 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of the toxic effects of microplastics based on studies on mammals and mammalian cell lines†
Kuok Ho Daniel
Tang

Department of Environmental Science, The University of Arizona, Tucson, Ariona 85721, USA. E-mail: daniel.tangkh@yahoo.com
First published on 8th October 2024
Abstract
Microplastics have raised global alarm because of their pervasiveness, potential human toxicity, and ecotoxicity. This paper reviews studies conducted on mammals and mammalian cell lines to illustrate the toxic effects of MPs and the MP levels causing or not causing an observable negative response. Most current studies in this area have been conducted on polystyrene with few studies dedicated to polyethylene and polypropylene. In vivo studies commonly use mice or rats as the experimental subjects and ingestion as the exposure mode, while in vitro studies use different types of cell lines, with intestinal cell models being the most common. The toxic effects of microplastics are size- and biomarker-dependent, with polystyrene microplastics at 1.49 × 106 to 4.55 × 107 particles per mouse not leading to observable negative effects but 0.01 mg day−1 to 0.15 mg day−1 per mouse yielding negative responses. For cell lines, polystyrene microplastics at 10 μg mL−1–20000 particles mL−1 did not induce negative effects but a level of 0.01 μg mL−1–5000 particles mL−1 caused negative effects, depending on the types of cells used. Polyethylene microplastics at 0.125 mg day−1 generally could cause mice to respond negatively, whereas polypropylene microplastics at 5000 particles mL−1 were observed to cause a negative response in THP-1 macrophages. The different units for the toxic doses used make comparison of the doses challenging. It is, therefore, recommended that a common unit is used in reporting the toxic levels of microplastics, particularly mg kg−1–bw day−1 for in vivo studies and μg mL−1 or mg L−1 for in vivo studies. Standardized biomarkers and bioindicators could also be used to facilitate comparison.
Environmental significanceMicroplastics have permeated all compartments of the environment and are increasing in abundance with higher plastic consumption. Numerous papers have highlighted the ecotoxicities of microplastics. However, it remains unclear how this emerging pollutant affects human health. Multiple in vitro and in vivo studies have been conducted on cell lines and rodents, respectively, to examine the toxicity of microplastics, but there are few reviews that bring the information on the toxic effects of microplastics together through a unique approach of presenting the microplastic levels producing no observable effects and those producing adverse effects on the mammalian systems. This review represents one of these very few attempts. Through reviewing the toxic levels and effects of microplastics on mammalian systems, it contributes to a better understanding of how microplastics could affect human health. |
1 Introduction
Widespread use of plastic items and plastic waste mismanagement have resulted in the detection of primary and secondary microplastics (MPs) in the environment.1 MPs have been extensively studied by the scientific community, particularly in terms of their occurrence, abundance, distribution, and ecotoxicity.2,3 It is now known that MPs have permeated all compartments of the environment from soil, and water to air, and they have contaminated remote and supposedly pristine environments.4–6 A study revealed an average abundance of approximately 0.02 MPs per gram of sand samples collected in the Kavir and Lut deserts of Iran.7 It was also reported that 98% of the wet and dry environmental samples collected from the most isolated parts of the US, such as the Grand Canyon, contained MPs.8 Detection of MPs in the snow samples in Fram Strait between Svalbard and Greenland pointed to the contamination of the Arctic atmosphere by MPs.3 Atmospheric transport was deemed the major reason for MP detection in these remote areas. Globally, an estimated 33.76 tons of MP fibers are churned out into the atmosphere.9The ubiquity of MPs in the environment means it is very likely for organisms to be exposed to MPs. Indeed, studies have shown the presence of MPs in different organisms ranging from plankton to whales, as well as in various food items.10,11 Feeding mechanisms and difficulty in differentiating plankton from MPs result in the ingestion of MPs by marine organisms.12 Ory et al. reported that Decapterus muroadsi in the South Pacific consumed blue MPs due to their resemblance to copepods.13 MPs have been found to contaminate a planktonic community at an abundance of 139 MPs m−3 and their sizes (predominantly 500–1000 μm) fell into the common planktonic size range.14 Similarly, 140–180 MPs m−3 were retrieved from a shallow lake in Argentina and their sizes (50–950 μm) overlapped with those of the dominant zooplankton comprising rotifers and cyclopoids (<200–600 μm), thus exposing aquatic organisms at higher trophic levels to MPs.15 Degradation of MPs produces smaller plastic particles, which could be ingested or entrained by plankton, especially the filter-feeding zooplankton with crucial roles in the marine food webs, leading to the transfer of MPs along the food webs.16 MPs were detected in 20% of the fish samples taken from the coast of Portugal17 and 23% of the edible fish samples comprising Merluccius merluccius and Mullus barbatus gathered from the Mediterranean Sea.18
Exposure to MPs has received much attention and studies on the effects of such exposure have been conducted.19,20 Exposure of zooplankton to MPs under laboratory conditions caused altered feeding behavior, retarded growth, and development, as well as decreased reproductivity and lifespan though a small number of studies reported no significant effect.16 Zebrafish (Danio rerio) exposed to polystyrene (PS) MPs in an experiment were observed to accumulate MPs in gills, livers, and guts. This triggered oxidative stress, elevated activities of superoxide dismutase and catalase, as well as inflammation and disrupted lipid metabolism in the liver.21 Accumulation of MPs in the guts of lugworms (Arenicola marina L.) resulted in increased rates of respiration, probably due to inflammation triggered by MP.22 MPs were found to affect the benthic assemblage structures by reducing species richness and abundance, as evident in the reduction of juvenile periwinkles (Littorina sp.) and an isopod (Idotea balthica).23 MPs have also been detected in humans. A study found human blood samples to contain polyethylene terephthalate (PET), polyethylene (PE), and PS primarily. The total concentration of MP particles in the samples averaged 1.6 μg mL−1.24 Montano et al. found human semen samples to contain 16 MP particles sized 2 to 6 μm. These included polypropylene (PP), PE, PET, PS, and polyvinyl chloride (PVC).25 MPs sized 4–15 μm were observed in human urine, and they were primarily polyethylene vinyl acetate (PVA), PVC, PP, and PE.26
In addition, MPs act as carriers of other environmental pollutants by providing the surfaces for the sorption of these chemicals.27 Alimi et al. found PE fragments to exhibit high sorption of environmental pollutants, and polychlorinated biphenyls (PCBs) adsorbed to colorless MPs better than colored plastics.28 Sorption of perfluorooctanesulfonamide (PFOSA) on PE and PVC was also reported. This results in co-exposure of living organisms to other environmental pollutants sorbed by MPs.29 Additionally, MPs can leach out additives, particularly phthalates, which are endocrine-disrupting substances.30,31 Due to a lack of strong covalent bonds between the additives and plastic polymers, they could be released from MPs, causing adverse health effects on organisms exposed to them. Human and animal exposures to phthalates have been associated with negative impacts on reproductive and cardiovascular systems.32
Currently, most of the human health impacts of MPs have been characterized through studies conducted on mice or rats and human or mammalian cell lines.33 Animal and cell line studies provide valuable information for the deduction of MP toxicity in humans. These studies reveal the toxic levels of MPs, particularly the levels at which there are no discernible effects and those at which adverse effects begin to show. This review aims to systematically present the toxicity of MPs based on the levels of MPs causing adverse effects on mammals or human cell line models.
2 Mammal and human cell line studies on MP toxicity
The toxic effects of MPs on mammals are typically observed either through in vitro experiments conducted on mammalian cell lines or in vivo experiments conducted on mice or rats (Fig. 1 and Table S1†).34,35 The duration of exposure and the concentrations of MPs used in in vitro and in vivo studies differ, with the former lasting usually for 24 hours and, in some cases, up to 96 hours36,37 and the latter lasting from 7 days to 90 days.38 Shorter cell line studies over 6 hours have also been conducted.39,40 Based on the duration of exposure, in vitro studies usually indicate the acute toxicity of MPs on the cells exposed, whereas in vivo studies commonly demonstrate the sub-chronic or chronic toxicity of MPs on the organisms exposed.41,42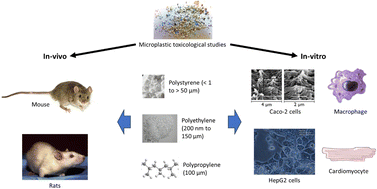 | ||
| Fig. 1 In vivo and in vitro toxicological studies of MPs were conducted mostly for PS, PE, and PP MPs. | ||
Caco-2 cells from human colorectal adenocarcinoma cells are most commonly used as a model of the intestinal epithelial barrier and they express tight junctions, microvilli as well as enzymes and transports typical of enterocytes (Fig. 1).43,44 Caco-2 cells can also be cultured together with HT29-MTX-E12 cell line, macrophages and/or dendritic cells to form different intestinal models.39,45 Other cell lines such as HepaRG, NIH/3T3 fibroblasts, cardiomyocytes, BeWo be30 and CHO-K1 have been employed to study the hepatoxicity, embryotoxicity, and cardiotoxicity of microplastics as well as the effects of microplastics on placental and intestinal barriers, respectively45–47 (Table S1†). While Caco-2, HepaRG, BeWo b30 and HT29-MTX-E12 are human cell lines, NIH/3T3 fibroblasts and ES-D3 come from mice.45,48 CHO-K1 originated from the ovary of an adult Chinese hamster.49 Raji-B cells, used in certain intestinal models as a type of lymphocytes, are also derived from humans.50 Mice and rats are typically used in in vivo studies, but they are different in numerous aspects.34 Mice are usually smaller than rats and have shorter lifespans. They have higher metabolic rates and are more genetically diverse than rats. Mice are more commonly used in microplastic toxicological studies than rats.51,52 Genetically identical inbred strains such as C57BL/6 are frequently used to reduce the effects of genetic variation on toxicological responses.53 Ingestion is the exposure mode in all the studies reviewed, except one involving intratracheal instillation.54
2.1. Levels of MPs producing no observable adverse effects
Numerous studies found MPs to have no significant effects on the experimental subjects.55,56 Stock et al. did not observe significant changes in body weights as well as the weights of the liver, spleen, kidney, heart, lungs, and testes when mice were fed daily with 4.55 × 107 PS MPs sized 1 μm and 4 μm respectively, and 1.49 × 106 PS MPs sized 10 μm (Table S1†).57 There was no significant effect on the activities of the β-galactosidase reporter and heme oxygenase 1-dependent reporter, both indicating oxidative stress and inflammation.57 PS MPs sized 0.5 μm were reported to not produce a significant effect on the volume of growing mice follicles at 0.15 mg day−1 over 90 days, but increasing the dose to 1.5 mg day−1 caused a decrease in the follicle volume.38 Having conducted a 28-day study on the effects of PS MPs on mice, Jin et al. found that mice exposed to the same dosage of 100 μL (10 mg mL−1 or 1 mg) PS MPs with different sizes produced different results with MPs of 0.5 μm not causing any noticeable weight change but those sized 4 μm and 10 μm decreased their body weights, indicating variable effects of MP sizes on the same bioindicator (Table S1†).58,59 Another study showed that exposure of mice to nano-PS 50 nm in diameter for 30 days at a dose of 10 mg kg−1 did not result in significant changes in their body weights, anxiety-like behavior, locomotor function as well as biomarkers related to intestinal inflammation, oxidative stress, intestinal epithelial cell tight junction proteins, inflammation and oxidative stress of cortex, lung, and liver, in addition to IL-1β, IL-6 and TNF-α levels in serum60 (Table S1†).Additionally, Domenech et al. reported that PS MPs sized 0.05–0.1 μm did not induce cytotoxicity in Caco-2/HT29 intestinal cells and Caco-2/HT29 + Raji B cells at 200 μg mL−1, neither did they significantly alter intestinal barrier integrity and permeability, as well as induce the production of intracellular reactive oxygen species (ROS) and oxidative deoxyribonucleic acid (DNA) damage at 100 μg mL−1 (Table S1†).50 Cortés et al. revealed exposure of the Caco-2 cell line to PS MPs sized 0.05–0.1 μm resulted in no significant cytotoxicity at 150 μm mL−1 after 48 hours of exposure, and no significant alteration in intracellular ROS levels, oxidative DNA damage, and chromosome damage at 100 μg mL−1 after 24 hours of exposure, in parallel to the findings of Domenech et al.36,50 However, 200 μg mL−1 of PS MPs induced mild toxicity, whereas 24-hour exposure to 50 μg mL−1 PS MPs and 48-hour exposure to 25 μg mL−1 PS MPs increased the expression of ROS-related genes.36 This implies that exposure duration and level influence the effect on a biomarker.61 Liu et al. found 20 μg mL−1 of transformed PS MPs sized 100 nm and 5 μm to yield no significant effects on lactate dehydrogenase of Caco-2 cells – an indicator of tissue damage, and intestinal transport elicited through changes in the permeability of paracellular marker Lucifer Yellow (Table S1†).37 Nonetheless, these biomarkers showed significant changes when exposed to 20 μg mL−1 original PS MPs of similar sizes for 96 hours.37 These findings indicate MP transformation via in vitro digestion could reduce MP toxicity as coronas develop on the surfaces of MPs, leading to potential changes in their charges and sizes.62
Similarly, exposure of the intestinal cell line, Caco-2 cells, to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 PP and PS particles mL−1, both fresh and weathered, did not produce a noticeable change in the levels of lactate dehydrogenase, IL-6, IL-8, and TNF-α.63 HepG2 cells exposed to the same dosage and type of PP and PS MPs also did not demonstrate significant changes in the biomarkers except that fresh PS MPs at 5000 particles mL−1 led to higher IL-6 but the changes did not seem to be dose-dependent (Table S1†). In THP-1 macrophage, only weathered PP MPs at 20
000 PP and PS particles mL−1, both fresh and weathered, did not produce a noticeable change in the levels of lactate dehydrogenase, IL-6, IL-8, and TNF-α.63 HepG2 cells exposed to the same dosage and type of PP and PS MPs also did not demonstrate significant changes in the biomarkers except that fresh PS MPs at 5000 particles mL−1 led to higher IL-6 but the changes did not seem to be dose-dependent (Table S1†). In THP-1 macrophage, only weathered PP MPs at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles mL−1 did not noticeably change lactate dehydrogenase and IL-1β levels (Table S1†). All types of MPs increased the MIP-1β level of THP-1 macrophage at 5000 particles mL−1.63 Hesler et al. conducted a study to test the toxicity of nanoscale (46.3 nm) and microscale (465.8 nm) COOH-modified PS on different cell lines and revealed that a 24-hour exposure to 100 μg mL−1 of nano-PS and micro-PS, respectively, did not noticeably alter the trans-epithelial electrical resistance of Caco-2/HT29-MTX-E12-culture representing intestinal barrier and BeWo b30 cells representing placental barrier (Table S1†).45 100 μg mL−1 of nano-PS did not cause cytotoxicity to NIH/3T3 fibroblast and mouse ES-D3, both of which were used to indicate embryotoxicity. 50 μg mL−1 nano- and 10 μg mL−1 micro-PS were not observed to result in substantial genotoxicity when tested on HepG2CDKN1A biosensor cells, whereas PS MPs of both sizes did not produce observable genotoxicity to CHO-K1 cells exposed at a dosage of 100 μg mL−1 over 24 hours (Table S1†).
000 particles mL−1 did not noticeably change lactate dehydrogenase and IL-1β levels (Table S1†). All types of MPs increased the MIP-1β level of THP-1 macrophage at 5000 particles mL−1.63 Hesler et al. conducted a study to test the toxicity of nanoscale (46.3 nm) and microscale (465.8 nm) COOH-modified PS on different cell lines and revealed that a 24-hour exposure to 100 μg mL−1 of nano-PS and micro-PS, respectively, did not noticeably alter the trans-epithelial electrical resistance of Caco-2/HT29-MTX-E12-culture representing intestinal barrier and BeWo b30 cells representing placental barrier (Table S1†).45 100 μg mL−1 of nano-PS did not cause cytotoxicity to NIH/3T3 fibroblast and mouse ES-D3, both of which were used to indicate embryotoxicity. 50 μg mL−1 nano- and 10 μg mL−1 micro-PS were not observed to result in substantial genotoxicity when tested on HepG2CDKN1A biosensor cells, whereas PS MPs of both sizes did not produce observable genotoxicity to CHO-K1 cells exposed at a dosage of 100 μg mL−1 over 24 hours (Table S1†).
Mice exposed to the PE MPs sized 16.9 μm for 90 days did not experience noticeable changes in their epididymis, duodenum, ileum, colon, heart, brain, uterus, and thymus tissues at a dose of 2 mg day−1, while lower doses ranging from 0.125 mg day−1 to 0.5 mg day−1 were found to affect lung, kidney and spleen tissues.54 This, again, points to the fact that different MP doses may induce changes in different biomarkers. Additionally, Park et al. observed that exposure of mice to PE for 90 days at a dose of 0.125 mg day−1 or 3.75 mg kg−1–bw day−1 did not yield observable pathological changes in the heart, intestinal, uterine, and brain tissues. However, they used larger PE MPs with sizes between 40–48 μg (Table S1†).64 A study on an intestinal cell model unveiled that healthy and inflamed cells have different susceptibility to PE sized 200–9000 nm where exposure of the inflamed culture to 50 μg cm−2 of PE did not increase inflammatory cytokine, IL-8, in contrast to the healthy culture65 (Table S1†). Both healthy and inflamed cultures did not exhibit a significant increase in pro-inflammatory cytokines comprising IL-1β, IL-6, and TNF-α as well as DNA damage at 50 μg cm−2 PE MPs.65 Huang et al. did not observe substantial fluctuation in the lactate dehydrogenase level of Caco-2 cells exposed to PE with a size range of 30–140 μm for 48 hours at 1000 mg L−1.66
2.2. Levels of MPs producing adverse effects
At least one adverse health effect associated with MPs has been reported in almost all the studies (Table S1†). 28-day exposure of mice to 0.01 mg day−1 of 5 μm and 20 μm PS MPs led to an observable decrease in adenosine triphosphate and total cholesterol (indicative of lipid metabolism) levels and an increase in lactate dehydrogenase.67 0.1 mg day−1 of the PS MPs significantly induced oxidative stress. Superoxide dismutase was, in fact, reduced by 0.01 mg day−1 of 20 μm PS MPs (Table S1†). Histological observations revealed inflammation and formation of liquid droplets in the liver at 0.5 mg day−1 of the PS MPs.67 Similarly, the research conducted by Lu et al. provides strong evidence that exposure to PS sized 0.5 μm and/or 50 μm at a concentration of 1000 μg L−1 leads to decreased body weight and relative liver and fat weights in mice. Decreased mucin secretion was reported after 5 weeks of exposure to 100 μg L−1 of the PS particles (Table S1†).68 Inhalation of 2.64 × 1014 nano-PS particles by rats for 24 hours caused lower fetal and placenta weights.54 Mice ingesting 0.01–0.1 mg day−1 of PS MPs sized 5–5.9 μm for 42 days had lower spermatogenic cells, sperm count, succinate dehydrogenase and lactate dehydrogenase activities, and testosterone levels, as well as higher oxidative stress and inflammatory factors.69 Ingestion of 0.15–1.5 mg day−1 PS particles with a size of 0.5 μm by rats for 90 days resulted in a decreased volume of growing follicles, the secretion of anti-Mullerian hormone, increased oxidative stress, apoptosis of granulosa cells and ovary fibrosis.38 Jin et al. (2021) found that 28-day exposure of mice to 10 mg L−1 of PS MPs sized 0.5 μm, 4 μm, and 10 μm led to decreased sperm quality and testosterone levels, increased testicular inflammatory factor and disrupted integrity of the blood-testis barrier.58 These findings imply the potentially deleterious effects of PS MPs on male and female reproductive health.Hou et al. exposed mice to 5 μm PS MPs for 35 days and observed decreased live sperm in epididymis as well as increased pro-apoptotic protein and inflammatory responses at 100 μg L−1 or 0.6–0.7 μg day−1. When the dosage was increased to 10 mg L−1 (60–70 μg day−1), increased sperm malformation and more inflammatory responses were observed (Table S1†).70 In contrast, mice exposed to PS sized 50 nm up to 30 days at a dosage of 10 mg kg−1 did not experience noticeable changes in body weight, anxiety-like behavior, inflammatory responses, and oxidative stress, except for a noticeable increase in Tff3 and Klf3, signaling intestinal mucus secretion.60 Rawle et al. reported that 80 μg kg−1 day−1 of PS MPs sized 1 μm triggered transcriptional changes and mild inflammatory signature in the colon of mice as well as increased inflammation and leukocytes over a 33- to 41-day exposure.71
In terms of cell model, nanoscale (46.3 nm) COOH-modified PS resulted in increased cell viability of an intestinal barrier model at 100 μg mL−1 while 5 g mL−1 nano-PS and 0.01 μg mL−1 micro-PS increased the cell viability of a placental barrier model.45 Embryotoxicity was triggered at 12.6 μg mL−1 micro-PS and cytotoxicity of cardiomyocytes was induced by 89.9 μg mL−1 nano-PS and 0.1 μg mL−1 micro-PS.45 A loss of the viability of Caco-2 was also reported at 1 × 107 pg mL−1 and 1 × 109 pg mL−1 PS MPs sized 1 μm and 4 μm respectively.57 Moreover, 12-hour exposure of Caco-2 cells to PS MPs sized 0.1 μm and 5 μm at 200 μg mL−1 was found to increase intracellular ROS.43 In terms of mitochondrial depolarization, 0.1 μm PS MPs caused an adverse effect at ≥20 μg mL−1, while 5 μm PS MPs caused an adverse effect at ≥1 μg mL−1 (Table S1†). This is in contrast to the findings of Stock et al., which showed smaller PS MPs exerted an adverse health effect at a lower level.43,57
Wang et al. revealed a mixture of PS MPs of different sizes (Table S1†) caused increased intracellular ROS at 120 μg mL−1, and this is lower than the 200 μg mL−1 reported by Wu et al. for PS MPs sized 0.1 μm and 5 μm.43,72 The study also revealed that 120 μm mL−1 of PS MPs of all sizes could induce mitochondrial depolarization, with larger MPs having a more pronounced effect, in parallel to the findings of Wu et al.43 Cortés et al. observed mild cytotoxicity in Caco-2 cell line exposed to 200 μg mL−1 PS MPs sized 0.05–0.1 μm for 24 and 48 hours and increased ROS at 25–50 μg mL−1.36 Original PS MPs at 20 μg mL−1 induced more adverse effects in Caco-2 cells than digested PS MPs, and these effects are related to cell membrane disruption and decreased intestinal transport.37 100 μm fresh PS MPs increased the level of IL-6 in HepG2 cells at 5000 particles mL−1 without a clear dose-dependence trend. The same dose of fresh PS MPs also increased lactate dehydrogenase, IL-1β, and MIP-1 β in THP-1 macrophage. These effects were only observed when treated with weathered PS MPs at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles mL−1.63 Upon exposing HepaRG cells to modified PS sized 20 nm, 40 nm, and 1 μm for 24 hours, Stock et al. found a dose of 1.5 × 1011 to 2.5 × 1012 μm2 PS particle surface per mL resulted in a slight increase in caspase-3 activity (a measure of apoptosis) with 20 nm carboxy (−) PS inducing a negative effect at the lowest level (Table S1†).73 The same PS particles are more potent than amino (+) PS particles in raising caspase-9 activity.73 500 μg mL−1 UV-treated PS MPs of 1.0–1.9 μm decreased cell viability and increased plasma membrane damage of Caco-2 cells.44
000 particles mL−1.63 Upon exposing HepaRG cells to modified PS sized 20 nm, 40 nm, and 1 μm for 24 hours, Stock et al. found a dose of 1.5 × 1011 to 2.5 × 1012 μm2 PS particle surface per mL resulted in a slight increase in caspase-3 activity (a measure of apoptosis) with 20 nm carboxy (−) PS inducing a negative effect at the lowest level (Table S1†).73 The same PS particles are more potent than amino (+) PS particles in raising caspase-9 activity.73 500 μg mL−1 UV-treated PS MPs of 1.0–1.9 μm decreased cell viability and increased plasma membrane damage of Caco-2 cells.44
As for PE MPs, healthy and inflamed intestinal cell models exposed to 50 μg cm−2 PE particles sized 200–9900 nm for 24 hours showed increased lactate dehydrogenase levels while the same dose increased IL-8 of the healthy cell model.65 Fresh PP MPs of 100 μm were also observed to increase lactate dehydrogenase and IL-1β levels in THP-1 macrophage at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles mL−1 and 5000 particles mL−1, respectively.63 Huang et al. observed that PE MPs of 30–140 μm decreased the viability of Caco-2 cells at 1000 mg L−1 and increased intracellular ROS at 100 mg L−1 after 48 hours of exposure.66 In mice, 600 μg PE MPs sized 10–150 μm caused intestinal inflammation after 5 weeks of exposure.53 90-day exposure of mice to PE MPs sized 16.9 μm at doses ranging from 0.125 mg day−1 to 0.5 mg day−1 led to lesions in tissues of multiple organs, germ cell degradation, and adverse changes in the ovary.54 Additionally, Park et al. also reported numerous changes in immune response after mice were exposed to 0.5 mg day−1 (15 mg kg−1–bw day−1) to 2 mg day−1 (60 mg kg−1–bw day−1) of PE MPs sized 40–48 μm for 90 days, in addition to reduced live births per dam and reduced body weight of pubs (2 mg day−1).64 Pathologically, 0.125 mg day−1 (3.75 mg kg−1–bw day−1) of the PE MPs caused degenerated testicular germ cells, stomach mucosal hypertrophy, ovary cyst, and deleterious changes in lung and kidney tissues.64 Behavioral changes in terms of reduced locomotion activity were noted when mice were exposed to 60 mg L−1 PE MPs with a mean diameter of 35.46 μm for 7 days.74
000 particles mL−1 and 5000 particles mL−1, respectively.63 Huang et al. observed that PE MPs of 30–140 μm decreased the viability of Caco-2 cells at 1000 mg L−1 and increased intracellular ROS at 100 mg L−1 after 48 hours of exposure.66 In mice, 600 μg PE MPs sized 10–150 μm caused intestinal inflammation after 5 weeks of exposure.53 90-day exposure of mice to PE MPs sized 16.9 μm at doses ranging from 0.125 mg day−1 to 0.5 mg day−1 led to lesions in tissues of multiple organs, germ cell degradation, and adverse changes in the ovary.54 Additionally, Park et al. also reported numerous changes in immune response after mice were exposed to 0.5 mg day−1 (15 mg kg−1–bw day−1) to 2 mg day−1 (60 mg kg−1–bw day−1) of PE MPs sized 40–48 μm for 90 days, in addition to reduced live births per dam and reduced body weight of pubs (2 mg day−1).64 Pathologically, 0.125 mg day−1 (3.75 mg kg−1–bw day−1) of the PE MPs caused degenerated testicular germ cells, stomach mucosal hypertrophy, ovary cyst, and deleterious changes in lung and kidney tissues.64 Behavioral changes in terms of reduced locomotion activity were noted when mice were exposed to 60 mg L−1 PE MPs with a mean diameter of 35.46 μm for 7 days.74
2.3. Implications of MP toxic levels and their influencing factors
It becomes apparent at this point that the abilities of MPs and smaller plastic particles to induce negative health effects vary considerably with different types and sizes of MPs, the biomarkers or bioindicators used, the types of cell models used, and in some instances, the gender of the experimental subjects (Tables S1† and 1). Frequently, the comparison between the MP levels producing adverse effects is complicated by the different units used, and most of the time, they are not convertible due to the varying methods used in determining the levels. Furthermore, weathering of MPs through digestion and ultraviolet irradiation could alter their toxicity. Table 1 attempts to present the levels of MPs producing no observable health effect and an adverse effect according to the types and sizes of MPs, taking the lowest or most conservative levels reported in Table S1.†| MP type | Size range | Level without observable effectsa | Level causing adverse effectsa |
|---|---|---|---|
| a These are conservative levels showing either the highest levels a specific experimental subject can be exposed to a particular type of MPs in a certain size range without any observable adverse effects or the lowest levels producing an observable adverse effect on a particular subject. | |||
| PS | ≤1 μm | • 10 μg mL−1 (HepG2CDKN1A) | • 100 μg L−1 (mice) |
| • 0.01 μg mL−1 (BeWo b30) | |||
| • 12.6 μg mL−1 (NIH/3T3 fibroblasts & mouse ES-D3) | |||
| • 89.0 μg mL−1 (cardiomyocytes) | |||
| • 10 μg mL−1 (Caco-2) | |||
| • 2.64 × 1014 particles (mice) | |||
| • 100 μg mL−1 (CHO-K1 cells) | • 0.15 mg day−1 (mice) | ||
| • 4.55 × 107 particles (mice) | • 1 μg mL−1 (rat granulosa cell) | ||
| • 100 μg mL−1 (intestinal cell model) | • 1.5 × 1011 μm2 particle surface per mL (HepaRG) | ||
| • 10 mg kg−1 (mice) | • 80 μg kg−1 day−1 (mice) | ||
| >1 μm to 20 μm | • 1.49 × 106 particles (mice) | • 0.6–0.7 μg day−1 (mice) | |
| • 1 μg mL−1 (Caco-2) | |||
| • 100 μg L−1 (mice) | |||
| >20 μm to 50 μm | — | • 100 μg L−1 (mice) | |
| • 0.01 mg day−1 (mice) | |||
| >50 μm | • 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles mL−1 (Caco-2) 000 particles mL−1 (Caco-2) |
• 5000 particles mL−1 (HepG2 & THP-1 macrophage) | |
| PE | 200–9900 nm | — | • 50 μg cm−2 (intestinal cell model) |
| 10–150 μm | — | • 600 μg (mice) | |
| 30–140 μm | — | • 100 mg L−1 (Caco-2) | |
| 16.9 μm | — | • 0.125 mg day−1 (mice) | |
| 40–48 μm | — | • 0.125 mg day−1 or 3.75 mg kg−1–bw day−1 (mice) | |
| PP | 100 μm | — | • 5000 particles mL−1 (THP-1 macrophage) |
| MPs akin to those of tires and polyolefins | 50–500 μm | • 1028.58 μg cm−2 (intestinal cell model) | — |
Modified MPs are treated as a subset of the major MP types; for instance, COOH-modified PS is considered as PS. PS particles ≤1 μm are those commonly in the nanoscale. Nano-PS at levels ranging from 10 μg mL−1 to 100 μg mL−1 did not produce an observable effect on certain cell line models (e.g., HepG2CDKN1A). From 4.55 × 107 particles up to 10 mg kg−1, nano-PS did not yield an observable effect in mice (Table 1). However, for other cell lines (e.g., BeWo b30), some studies revealed that nano-PS began to cause a negative response at a level of 0.01 μg mL−1 to 89.0 μg mL−1, while the levels needed to be at 100 μg L−1, 0.15 mg day−1, 2.64 × 1014 particles, or 80 μg kg−1 day−1 to produce adverse effects in mice (note the different units used in different studies due to the different methods in measuring MP abundance) (Table 1).
For PS MPs with sizes ranging from more than 1 μm to 20 μm, a study revealed that ingestion of 1.49 × 106 particles did not induce an observable negative response in mice57 (Table 1). The deduced levels inducing adverse effects are 0.6–0.7 μg day−1 or 100 μg L−1 for mice, and 1 μg mL−1 for Caco-2 cells (Table 1). PS MPs in the size range of more than 20 μm to 50 μm produced negative effects in mice at 100 μg L−1 or 0.01 mg day−1 whereas PS MPs sized more than 50 μm induced negative responses in HepG2 cells and THP-1 macrophage at 5000 particles mL−1 (Table 1). Due to limited studies on PE MPs, the size ranges used in those studies are presented as they are. There is much overlapping in the MP size range of 10–150 μm and the MP level causing a negative response for this size range was deduced at 600 μg or 0.125 mg day−1 for mice (Table 1). As for Caco-2 cells, the level is 100 mg L−1. Exposure to PE particles of 200–9900 nm at 50 μg cm−2 generally gives rise to a negative effect in intestinal cell models. PP MPs sized 100 μm were deduced to produce a negative effect at 5000 particles mL−1, while MPs resembling those of tires and polyolefins might cause an adverse response at 1028.58 μg cm−2 (Table 1).
These levels without or with observation health effects were deduced from MP toxicological studies conducted on mice and cell models. These levels were not explicitly stated in those studies and the deductions were made based on statistically significant changes in the biomarkers reported in the studies. If a particular level of MPs induced a statistically significant alteration in the biomarker, the level is deemed to cause an adverse effect. In some studies, however, even though an MP dose produced a substantial change in the biomarker, a clear dose–response relationship after the dose may not be readily discernible or could only be observed up to a certain extent.45,69,73 In these instances, the levels at which the negative effects started to show were noted. A lack of dose–response relationship is an obvious limitation in some toxicological studies on MPs.
Besides, certain crucial information, such as the weights of mice, were not made available, making the conversion of dose units challenging. A lack of standardized biomarkers adds to the complexity in determining the levels of MPs causing adverse effects. Currently, a wide range of biomarkers have been used and they tend to give different MP levels with or without adverse effects even with the same type and size of MPs tested (Table S1†). Certain biomarkers examine changes at enzymatic and genetic levels while others gauge changes at cellular and organ levels. Measuring mortality and acute lethal toxicity caused by MPs may require unrealistically large doses of MPs due to their inherently low toxicity.
In the environment, MPs could come from a variety of sources and undergo different degrees of weathering, which alter their toxicity. Similarly, it is likely for environmental MPs to interact with a gamut of chemicals, each with different toxicity. Weathering may result in the physical and chemical changes of MPs, thus changing their brittleness, density, size, shape, and surface charges. This was elucidated by the study of Liu et al., which transformed MPs through an in vitro digestive process and found the transformed MPs to have a lower toxic effect on membrane integrity and permeability of Caco-2 cells (Table S1†).37 The authors attributed the alteration to the formation of corona on the surfaces of transformed MPs that could change their sizes, zeta potentials, and sorption potentials. The zeta potentials of MPs were marginally increased after digestion, causing reduced electrostatic repulsion between particles, and this potentially facilitated their agglomeration.37 Such agglomeration was evidenced by an increase in the average sizes of the transformed MPs, making their internalization by Caco-2 cells more difficult. Another possible reason for this is that the corona contained proteins that might trigger an immune response, hence repulsion by the cell membrane.75 Jeon et al. also reported lower toxicity of weathered MPs on THP-1 macrophage, particularly its cytotoxicity and pro-inflammatory responses because of a decline in ROS generation, possibly due to the higher affinity of weathered MPs to serum protein which scavenges ROS.63 Stock et al. observed that the sizes and surface charges of nano-PS particles influenced their cellular uptake and transport, where the smallest particles (20 nm) and those with positively charged surfaces had higher cellular uptake by and transport into an intestinal cell model. PS particles of 20 nm with carboxy groups (−) and those of 100 nm with amine groups (+) were also found to induce intrinsic apoptosis mechanism more notably than neutral 100 nm PS in HepaRG cells.73
While sizes of nanoplastics have been shown to affect their toxicity with those of smaller sizes tending to have larger toxicity, the correlation is less consistent in MPs. For instance, PS MPs sized 5 μm were reported to induce a negative response at a lower level than those sized 0.1 μm when tested on Caco-2 cells.43 Upon comparing the findings of two separate studies on Caco-2 cells, PS MPs sized 0.05–0.1 μm seemed to have lower toxicity than PS MPs sized 5 μm.37,50 In addition, Table S1† shows a lack of size dependence in the levels of PS MPs yielding adverse effects on mice, with a level of 100 μg L−1 reported across three size ranges.
3 Conclusion
This review presents the toxicity of MPs based on diverse in vivo studies conducted primarily on mice and in vitro studies on different cell models, especially intestinal cells. It is crucial as there are currently very few reviews that aim to provide a better understanding of MP toxicity from the perspective of the MP levels causing or not causing adverse effects on the experimental subjects. Examining MP toxicity in mammals and cell models enables the toxic effects of MPs on humans to be better characterized. The levels of MPs producing or not producing an observable negative effect deduced from the studies reviewed widely differ due to the different sizes of MPs used, different cell lines tested, and the different biomarkers/bioindicators employed. A lack of uniformity in the units of dosage due to the variations in MP preparation and, in some instances, a lack of weight measurement of the experimental animals makes the comparison of these levels challenging. This review, therefore, proposes a standardization of the methods for measuring MP toxicity. Specifically, it recommends the following:• Use a common unit in reporting the toxic levels of MPs. For in vivo studies, mg kg−1–bw day−1 is recommended since this unit is widely used in reporting toxicological doses. For in vitro studies, the commonly used unit based on the studies reviewed is mg L−1 or μg mL−1.
• To allow the reporting of dosage in mg kg−1–bw day−1 for in vivo studies, the weights of MPs need to be obtained or deduced from the number of particles administered. This involves better particle characterization of MPs. The weights of the experimental animals need to be measured.
• Use standardized biomarkers and bioindicators. For in vitro studies, the biomarkers/bioindicators may encompass behavioral changes, liver and brain immune markers, blood glucose metabolism, lipid metabolism, reproductive function, inflammatory cytokine level, digestive tract histopathology, and nervous system. For in vivo studies, the biomarkers may include cytotoxicity, oxidative stress, immune response, membrane integrity, and gene expression.
• More experiments need to be conducted on different types of MPs, including bioplastics and biodegradable plastics, particularly polyethylene terephthalate, PE, PP, polyurethane, polyamide, polymethyl methacrylate, styrene acrylate, polyhydroxyalkanoates, polylactic acid and polyesteramides.
• For each of the MP types, the effects of different environmentally relevant sizes of MPs should be tested. This may require the use of standardized size ranges to enable comparison across different studies.
•More studies on weathered MPs are needed. Since MPs undergo different degrees of weathering and interactions with other environmental pollutants upon entering the environment, toxicological studies on weathered MPs and MPs interacted with environmental pollutants are important.
Data availability
No primary research results have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author wishes to thank the University of Arizona for the administrative support given.References
- Y. Li, C. Liu, H. Yang, W. He, B. Li, X. Zhu, S. Liu, S. Jia, R. Li and K. H. D. Tang, Leaching of chemicals from microplastics: A review of chemical types, leaching mechanisms and influencing factors, Sci. Total Environ., 2024, 906, 167666 CrossRef CAS.
- F. D. L. Leusch, H.-C. Lu, K. Perera, P. A. Neale and S. Ziajahromi, Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices, Environ. Pollut., 2023, 319, 120984 CrossRef.
- K. H. D. Tang, Microplastics in and Near Landlocked Countries of Central and East Asia: A Review of Occurrence and Characteristics, Trop. Aquat. Soil Pollut., 2023, 3, 120–130 CrossRef.
- K. H. D. Tang, Abundance of Microplastics in Wastewater Treatment Sludge, J. Hum. Earth Future, 2022, 3, 138–146 CrossRef.
- A. Hayes, K. P. Kirkbride and S. C. Leterme, Variation in polymer types and abundance of microplastics from two rivers and beaches in Adelaide, South Australia, Mar. Pollut. Bull., 2021, 172, 112842 CrossRef.
- S. Padha, R. Kumar, A. Dhar and P. Sharma, Microplastic pollution in mountain terrains and foothills: A review on source, extraction, and distribution of microplastics in remote areas, Environ. Res., 2022, 207, 112232 CrossRef PubMed.
- S. Abbasi, A. Turner, M. Hoseini and H. Amiri, Microplastics in the Lut and Kavir Deserts, Iran, Environ. Sci. Technol., 2021, 55, 5993–6000 CrossRef PubMed.
- J. Brahney, M. Hallerud, E. Heim, M. Hahnenberger and S. Sukumaran, Plastic rain in protected areas of the United States, Science, 2020, 368, 1257–1260 CrossRef CAS.
- X. Zhao, Y. Zhou, C. Liang, J. Song, S. Yu, G. Liao, P. Zou, K. H. D. Tang and C. Wu, Airborne microplastics: Occurrence, sources, fate, risks and mitigation, Sci. Total Environ., 2023, 858, 159943 CrossRef CAS.
- R. C. Moore, L. Loseto, M. Noel, A. Etemadifar, J. D. Brewster, S. MacPhee, L. Bendell and P. S. Ross, Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea, Mar. Pollut. Bull., 2020, 150, 110723 CrossRef CAS PubMed.
- R.-F. Shiu, C. I. Vazquez, C.-Y. Chiang, M.-H. Chiu, C.-S. Chen, C.-W. Ni, G.-C. Gong, A. Quigg, P. H. Santschi and W.-C. Chin, Nano- and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton, Sci. Total Environ., 2020, 748, 141469 CrossRef CAS PubMed.
- W. Courtene-Jones, N. J. Clark, A. C. Fischer, N. S. Smith and R. C. Thompson, in Plastics and the Ocean, 2022, pp. 349–366 Search PubMed.
- N. C. Ory, P. Sobral, J. L. Ferreira and M. Thiel, Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre, Sci. Total Environ., 2017, 586, 430–437 CrossRef CAS PubMed.
- R. S. Pazos, D. E. Bauer and N. Gómez, Microplastics integrating the coastal planktonic community in the inner zone of the Río de la Plata estuary (South America), Environ. Pollut., 2018, 243, 134–142 CrossRef CAS PubMed.
- M. B. Alfonso, A. H. Arias and en M. C. Piccolo, Microplastics integrating the zooplanktonic fraction in a saline lake of Argentina: influence of water management, Environ. Monit. Assess., 2020, 192, 117 CrossRef CAS.
- Z. L. R. Botterell, N. Beaumont, T. Dorrington, M. Steinke, R. C. Thompson and P. K. Lindeque, Bioavailability and effects of microplastics on marine zooplankton: A review, Environ. Pollut., 2019, 245, 98–110 CrossRef CAS PubMed.
- D. Neves, P. Sobral, J. L. Ferreira and T. Pereira, Ingestion of microplastics by commercial fish off the Portuguese coast, Mar. Pollut. Bull., 2015, 101, 119–126 CrossRef CAS.
- D. Giani, M. Baini, M. Galli, S. Casini and M. C. Fossi, Microplastics occurrence in edible fish species (Mullus barbatus and Merluccius merluccius) collected in three different geographical sub-areas of the Mediterranean Sea, Mar. Pollut. Bull., 2019, 140, 129–137 CrossRef CAS.
- K. H. D. Tang, R. Li, Z. Li and D. Wang, Health risk of human exposure to microplastics: a review, Environ. Chem. Lett., 2024, 22, 1155–1183 CrossRef CAS.
- K. H. D. Tang, Ecotoxicological Impacts of Micro and Nanoplastics on Marine Fauna, Exam. Mar. Biol. Oceanogr., 2020, 3, 1–5 Search PubMed.
- Y. Lu, Y. Zhang, Y. Deng, W. Jiang, Y. Zhao, J. Geng, L. Ding and H. Ren, Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver, Environ. Sci. Technol., 2016, 50, 4054–4060 CrossRef CAS.
- D. Gola, P. Kumar Tyagi, A. Arya, N. Chauhan, M. Agarwal, S. K. Singh and S. Gola, The impact of microplastics on marine environment: A review, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100552 CAS.
- D. S. Green, Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities, Environ. Pollut., 2016, 216, 95–103 CrossRef CAS PubMed.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Discovery and quantification of plastic particle pollution in human blood, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- L. Montano, E. Giorgini, V. Notarstefano, T. Notari, M. Ricciardi, M. Piscopo and O. Motta, Raman Microspectroscopy evidence of microplastics in human semen, Sci. Total Environ., 2023, 901, 165922 CrossRef CAS PubMed.
- C. Pironti, V. Notarstefano, M. Ricciardi, O. Motta, E. Giorgini and L. Montano, First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body, Toxics, 2023, 11(1), 40 CrossRef CAS.
- N. Rafa, B. Ahmed, F. Zohora, J. Bakya, S. Ahmed, S. F. Ahmed, M. Mofijur, A. A. Chowdhury and F. Almomani, Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects, Environ. Pollut., 2024, 343, 123190 CrossRef CAS.
- O. S. Alimi, J. Farner Budarz, L. M. Hernandez and N. Tufenkji, Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport, Environ. Sci. Technol., 2018, 52, 1704–1724 CrossRef CAS PubMed.
- K. H. D. Tang, Environmental Co-existence of Microplastics and Perfluorochemicals: A Review of Their Interactions, Biointerface Res. Appl. Chem., 2023, 13, 587 CAS.
- A. T. N. Do, Y. Ha and J.-H. Kwon, Leaching of microplastic-associated additives in aquatic environments: A critical review, Environ. Pollut., 2022, 305, 119258 CrossRef CAS PubMed.
- C. Li and K. H. D. Tang, Effects of pH and Temperature on the Leaching of Di (2-Ethylhexyl) Phthalate and Di-n-butyl Phthalate from Microplastics in Simulated Marine Environment, Biointerface Res. Appl. Chem., 2023, 13, 269 CAS.
- M. Mariana, J. Feiteiro, I. Verde and E. Cairrao, The effects of phthalates in the cardiovascular and reproductive systems: A review, Environ. Int., 2016, 94, 758–776 CrossRef CAS.
- Q. Shi, J. Tang, R. Liu and L. Wang, Toxicity in vitro reveals potential impacts of microplastics and nanoplastics on human health: A review, Crit. Rev. Environ. Sci. Technol., 2022, 52, 3863–3895 CrossRef.
- W. Liu, B. Zhang, Q. Yao, X. Feng, T. Shen, P. Guo, P. Wang, Y. Bai, B. Li, P. Wang, R. Li, Z. Qu and N. Liu, Toxicological effects of micro/nano-plastics on mouse/rat models: a systematic review and meta-analysis, Front. Public Health, 2023, 11, 1103289 CrossRef.
- R. Gautam, J. Jo, M. Acharya, A. Maharjan, D. Lee, K. C. Pramod Bahadur, C. Kim, K. Kim, H. Kim and Y. Heo, Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines, Sci. Total Environ., 2022, 838, 156089 CrossRef PubMed.
- C. Cortés, J. Domenech, M. Salazar, S. Pastor, R. Marcos and A. Hernández, Nanoplastics as a potential environmental health factor: effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells, Environ. Sci.: Nano, 2020, 7, 272–285 RSC.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the digestive process on intestinal toxicity of polystyrene microplastics as determined by in vitro Caco-2 models, Chemosphere, 2020, 256, 127204 CrossRef PubMed.
- R. An, X. Wang, L. Yang, J. Zhang, N. Wang, F. Xu, Y. Hou, H. Zhang and L. Zhang, Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats, Toxicology, 2021, 449, 152665 CrossRef PubMed.
- R. Lehner, W. Wohlleben, D. Septiadi, R. Landsiedel, A. Petri-Fink and B. Rothen-Rutishauser, A novel 3D intestine barrier model to study the immune response upon exposure to microplastics, Arch. Toxicol., 2020, 94, 2463–2479 CrossRef CAS PubMed.
- S. Palaniappan, C. M. Sadacharan and B. Rostama, Polystyrene and Polyethylene Microplastics Decrease Cell Viability and Dysregulate Inflammatory and Oxidative Stress Markers of MDCK and L929 Cells In Vitro, Exposure Health, 2022, 14, 75–85 CrossRef CAS.
- R. Sun, K. Xu, L. Yu, Y. Pu, F. Xiong, Y. He, Q. Huang, M. Tang, M. Chen, L. Yin, J. Zhang and Y. Pu, Preliminary study on impacts of polystyrene microplastics on the hematological system and gene expression in bone marrow cells of mice, Ecotoxicol. Environ. Saf., 2021, 218, 112296 CrossRef CAS.
- S. Lee, D. Kim, K.-K. Kang, S.-E. Sung, J.-H. Choi, M. Sung, C.-H. Shin, E. Jeon, D. Kim, D. Kim, S. Lee, H.-K. Kim and K. Kim, Toxicity and Biodistribution of Fragmented Polypropylene Microplastics in ICR Mice, Int. J. Mol. Sci., 2023, 24(10), 8463 CrossRef CAS PubMed.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells, Chemosphere, 2019, 221, 333–341 CrossRef CAS.
- X. Yu, M. Lang, D. Huang, C. Yang, Z. Ouyang and X. Guo, Photo-transformation of microplastics and its toxicity to Caco-2 cells, Sci. Total Environ., 2022, 806, 150954 CrossRef CAS PubMed.
- M. Hesler, L. Aengenheister, B. Ellinger, R. Drexel, S. Straskraba, C. Jost, S. Wagner, F. Meier, H. von Briesen, C. Büchel, P. Wick, T. Buerki-Thurnherr and Y. Kohl, Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro, Toxicol. In Vitro, 2019, 61, 104610 CrossRef CAS PubMed.
- M. B. Paul, L. Böhmert, I.-L. Hsiao, A. Braeuning and H. Sieg, Complex intestinal and hepatic in vitro barrier models reveal information on uptake and impact of micro-, submicro- and nanoplastics, Environ. Int., 2023, 179, 108172 CrossRef CAS.
- T. H. A. Truong, S. R. Mothe, J. L. Min, H. M. Tan, A. W. Jackson, D. T. Nguyen, D. K. J. Ye, P. Kanaujia, P. Thoniyot and T. T. Dang, Immuno-Modulatory Effects of Microparticles Formulated from Degradable Polystyrene Analogue, Macromol. Biosci., 2022, 22, 2100472 CrossRef CAS PubMed.
- A. Banerjee, L. O. Billey and W. L. Shelver, Uptake and toxicity of polystyrene micro/nanoplastics in gastric cells: Effects of particle size and surface functionalization, PLoS One, 2022, 16, e0260803 CrossRef.
- S. Ballesteros, J. Domenech, I. Barguilla, C. Cortés, R. Marcos and A. Hernández, Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics, Environ. Sci.: Nano, 2020, 7, 3431–3446 RSC.
- J. Domenech, A. Hernández, L. Rubio, R. Marcos and C. Cortés, Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier, Arch. Toxicol., 2020, 94, 2997–3012 CrossRef CAS.
- Y. Zhang, X. Wang, Y. Zhao, J. Zhao, T. Yu, Y. Yao, R. Zhao, R. Yu, J. Liu and J. Su, Reproductive toxicity of microplastics in female mice and their offspring from induction of oxidative stress, Environ. Pollut., 2023, 327, 121482 CrossRef CAS PubMed.
- Z. Liu, Q. Zhuan, L. Zhang, L. Meng, X. Fu and Y. Hou, Polystyrene microplastics induced female reproductive toxicity in mice, J. Hazard. Mater., 2022, 424, 127629 CrossRef CAS PubMed.
- B. Li, Y. Ding, X. Cheng, D. Sheng, Z. Xu, Q. Rong, Y. Wu, H. Zhao, X. Ji and Y. Zhang, Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice, Chemosphere, 2020, 244, 125492 CrossRef CAS PubMed.
- S. B. Fournier, J. N. D'Errico, D. S. Adler, S. Kollontzi, M. J. Goedken, L. Fabris, E. J. Yurkow and P. A. Stapleton, Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy, Part. Fibre Toxicol., 2020, 17, 55 CrossRef CAS PubMed.
- Z. Wei, Y. Wang, S. Wang, J. Xie, Q. Han and M. Chen, Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice, Toxicology, 2022, 465, 153059 CrossRef CAS PubMed.
- W. Yung-Li, L. Yu-Hsuan, H. Yung-Ho, C. I-Jen, H. C. Chia-Yu, H. Chih-Chia, C. Zi-Chun, L. Chung-Pei, L. Yuh-Feng and C. Hui-Wen, The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice, Environ. Health Perspect., 2024, 129, 57003 Search PubMed.
- V. Stock, L. Böhmert, E. Lisicki, R. Block, J. Cara-Carmona, L. K. Pack, R. Selb, D. Lichtenstein, L. Voss, C. J. Henderson, E. Zabinsky, H. Sieg, A. Braeuning and en A. Lampen, Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo, Arch. Toxicol., 2019, 93, 1817–1833 CrossRef PubMed.
- H. Jin, T. Ma, X. Sha, Z. Liu, Y. Zhou, X. Meng, Y. Chen, X. Han and J. Ding, Polystyrene microplastics induced male reproductive toxicity in mice, J. Hazard. Mater., 2021, 401, 123430 CrossRef.
- W. A. da Silva Brito, D. Singer, L. Miebach, F. Saadati, K. Wende, A. Schmidt and S. Bekeschus, Comprehensive in vitro polymer type, concentration, and size correlation analysis to microplastic toxicity and inflammation, Sci. Total Environ., 2023, 854, 158731 CrossRef.
- J. Xiao, X. Jiang, Y. Zhou, G. Sumayyah, L. Zhou, B. Tu, Q. Qin, J. Qiu, X. Qin, Z. Zou and C. Chen, Results of a 30-day safety assessment in young mice orally exposed to polystyrene nanoparticles, Environ. Pollut., 2022, 292, 118184 CrossRef PubMed.
- E. Danopoulos, M. Twiddy, R. West and J. M. Rotchell, A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells, J. Hazard. Mater., 2022, 427, 127861 CrossRef PubMed.
- T. Witzmann, A. F. R. M. Ramsperger, S. Wieland, C. Laforsch, H. Kress, A. Fery and G. K. Auernhammer, Repulsive Interactions of Eco-corona-Covered Microplastic Particles Quantitatively Follow Modeling of Polymer Brushes, Langmuir, 2022, 38, 8748–8756 Search PubMed.
- S. Jeon, D.-K. Lee, J. Jeong, S. I. Yang, J.-S. Kim, J. Kim and W.-S. Cho, The reactive oxygen species as pathogenic factors of fragmented microplastics to macrophages, Environ. Pollut., 2021, 281, 117006 Search PubMed.
- E.-J. Park, J.-S. Han, E.-J. Park, E. Seong, G.-H. Lee, D.-W. Kim, H.-Y. Son, H.-Y. Han and B.-S. Lee, Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation, Toxicol. Lett., 2020, 324, 75–85 CrossRef CAS PubMed.
- M. Busch, A. A. M. Kämpfer and R. P. F. Schins, An inverted in vitro triple culture model of the healthy and inflamed intestine: Adverse effects of polyethylene particles, Chemosphere, 2021, 284, 131345 CrossRef CAS PubMed.
- W. Huang, H. Yin, Y. Yang, L. Jin, G. Lu and Z. Dang, Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: Simulation in vitro with human cell Caco-2 and gut microbiota, Sci. Total Environ., 2021, 778, 146264 CrossRef.
- Y. Deng, Y. Zhang, B. Lemos and H. Ren, Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure, Sci. Rep., 2017, 7, 46687 CrossRef.
- L. Lu, Z. Wan, T. Luo, Z. Fu and Y. Jin, Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice, Sci. Total Environ., 2018, 631–632, 449–458 CrossRef PubMed.
- X. Xie, T. Deng, J. Duan, J. Xie, J. Yuan and M. Chen, Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway, Ecotoxicol. Environ. Saf., 2020, 190, 110133 CrossRef PubMed.
- B. Hou, F. Wang, T. Liu and Z. Wang, Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice, J. Hazard. Mater., 2021, 405, 124028 CrossRef PubMed.
- D. J. Rawle, T. Dumenil, B. Tang, C. R. Bishop, K. Yan, T. T. Le and A. Suhrbier, Microplastic consumption induces inflammatory signatures in the colon and prolongs a viral arthritis, Sci. Total Environ., 2022, 809, 152212 CrossRef PubMed.
- Q. Wang, J. Bai, B. Ning, L. Fan, T. Sun, Y. Fang, J. Wu, S. Li, C. Duan, Y. Zhang, J. Liang and Z. Gao, Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells, Chemosphere, 2020, 254, 126788 CrossRef CAS.
- V. Stock, L. Böhmert, G. Coban, G. Tyra, M.-L. Vollbrecht, L. Voss, M. B. Paul, A. Braeuning and H. Sieg, Microplastics and nanoplastics: Size, surface and dispersant – What causes the effect?, Toxicol. In Vitro, 2022, 80, 105314 CrossRef CAS PubMed.
- A. P. da Costa Araújo and G. Malafaia, Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish, J. Hazard. Mater., 2021, 401, 123263 CrossRef.
- V. H. Nguyen, N. M. Meghani, H. H. Amin, T. T. D. Tran, P. H. L. Tran, C. Park and B.-J. Lee, Modulation of serum albumin protein corona for exploring cellular behaviors of fattigation-platform nanoparticles, Colloids Surf., B, 2018, 170, 179–186 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00227j |
| This journal is © The Royal Society of Chemistry 2024 |

