Furan and benzochalcogenodiazole based multichromic polymers via a donor–acceptor approach†
Merve İçli-Özkuta, Halil İpekb, Baris Karabayc, Atilla Cihaner*c and Ahmet M. Önal*b
aDepartment of Chemistry, Yüzüncü Yıl University, 65080 Van, Turkey
bDepartment of Chemistry, Middle East Technical University, TR-06800 Ankara, Turkey. E-mail: aonal@metu.edu.tr; Fax: +903122103200; Tel: +903122103188
cChemical Engineering and Applied Chemistry, Atilim Optoelectronic Materials and Solar Energy Laboratory (ATOMSEL), Atilim University, TR-06836 Ankara, Turkey. E-mail: cihaner@atilim.edu.tr; Fax: +903125868091; Tel: +903125868304
First published on 21st January 2013
Abstract
Two new furan and benzochalcogenodiazole based monomers, namely 4,7-di(furan-2-yl)benzo[c][1,2,5]selenadiazole (FSeF) and 4,7-di(furan-2-yl)benzo[c][1,2,5]thiadiazole (FSF), were designed and synthesized via a donor–acceptor–donor approach. The monomers were electrochemically polymerized via potentiodynamic or potentiostatic methods. The monomers and their polymers exhibited lower oxidation potentials (1.16 V and 1.06 V for monomers; 0.93 V and 0.80 V for polymers vs. Ag/AgCl) and red shifts of the whole dual-band absorption spectra upon moving from S to Se. Intramolecular charge transfer properties of the monomers and the polymers were demonstrated by using electroanalytical and optical methods. Also, the polymers PFSeF and PFSF were multicolored at different redox states and have low band gaps of 1.43 eV and 1.61 eV, respectively.
Introduction
After the investigation of the precursor conjugated polymer polyacetylene opened a new area of conducting polymers in the 1970s,1,2 conjugated polyaromatics have been investigated extensively. Among them, myriad of studies have been reported on five-membered heterocycles containing polymers such as polypyrroles, polythiophenes and their derivatives3 due to their susceptible electronic and optical properties. Very recently, many studies also have been performed on selenophene based polymers.4–10 Among these studies, unfortunately the studies that contain furan and its derivatives have remained outnumbered due to the susceptibility of the furans on the side reactions during the polymerization and low stability of the resulting polymers.11–14 In order to overcome these disadvantages, many methods have been tried including the use of boron trifluoride-diethyl ether to reduce the aromaticity of the furan ring to avoid overoxidation15 and making the copolymers of furan with different monomeric units such as thiophene.16 However, the corresponding polymers were not fully conjugated. The pursuit of lowering the oxidation potential of furan, i.e., obtaining fully conjugated polyfurans, hybrid conjugated monomers have been started to be synthesized. Among them donor (D, electron-rich) and acceptor (A, electron-deficient) type monomers give intriguing results3,17 since it is possible to tune the band gap of the materials, which determines the optical and electronic properties, by the combination of D and A units in one pot. Generally, by using this approach, the highest occupied molecular orbital (HOMO) energy level of the D unit increases and the lowest unoccupied molecular orbital (LUMO) energy level of the A unit decreases and therefore, the gap between HOMO and LUMO levels decreases in a new hybrid monomer. If D and A units are combined under the same umbrella, an intramolecular charge transfer is generally observed which might affect the optical and electronic properties of their corresponding π-conjugated polymers.18,19 The degree of charge density difference determines the charge transfer between the D and A units, which can be easily monitored using spectroscopic analysis.20Recent studies showed that furan containing polymers have some priorities over thiophene based ones for the application of polymer organics in advanced technological applications. For instance, the greater electron withdrawing ability of furan is capable of reducing the HOMO energy level of the D parts in the solar cells which results in the high open circuit voltage.17 Furthermore, the field-effect mobilities of furan derivatives are very similar to the thiophene based one, and this property together with natural feed-stock and higher open circuit voltage when compared its five-membered analogues make furan based polymers outstanding candidates for organic electronics.21 There are also some examples of furan containing polymers, emitting blue light, obtained via polycondensation reactions.22,23
Keeping all this in mind, we have synthesized and characterized two new furan and benzochalcogenodiazole based monomers, namely 4,7-di(furan-2-yl)benzo[c][1,2,5]selenadiazole (FSeF) and 4,7-di(furan-2-yl)benzo[c][1,2,5]thiadiazole (FSF), via D–A–D approach. Then, the corresponding conjugated polymers, namely poly(4,7-di(furan-2-yl)benzo[c][1,2,5]selenadiazole) (PFSeF) and poly(4,7-di(furan-2-yl)benzo[c][1,2,5]thiadiazole) (PFSF), were electrochemically synthesized (Scheme 1) and also their optical and electrochemical properties were investigated. In addition, the intramolecular charge transfer properties of the monomers and their polymers were demonstrated by using electroanalytical and optical methods.
 | ||
| Scheme 1 Chemical structures of FSeF, FSF monomers and their corresponding polymers PFSeF and PFSF. | ||
Results and discussion
Two novel monomers, FSeF and FSF, having the same D units as furan and different A units of benzoselenadiazole and benzothiadiazole, were synthesized via Stille coupling reaction. Their characterizations were done by 1H, 13C NMR, FTIR and mass measurements (see ESI†).First of all, in order to understand the structure–property relationships in D–A–D type monomers, FSeF and FSF, where a single atom was changed in the A unit, the optical and electrochemical properties were investigated. As shown in Fig. 1a, the monomers exhibit dual-band absorptions due to the charge transfer between the D and A units since this is a characteristic signature generally observed in D–A type monomers. When compared to FSF, the entire dual-band spectrum of FSeF is shifted to a lower energy due to the heavier chalcogen atom Se into the A unit. In the literature the absorption band at a longer wavelength was attributed to the intramolecular charge transfer in D–A type materials.20,24,25 The low energy band at 452 nm for FSF was shifted to a longer wavelength at 481 nm for FSeF with decreasing band intensity, which may be due to the poor acceptor nature of the benzoselenadiazole unit containing the less electronegative Se atom since it is hardly be able to separate the charge between the D and A units (Fig. 1a).20,26 Therefore, there is a poor intramolecular charge transfer in FSeF, which results in a red-shift absorption band with a low intensity. The emission colors of the monomers changed from greenish yellow to orange when moving from S to Se (Fig. 1b).
 | ||
| Fig. 1 (a) Absorption and (b) emission spectra of FSF and FSeF in toluene. Inset: the colors of FSF and FSeF in toluene under (a) day light and (b) handheld UV lamp. | ||
In order to support the intramolecular charge transfer between the D and A units, solvatochromic experiments were performed.20 By changing the polarity of the solvents, the stability of the excited state must be checked and if there is enough charge separation in the monomer, the emission spectrum must shift to a longer wavelength (a red-shift) by increasing the polarity of the solvents. The emission spectra of FSF were tested in solvents of increasing dielectric constants: toluene (2.38), THF (7.58) and CH2Cl2 (8.93).27 As shown in Fig. 2, the emission spectra were shifted to longer wavelengths from 552.2 nm (toluene, greenish yellow) to 556.9 nm (THF, yellow) and to 562.7 nm (CH2Cl2, yellow). This red shift in the emission proves that the low-energy band present in the absorption spectrum is originating from intramolecular charge transfer.
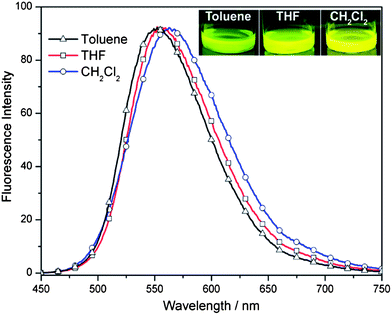 | ||
| Fig. 2 Emission spectra of FSF in toluene (greenish yellow), THF (yellow) and CH2Cl2 (yellow). | ||
On the other hand, the emission spectra of FSeF did not show any shift or change by increasing the solvent polarity, indicating the poor intramolecular charge transfer between furan and benzoselenadiazole units due to the poor electron acceptor power of the A unit (see ESI, Fig. S1†).20,26
After optical properties, the electrochemical behaviours of the monomers were investigated in an electrolyte solution consisting of 0.1 M TBAH dissolved in ACN. The FSeF and FSF monomers showed single irreversible oxidation peaks around 1.06 V and 1.16 V (vs. Ag/AgCl), respectively (Fig. 3). This difference was expected since the acceptor power of the benzothiadiazole is larger than the benzoselenadiazole due to the higher electronegativity of the S atom when compared to Se. Therefore, FSeF has higher electron rich nature than FSF, resulting in a lower oxidation potential. On the other hand, the benzotriazole containing monomer with the same D unit (2-dodecyl-4,7-di(furan-2-yl)-2H-benzo[d][1,2,3]triazole, FNT) synthesized in the literature has an irreversible oxidation potential at 1.04 V (after correcting for Ag/AgCl) due to the poor electron deficient nature of the A unit when compared to the benzoselenadiazole.28 As a consequence, the oxidation potential values of the monomers depend on the electron deficient nature of the acceptor units by increasing order of N, Se and S. Unfortunately, there is no systematic relationship between redox behaviour and the A moiety of the monomers. The reduction behaviour of the monomers, for example, supported this observation since reduction potential values were opposite to the expected values. For example, in the same D–A–D system, if FSeF has a lower oxidation potential than FSF, its reduction potential must have a more negative value, but the monomers, FSeF and FSF, exhibited reversible reduction peaks with half wave potentials of Eredm,1/2 = −1.13 V and Eredm,1/2 = −1.23 V, respectively. In other words, FSeF is more easily reduced. The results are consistent with the data obtained in the literature with 3,4-propyledioxythiophene based (as D group) analogues of these polymers.29 As a result, this difference observed in the cathodic region may not only be explained by the powers of the acceptor units but also depends on the factors such as heavy atom substitution, electronegativity, bond length and acceptor aromaticity.20
 | ||
| Fig. 3 Cyclic voltammograms of 3.3 × 10−3 M of FSeF and 4.1 × 10−3 M of FSF in 0.1 M TBAH/ACN electrolyte solution at a scan rate of 100 mV s−1vs. Ag/AgCl. | ||
After determining the electrochemical behavior of FSeF and FSF, electrochemical polymerizations were carried out in ACN containing 0.1 M TBAH. During the repetitive scanning, new reversible redox couples were observed, which is a characteristic behavior of conducting polymer films, showing the formation of an electroactive polymer film on the electrode surface. Moreover, after each successive cycle the peak current values of the redox couples intensified, which confirmed an increase in the polymer film thicknesses (Fig. 4). The polymer films, PFSeF and PFSF, coated on the electrode surface were also scanned at different scan rates. The linear increase as a function of the scan rates proved that the redox processes were non-diffusional controlled and also the polymer films were well-adhered on the electrode surface for both polymers (see ESI, Fig. S2 and S3†).
 | ||
| Fig. 4 Electropolymerization of (a) 2.3 × 10−3 M of FSeF and (b) 3.1 × 10−3 M of FSF in 0.1 M TBAH/ACN at a scan rate of 100 mV s−1 by potential scanning to give PFSeF and PFSF, respectively. | ||
In endeavours to prove that there is n-type doping as well as the p-type one, the polymer films were scanned cathodically and anodically. When compared to S containing polymer PFSF, the n-type doping process of Se containing polymer PFSeF was easily observed (Fig. 5), which is consistent with the literature.29
 | ||
| Fig. 5 Cyclic voltammograms of (a) PFSeF and (b) PFSF in 0.1 M TBAH/ACN electrolyte solution at a scan rate of 100 mV s−1vs. Ag/AgCl. | ||
During the anodic scan, reversible oxidation peaks were observed with half wave potentials of Eoxp,1/2 = 0.80 V for PFSeF and Eoxp,1/2 = 0.93 V for PFSF. These data were compatible with those obtained from the monomers. Their benzotriazole based polymer analogue, namely (poly(2-dodecyl-4,7-di(furan-2-yl)-2H-benzo[d][1,2,3]triazole), PFNT) has a half wave potential at Eoxp,1/2 = 0.61 V vs. Ag/AgCl.28 For the sake of comparison, the potential values of the p-type doping processes agree well with the electron acceptor power of the A unit increasing upon moving from N to Se to S.
During the cathodic scan, polymers, PFSeF and PFSF showed half wave potentials of Eredp,1/2 = −1.16 V and Eredp,1/2 = −1.24 V, respectively. n-type doping of PFNF was reported as Eredp,1/2 = −1.79 V vs. Ag/AgCl.28 The potentials of the n-type doping processes shifted to more negative potentials upon changing Se to S to N. In this sequence Se was expected to be between S and N atoms due to the power of the acceptor units, whereas this difference can be explained by the stabilization of the LUMO. As explained by Das et al. the degree of bond length alternation of S–N and Se–N in the A units increases on moving from S to Se, which decreases the aromaticity and leads to stabilization of the LUMO.20,26 The HOMO, LUMO levels and Eg values were calculated from the cyclic voltammetry (CV) and are tabulated in Table 1 together with HOMO, LUMO levels of their corresponding monomers. As it is seen from Table 1 the HOMO energy levels of polymers increase in the order of N, S and Se.
It is interesting to note that, while thiophene analogues have p- and n-type doping similar to FSeF and PFSF, pyrrole analogues have only p-type doping behaviour, which may be explained as due to the electron rich nature of the pyrrole unit when compared to furan.29–31
Another method to investigate the redox properties of the polymers is differential pulse voltammetry (DPV) method (see ESI, Fig. S4†). Band gaps calculated from DPV are in agreement with those calculated from CV (Table 1). On the other hand, the optical band gaps of the polymer films PFSeF and PFSF were calculated as 1.54 eV and 1.65 eV from the onset of the low energy band, respectively, shown in Fig. 6.
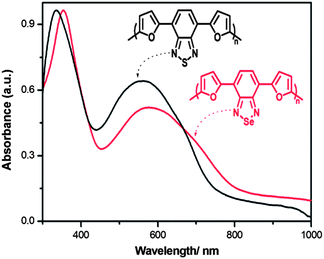 | ||
| Fig. 6 Absorption spectra of neutral state PFSeF and PFSF films on ITO in ACN. | ||
These values are relatively bigger than their thiophene (1.46–1.50 eV) and pyrrole analogues (1.08–1.12 eV), which shows that optical spectra of the polymers red shift according to increasing power of donor unit.30,31 The results are again consistent with the band gaps calculated from CV (1.43 eV for PFSeF and 1.69 eV for PFSF) and DPV (1.43 eV for PFSeF and 1.61 eV for PFSF).
The entire dual-band absorption spectrum of PFSeF shifts to lower energy and the absorption band at longer wavelength of the corresponding polymer is attributed to the intramolecular charge transfer as in the case of monomer. When compared to PFSF, the low energy band of PFSeF has a red shift with lower intensity due to the presence of heavier atom (lower ionization energy of Se) and the poor acceptor nature of benzoselenadiazole. As a consequence, a band gap reduction was observed on moving from S to Se. On the other hand, PFNF film (ESPELg = 1.9 eV) exhibited only one broad absorption band centred at 524 nm,28 which could be attributed to the strength of the acceptors, among which benzotriazole is the poorest A unit and there is no enough ability for the charge separation between D and A units in polymer backbone.26 Similar behaviour was also observed in our previous work about 3,4-propylenedioxythiophene and benzotriazole based polymers.29 On the other hand, the polymers containing benzothiadiazole and benzoselenadiazole units as acceptor and thiophene and pyrrole units as donor exhibit dual band spectra as reported previously.30,31
Upon oxidation the transition band of PFSeF at 355 nm started to decrease with a concomitant increase in the intensity of the band at 577 nm. Also, the formation of a new band beyond 700 nm was observed confirming the formation of the charge carriers in Fig. 7a. During the oxidation, the color of the PFSeF changed from dark gray (0.0 V) to dark cyan (0.2 V) and to blue (1.15 V). Furthermore, during p-type doping, an isosbestic point at 410 nm indicates that only two phases coexist. On the other hand, the transition band at 335 nm shifted to a longer wavelength and the band at 565 nm firstly began to increase and then decrease upon applied higher oxidation potentials. An increase in the intensity of new absorption band beyond 700 nm also represented charge carrier formation (Fig. 7b). Upon oxidation the color of the PFSF turned from wine (0.0 V) to violet (0.2 V) and to dark gray (1.05 V). It should be also mentioned that the color of the PFSeF and PFSF were dark yellow and light gray in the reduced states, respectively (Table 2 and see ESI, Fig. S5†).
 | ||
| Fig. 7 Electronic absorption spectra of the (a) PFSeF and (b) PFSF on ITO in 0.1 M TBAH/ACN at various applied potentials along the anodic region. | ||
| Polymers | λmax,1 (nm) | λmax,2 (nm) | Colorimetric results | Colors at different oxidation states | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 V | 0.2 V | 1.15 V | −1.3 V | 0.0 V | 0.2 V | 1.15 V | −1.3 V | ||||
| PFSeF | 355 | 577 | L | 39.20 | 53.62 | 42.83 | 54.84 |  |  |  |  |
| a | 0.015 | −0.20 | 4.99 | −2.59 | |||||||
| b | 13.82 | −17.46 | −17.79 | 11.96 | |||||||
| Polymers | λmax,1 (nm) | λmax,2 (nm) | Colorimetric results | Colors at different oxidation states | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 V | 0.2 V | 1.05 V | −1.4 V | 0.0 V | 0.2 V | 1.05 V | −1.4 V | ||||
| PFSF | 335 | 565 | L | 30.02 | 51.88 | 47.55 | 59.48 |  |  |  |  |
| a | 11.59 | 16.89 | 7.71 | 0.83 | |||||||
| b | 9.39 | −14.46 | −9.43 | −7.94 | |||||||
Stability under ambient conditions is an important parameter for any material to be used in advanced technological applications. Also, the stability of the polymer films was carried out under atmospheric conditions (without purging and sweeping the electrolyte solution with inert gas) between their redox states by using cyclic voltammetry method (see ESI, Fig. S6†). It was observed that PFSeF retained 85% of its electroactivity whereas PFSeF retained just 39%.
Conclusions
Two new furan based D–A–D type monomers, FSeF and FSF and their corresponding polymers, PFSeF and PFSF, were synthesized and also their optical and electrochemical properties were investigated. The intramolecular charge transfer properties of the monomers and their polymers were demonstrated by using electroanalytical (CV and DPV) and optical (UV-vis and fluorescence) methods. It can be easily conjectured that the power of the acceptor moieties, heavy atom substitution, electronegativity, ionization energy, bond length alternation and acceptor aromaticity have important roles in electrochemical and optical studies. Also, the polymers were multicolored at different redox states and they have low band gaps (1.43 eV for PFSeF and 1.61 eV for PFSF).Acknowledgements
The authors gratefully acknowledge the financial support from the Scientific and Technical Research Council of Turkey (TUBITAK-111T976), Atilim University (ATU-ALP-1011-02) and The Turkish Academy of Sciences (TUBA).References
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. MacDiarmid, Phys. Rev. Lett., 1977, 39, 1098 CrossRef CAS.
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. MacDiarmid, Phys. Rev. Lett., 1978, 40, 1472 CrossRef.
- T. A. Skotheim and J. R. Reynolds, in Handbook of Conducting Polymers – Conjugated Polymers: Synthesis, Properties and Characterization, CRC Press, Boca Raton, FL, 2007 Search PubMed.
- A. Patra, Y. H. Wijsboom, S. S. Zade, M. Li, Y. Sheynin, G. Leitus and M. Bendikov, J. Am. Chem. Soc., 2008, 130, 6734 CrossRef CAS.
- A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC.
- M. Li, A. Patra, Y. Sheynin and M. Bendikov, Adv. Mater., 2009, 21, 1707 CrossRef CAS.
- M. Li, Y. Sheynin, A. Patra and M. Bendikov, Chem. Mater., 2009, 21, 2482 CrossRef CAS.
- S. Atak, M. İçli-Özkut, A. M. Önal and A. Cihaner, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4398 CrossRef CAS.
- M. İçli-Özkut, S. Atak, A. M. Önal and A. Cihaner, J. Mater. Chem., 2011, 21, 5268–5272 RSC.
- M. İçli-Özkut, J. Mersini, A. M. Önal and A. Cihaner, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 615 CrossRef.
- R. M. McConnell, W. E. Godwin, S. E. Baker, K. Powell, M. Baskett and A. Morara, Int. J. Polym. Mater., 2004, 53, 697 CrossRef CAS.
- G. Distefano, D. Jones, M. Guerra, L. Favaretto, A. Modelli and G. Mengoli, J. Phys. Chem., 1991, 95, 9746 CrossRef CAS.
- B. Demirboğa and A. M. Önal, Synth. Met., 1999, 99, 237 CrossRef.
- S. Tirkeş and A. M. Önal, J. Appl. Polym. Sci., 2007, 103, 871 CrossRef.
- X. Wan, F. Yan, S. Jin, X. Liu and G. Xue, Chem. Mater., 1999, 11, 2400 CrossRef CAS.
- J. P. Ferrais and T. R. Hanlon, Polymer, 1989, 30, 1319 CrossRef.
- X. Wang, S. Chen, Y. Sun, M. Zhang, Y. Li, X. Li and H. Wang, Polym. Chem., 2011, 2, 2872 RSC.
- I. Yamaguchi and H. Mitsuno, React. Funct. Polym., 2011, 71, 140 CrossRef CAS.
- Y.-J. Hwang, F. S. Kim, H. Xin and S. A. Jenekhe, Macromolecules, 2012, 45, 3732 CrossRef CAS.
- G. L. Gibson, T. M. McCormick and D. S. Seferos, J. Am. Chem. Soc., 2012, 134, 539 CrossRef CAS.
- O. Gidron, A. Dadvand, Y. Sheynin, M. Bendikov and D. F. Perepichka, Chem. Commun., 2011, 47, 1976 RSC.
- S. M. Pyo, S. I. Kim, T. J. Shin, H. K. Park, M. Ree, K. H. Park and J. S. Kang, Macromolecules, 1998, 31, 4777 CrossRef CAS.
- S. M. Pyo, S. I. Kim, T. J. Shin, M. Ree, K. H. Park and J. S. Kang, Polymer, 1998, 40, 125 CrossRef.
- S. A. Jenekhe, L. Lu and M. M. Alam, Macromolecules, 2001, 34, 7315 CrossRef CAS.
- A. P. Kulkarni, Y. Zhu, A. Babel, P.-T. Wu and S. A. Jenekhe, Chem. Mater., 2008, 20, 4212 CrossRef CAS.
- S. Das, P. B. Pati and S. S. Zade, Macromolecules, 2012, 45, 5410 CrossRef CAS.
- R. B. Jordan, in Reaction Mechanisms of Inorganic and Organometallic Systems, Oxford University Press, USA, 3rd edn, 1999, ch. 3, p. 66 Search PubMed.
- N. Akbaşoğlu, A. Balan, D. Baran, A. Cirpan and L. Toppare, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5603 CrossRef.
- M. İçli, M. Pamuk, F. Algi, A. M. Önal and A. Cihaner, Chem. Mater., 2010, 22, 4034 CrossRef.
- A. Cihaner and F. Algi, Adv. Funct. Mater., 2008, 18, 3583 CrossRef CAS.
- D. Baran, G. Oktem, S. Celebi and L. Toppare, Macromol. Chem. Phys., 2011, 212, 799 CrossRef CAS.
- Y. F. Li, Y. Cao, J. Gao, D. L. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS.
- Q. J. Sun, H. Q. Wang, C. H. Yang and Y. F. Li, J. Mater. Chem., 2003, 13, 800 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR, FTIR and mass spectrometry analysis of monomers, some optical, electrochemical data of polymers. See DOI: 10.1039/c3py21061h |
| This journal is © The Royal Society of Chemistry 2013 |