v/v) as an extraction solvent exhibits higher efficiency than n-hexane and acetone. Moreover, complete extraction of DDTr for n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) is achieved within 4 hours. Meanwhile, the liquid extract from DDTr contaminated soil with n-hexane–acetone (49
51, v/v) is achieved within 4 hours. Meanwhile, the liquid extract from DDTr contaminated soil with n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) as solvent is colourless. These results indicate that n-hexane–acetone (49
51, v/v) as solvent is colourless. These results indicate that n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) is much better solvent for solvent extraction compared with n-hexane and acetone. Therefore, n-hexane–acetone (49
51, v/v) is much better solvent for solvent extraction compared with n-hexane and acetone. Therefore, n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) solvent system was used as the solvent for extraction in the following research.
51, v/v) solvent system was used as the solvent for extraction in the following research.
Furthermore, solvent extraction for n-hexane–acetone (49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v) was repeated 3 times to ascertain the extraction quantity of DDTr from soil sample (Table 1). It can be seen that about 0.5 g extract was obtained from 20.0 g DDTr contaminated soil, and the average extraction quantity of 20.0 g soil samples was 0.5019 g DDTr within 4 hours. In order to determine composition of the extract, liquid samples of the extract were filtered through Millipore membrane and analyzed by GC-MS. As displayed in Fig. 2, there are six peaks on the total ion chromatogram of the extract from DDTr contaminated soil. According to mass spectrum of the six peaks, they are respectively identified as p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP (Table 2), which are further verified via the total ion chromatograms of p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, o,p′-DDD, and DCBP standards. This indicates that the extract from DDTr contaminated soil are mainly composed of p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP. Moreover, the contents of these compounds in the extract from DDTr contaminated soil are 1.8%, 30.6%, 25.3%, 29.2%, 4.7%, and 8.4%, respectively. It is believed that DDE, DDD, and DBP in the extract might be formed by environmental degradation of DDT. Foght et al. proposed that DDE was predominantly produced from dehydrochlorination of DDT under aerobic soil condition.3 Boul et al. considered that DDD was form by reductive dechlorination of DDT in anaerobic soil.55 Moreover, DDT, DDE, and DDD may also be directly transformed to DCBP via the involvement of Fenton reaction.21 Yet, no further degradation product was detected in the extract from DDTr contaminated soil because DDE and DDD are viewed as recalcitrant compounds.1 On the other hand, DDTr were just transferred into the extract, and their toxicity was not reduced at all. Hence, solvent extraction method needs to be integrated with complementary technology to reduce the toxicity of DDTr. In this case, catalytic HDC of DDTr was carried out in the following research.
51, v/v) was repeated 3 times to ascertain the extraction quantity of DDTr from soil sample (Table 1). It can be seen that about 0.5 g extract was obtained from 20.0 g DDTr contaminated soil, and the average extraction quantity of 20.0 g soil samples was 0.5019 g DDTr within 4 hours. In order to determine composition of the extract, liquid samples of the extract were filtered through Millipore membrane and analyzed by GC-MS. As displayed in Fig. 2, there are six peaks on the total ion chromatogram of the extract from DDTr contaminated soil. According to mass spectrum of the six peaks, they are respectively identified as p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP (Table 2), which are further verified via the total ion chromatograms of p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, o,p′-DDD, and DCBP standards. This indicates that the extract from DDTr contaminated soil are mainly composed of p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP. Moreover, the contents of these compounds in the extract from DDTr contaminated soil are 1.8%, 30.6%, 25.3%, 29.2%, 4.7%, and 8.4%, respectively. It is believed that DDE, DDD, and DBP in the extract might be formed by environmental degradation of DDT. Foght et al. proposed that DDE was predominantly produced from dehydrochlorination of DDT under aerobic soil condition.3 Boul et al. considered that DDD was form by reductive dechlorination of DDT in anaerobic soil.55 Moreover, DDT, DDE, and DDD may also be directly transformed to DCBP via the involvement of Fenton reaction.21 Yet, no further degradation product was detected in the extract from DDTr contaminated soil because DDE and DDD are viewed as recalcitrant compounds.1 On the other hand, DDTr were just transferred into the extract, and their toxicity was not reduced at all. Hence, solvent extraction method needs to be integrated with complementary technology to reduce the toxicity of DDTr. In this case, catalytic HDC of DDTr was carried out in the following research.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, v/v)
51, v/v)
| Entry | Weight of soil (g) | Weight of the extract (g) |
|---|---|---|
| 1 | 20.3081 | 0.5159 |
| 2 | 20.1912 | 0.4984 |
| 3 | 20.1544 | 0.4915 |
| Entry | Compounds | Major ions (m/z) |
|---|---|---|
| 1 | DCPB | 250, 215, 139, 111, 75 |
| 2 | o,p′-DDE | 316, 246, 210, 176 |
| 3 | p,p′-DDE | 316, 246, 210, 176 |
| 4 | o,p′-DDD | 318, 235, 165 |
| 5 | o,p′-DDT | 352, 282, 235, 165 |
| 6 | p,p′-DDT | 352, 282, 235, 165 |
3.2. Catalytic HDC of the extract from DDTr contaminated soil
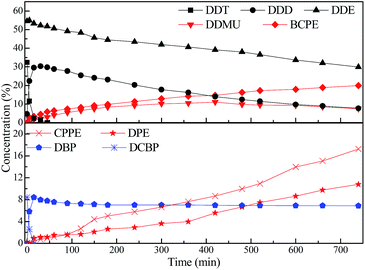
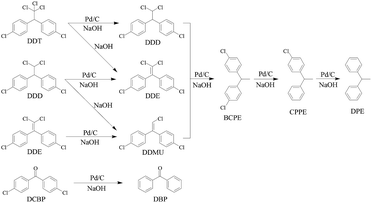
In our previous work, it was found that in situ produced inorganic salt would accumulate on surface of the catalyst, and thus would cause decline in activity of the catalyst in organic solvent.56,57 The addition of water in organic solvents could prevent inorganic salt from accumulating on surface of the catalyst and thereby enabled the catalyst to keep high activity in liquid-phase HDC. On the basis of these researches, alcohol–water homogeneous solvent system was developed to hydro-treat high concentration COCs, in which RANEY® Ni and Pd/C catalyst keep high activity and stability.56–60 Thus, 70% isopropanol–water (70/30, v/v) solvent system was applied to liquid-phase HDC of the extract from DDTr contaminated soil over 5% Pd/C catalyst.The catalytic HDC of the extract from DDTr contaminated soil was performed in a solution of NaOH over 5% Pd/C catalyst under mild condition. In this paper, the HDC process of DDTr was surveyed by monitoring the GC-MS analysis. Progress of product and intermediate distributions versus reaction time are given in Fig. 3. The concentrations of DDT and DCBP decrease sharply with the reaction time, and the concentrations of DDD, DDE, and 1-chloro-2,2-bis(chlorophenyl)ethylene (DDMU) increase to maximums and then decrease sluggishly. The concentrations of 1,1-bis(chlorophenyl)ethane (BCPE), 1-chlorophenyl-1-phenylethane (CPPE), and DPE increase gradually with the reaction time. The HDC reaction profiles of extract from DDTr contaminated soil imply that DDD, DDE, DDMU, BCPE, and CPPE are all intermediate product in the HDC of the extract. For the dechlorination of DDTr in 70% isopropanol–water (70/30, v/v), DDD and DDE as intermediate products were produced from HDC and dehydrochlorination of DDT, respectively.61 On the other hand, DDMU as an intermediate product could be formed via HDC of one aliphatic chlorine in DDE and via dehydrochlorination of one aliphatic chlorine in DDD.10 Hence, the reaction pathway for catalytic HDC of the extract from DDTr contaminated soil involves multisteps, as illustrated in Scheme 2.
After the HDC reaction, the contents of DDE, DDD, DDMU, BCPE, and CPPE are 29.85%, 7.69%, 7.49%, 19.91%, and 17.26% respectively observed in the samples by GC-MS analysis (Fig. 3). Yet, DDT of the extract is completely hydrodechlorinated within 45 min. This suggests that DDE and DDD are rather difficult to be completely hydrodechlorinated within 740 min under mild condition (40 °C, 0.1 MPa). Moreover, it can be seen from Fig. 4 that the chloride atom removal ratio of the extract is only 44.7% within 740 min using Pd/C catalyst. In order to completely reduce toxicity of the extract from DDTr contaminated soil, liquid-phase HDC of the extract was carried out under higher pressure (0.6 MPa) and higher temperature (80 °C) over Pd/C catalyst. It was found that DDTr extracted from contaminated soil can be completely dechlorinated within 180 min under higher pressure and temperature (Fig. 4). These results indicate that the combination technique of solvent extraction and catalytic HDC is a practical and efficient disposal method, which can effectively remove and degradate DDTr of the heavy pollution sites.
4. Conclusions
In summary, we developed a combination technique of solvent extraction and catalytic HDC, and designed an experimental apparatus for the remediation of DDTr contaminated soil. DDTr could be completely extracted from the soil sample at ambient temperature and pressure. Moreover, the extract from DDTr contaminated soil are mainly composed of p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE, p,p′-DDD, and DCBP based on GC-MS analysis. Then the extract from DDTr contaminated soil was effectively hydrodechlorinated over Pd/C with GC-MS analysis monitoring the HDC process of DDTr. The combination method described in this manuscript is a good way for lab detection and removing of the contamination. It provides a practical strategy and direction for rapid abatement of POPs contaminated soil under mild condition. However, for big scale application in industry or for a polluted soil field, this method might still need further investigation.Acknowledgements
This study was funded and conducted by the National Science Foundation of China (no. 21377162 and 21007088).Notes and references
- J. F. Quensen III, S. A. Mueller, M. K. Jain and J. M. Tiedje, Science, 1998, 280, 722–724 CrossRef.
- Stockholm Conversion on Persistent Organic Pollutants, 2001, http://www.pops.int/documents/convtext/convtext_en.pdf.
- J. Foght, T. April, K. Biggar and J. Aislabie, Biorem. J., 2001, 5, 225–246 CrossRef CAS PubMed.
- A. I. Lunney, B. A. Zeeb and K. J. Reimer, Environ. Sci. Technol., 2004, 38, 6147–6154 CrossRef CAS.
- Z. Lin, X. M. Li, Y. T. Li, D. Y. Huang, J. Dong and F. B. Li, J. Environ. Monit., 2012, 14, 1551–1558 RSC.
- M. D. Engelmann, J. G. Doyle and I. F. Cheng, Chemosphere, 2001, 43, 195–198 CrossRef CAS.
- A. Binelli and A. Provini, Chemosphere, 2003, 52, 717–723 CrossRef CAS.
- D. Carrizo, J. O. Grimalt, N. Ribas-Fito, J. Sunyer and M. Torrent, Environ. Sci. Technol., 2006, 40, 1420–1426 CrossRef CAS.
- Y. Guo, H. Y. Yu and E. Y. Zeng, Environ. Pollut., 2009, 157, 1753–1763 CrossRef CAS PubMed.
- Y. Ukisu, J. Hazard. Mater., 2008, 152, 287–292 CrossRef CAS PubMed.
- M. Yoshikane, W. R. Kay, Y. Shibata, M. Inoue, T. Yanai, R. Kamata, J. S. Edmonds and M. Morita, J. Environ. Monit., 2006, 8, 649–661 RSC.
- T. Lin, Z. Hu, G. Zhang, X. Li, W. Xu, J. Tang and J. Li, Environ. Sci. Technol., 2009, 43, 8033–8038 CrossRef CAS PubMed.
- L. J. Fitzpatrick, J. R. Dean, M. H. I. Comber, K. Harradine and K. P. Evans, J. Chromatogr. A, 2000, 874, 257–264 CrossRef CAS.
- S. E. Hale, J. E. Tomaszewski, R. G. Luthy and D. Werner, Water Res., 2009, 43, 4336–4346 CrossRef CAS PubMed.
- H. Tian, J. J. Li, Q. Shen, H. L. Wang, Z. P. Hao, L. D. Zou and Q. Hu, J. Hazard. Mater., 2009, 171, 459–464 CrossRef CAS PubMed.
- B. Ahling, Sci. Total Environ., 1978, 9, 117–124 CrossRef CAS.
- K.-H. Kim and S.-K. Ihm, J. Hazard. Mater., 2011, 186, 16–34 CrossRef CAS PubMed.
- A. R. Ribeiro, O. C. Nunes, M. F. R. Pereira and A. M. T. Silva, Environ. Int., 2015, 75, 33–51 CrossRef CAS PubMed.
- H. Shimakoshi, M. Tokunaga, T. Baba and Y. Hisaeda, Chem. Commun., 2004, 1806–1807 RSC.
- F. Cao, T. X. Liu, C. Y. Wu, F. B. Li, X. M. Li, H. Y. Yu, H. Tong and M. J. Chen, J. Agric. Food Chem., 2012, 60, 11238–11244 CrossRef CAS PubMed.
- A. S. Purnomo, T. Mori, I. Kamei and R. Kondo, Int. Biodeterior. Biodegrad., 2011, 65, 921–930 CrossRef CAS PubMed.
- A. S. Purnomo, T. Mori, I. Kamei, T. Nishii and R. Kondo, Int. Biodeterior. Biodegrad., 2010, 64, 397–402 CrossRef CAS PubMed.
- S. Suresh, Open Waste Manage. J., 2009, 2, 6–16 CrossRef CAS.
- S. K. Gautam and S. Suresh, J. Hazard. Mater., 2007, 139, 146–153 CrossRef CAS PubMed.
- Y. S. El-Temsah and E. J. Joner, Chemosphere, 2013, 92, 131–137 CrossRef CAS PubMed.
- W. Piechocki, G. Gryglewicz and S. Gryglewicz, J. Hazard. Mater., 2009, 163, 1397–1402 CrossRef CAS PubMed.
- F. Murena and F. Gioia, J. Hazard. Mater., 2004, 112, 151–154 CrossRef CAS PubMed.
- Y. Monguchi, A. Kume and H. Sajiki, Tetrahedron, 2006, 62, 8384–8392 CrossRef CAS PubMed.
- X. Y. Cao, H. Y. Han, G. P. Yang, X. F. Gong and J. N. Jing, Mar. Pollut. Bull., 2011, 62, 2370–2376 CrossRef CAS PubMed.
- R. DeVor, K. Carvalho-Knighton, B. Aitken, P. Maloney, E. Holland, L. Talalaj, S. Elsheimer, C. A. Clausen and C. L. Geiger, Chemosphere, 2009, 76, 761–766 CrossRef CAS PubMed.
- M. Antonopoulou, E. Evgenidou, D. Lambropoulou and I. Konstantinou, Water Res., 2014, 53, 215–234 CrossRef CAS PubMed.
- Q. F. Chen, C. C. Chen, H. W. Ji, W. H. Ma and J. C. Zhao, RSC Adv., 2013, 3, 17559–17566 RSC.
- L. Y. Huang, G. J. Su, Y. X. Liu, L. W. Li, S. Liu, H. J. Lu and M. H. Zheng, RSC Adv., 2014, 4, 25453–25460 RSC.
- X. J. Lang, W. R. Leow, J. C. Zhao and X. D. Chen, Chem. Sci., 2015, 6, 1075–1082 RSC.
- S. K. Gautam and S. Suresh, J. Colloid Interface Sci., 2006, 304, 144–151 CrossRef CAS PubMed.
- Y. Huang, X. Zhao and S. J. Luan, Sci. Total Environ., 2007, 385, 235–241 CrossRef CAS PubMed.
- F. J. Urbano and J. M. Marinas, J. Mol. Catal. A: Chem., 2001, 173, 329–345 CrossRef CAS.
- M. A. Keane, J. Chem. Technol. Biotechnol., 2005, 80, 1211–1222 CrossRef CAS PubMed.
- M. A. Keane, ChemCatChem, 2011, 3, 800–821 CrossRef CAS PubMed.
- A. Dixit, A. J. Tirpude, A. K. Mungray and M. Chakraborty, Desalination, 2011, 272, 265–269 CrossRef CAS PubMed.
- I. Oller, S. Malato and J. A. Sánchez-Pérez, Sci. Total Environ., 2011, 409, 4141–4166 CrossRef CAS PubMed.
- S. W. Zhou, X. Jin, F. F. Sun, H. Zhou, C. Y. Yang and C. H. Xia, Water Sci. Technol., 2012, 65, 780–786 CrossRef CAS PubMed.
- Y. Zhou, Y. Kuang, W. Y. Li, Z. L. Chen, M. Megharaj and R. Naidu, Chem. Eng. J., 2013, 223, 68–75 CrossRef CAS PubMed.
- M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Water Res., 2013, 47, 3070–3080 CrossRef CAS PubMed.
- M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2014, 150–151, 197–203 CrossRef CAS PubMed.
- F. Murena and F. Gioia, J. Hazard. Mater., 2009, 162, 661–667 CrossRef CAS PubMed.
- Z. M. de Pedro, E. Diaz, A. F. Mohedano, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2011, 103, 128–135 CrossRef CAS PubMed.
- S. P. Singh, P. Bose, S. Guha, S. K. Gurjar and S. Bhalekar, Chemosphere, 2013, 92, 811–820 CrossRef CAS PubMed.
- M. Gfrerer and E. Lankmayr, J. Chromatogr. A, 2005, 1072, 117–125 CrossRef CAS PubMed.
- A. Silva, C. Delerue-Matos and A. Fiúza, J. Hazard. Mater., 2005, 124, 224–229 CrossRef CAS PubMed.
- Y. B. Si, J. Zhou, H. M. Chen, D. M. Zhou and Y. D. Yue, Chemosphere, 2004, 56, 967–972 CrossRef CAS PubMed.
- M. J. Chen, F. Cao, F. B. Li, C. S. Liu, H. Tong, W. J. Wu and M. Hu, J. Agric. Food Chem., 2013, 61, 2224–2233 CrossRef CAS PubMed.
- H. H. Lee, Azeotropic Data—III, American Chemical Society, 1973 Search PubMed.
- US Environmental Protection Agency, Soxhlet Extraction, Test Methods for Evaluating Solid Waste, Method 3540C, Physical/Chemical Methods, third edn, EPA/SW-846, 1996.
- H. L. Boul, Chemosphere, 1996, 32, 855–866 CrossRef CAS.
- X. X. Ma, Y. Liu, S. J. Liu and C. H. Xia, Appl. Catal., B, 2014, 144, 580–587 CrossRef CAS PubMed.
- X. X. Ma, Y. Liu, X. Q. Li, J. G. Xu, G. D. Gu and C. H. Xia, Appl. Catal., B, 2015, 165, 351–359 CrossRef CAS PubMed.
- C. H. Xia, J. Xu, W. Z. Wu and X. M. Liang, Catal. Commun., 2004, 5, 383–386 CrossRef CAS PubMed.
- C. H. Xia, Y. Liu, S. W. Zhou, C. Y. Yang, S. J. Liu, J. Xu, J. B. Yu, J. P. Chen and X. M. Liang, J. Hazard. Mater., 2009, 169, 1029–1033 CrossRef CAS PubMed.
- D. R. Fang, W. J. Li, J. B. Zhao, S. Liu, X. X. Ma, J. G. Xu and C. H. Xia, RSC Adv., 2014, 4, 59204–59210 RSC.
- S. S. Zinovyev, N. A. Shinkova, A. Perosa and P. Tundo, Appl. Catal., B, 2005, 55, 39–48 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |