Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Size determination of nanoparticles by ICP-ToF-MS using isotope dilution in microdroplets
Marcus von der
Au
,
Sebastian
Faßbender
 ,
Michail Ioannis
Chronakis
,
Michail Ioannis
Chronakis
 ,
Jochen
Vogl
,
Jochen
Vogl
 and
Björn
Meermann
and
Björn
Meermann
 *
*
Division 1.1 – Inorganic Trace Analysis, Federal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Straße 11, 12489 Berlin, Germany. E-mail: bjoern.meermann@bam.de
First published on 9th May 2022
Abstract
Within this work, the combination of a microdroplet generator and an ICP-ToF-MS for nanoparticle analysis is presented. For the size determination of platinum nanoparticles an on-line isotope dilution analysis approach was developed. The 194Pt/195Pt isotopic ratio was used for the characterization of the particles, while the 182W/183W isotopic ratio was monitored simultaneously for mass bias correction. The on-line ID-MDG-sp-ICP-ToF-MS approach was deployed for the size determination of three platinum nanoparticle samples (50 nm, 63 nm, 70 nm); for validation, complementary size characterization techniques (sp-ICP-ToF-MS and TEM) were used. The robustness of this technique was evidenced, by using sodium chloride concentrations up to 100 mg L−1 as a matrix component. Our new on-line ID MDG-sp-ICP-ToF-MS approach is a promising tool for the fast and reliable determination of nanoparticles' size in severe matrix concentrations, e.g., environmental samples.
Introduction
The incorporation of nanoparticles in a variety of products, as well as the release of such particles in the environment, has dramatically increased during the past years.1 Therefore, small particles in the nanometer range have become a major concern in terms of ecotoxicology and human health.2,3 Furthermore, nanoparticles find their way into regulations and thus, uniform definitions.4 Hence, the presence of nanoparticles (NP) and their composition must be monitored, and rapid and reliable techniques are essential for this purpose. A detector which allows for an extremely sensitive elemental detection of single particles in short transient signals is ICP-MS in single particle mode, sp-ICP-MS.5 The utilization of this easy-to-use technique, which is mainly based on the appropriate dilution of a nanoparticle suspension, increased rapidly after its introduction in 1993 by Nomizu et al. and developed further by Degueldre et al. in 2003.6–8 Although the collection of data is simple, the quantification as well as deviation of nanoparticles' size information is difficult.9 One of the key aspects, which makes this technique difficult to use, is the determination of the transport efficiency (TE). Since most approaches are using liquid standards to quantify the metal content of the nanoparticles, the TE of the sample introduction system being used must be determined. For this purpose, adequately characterized standards are needed.9 The microdroplet generator (MDG), as a sample introduction system, is an upcoming technique that eliminates the need for these standards.10–16 This technique allows for calibration of the system by introducing a liquid in the form of monodisperse microdroplets resulting in the same type of signal as nanoparticles.14 By producing monodisperse droplets, the volume of the droplets and therefore the absolute mass of introduced calibrants are known and the system can be calibrated, since a TE for a droplet of 100% can be achieved.14The frequency of the generation of microdroplets can be adjusted in the range of a few Hz up to the kHz range. Since the ion cloud of the droplet is within the time range of the typical nanoparticle's ion cloud, fast ion counting, and data acquisition is necessary. With classic ICP-MS (scanning) mass analyzers, the number of isotopes which can be determined in a single event is limited. With the latest development of the ICP-ToF-MS, this limitation can be overcome and simultaneous information about nearly the whole periodic table can be acquired in short transient signals/single nanoparticles.17 Due to the (quasi) simultaneous detection of all elements/all isotopes at the same time also isotope dilution analysis (IDA) is possible within single particle events.18 With the monodisperse droplets generated by a microdroplet generator, the absolute mass of the isotopic standard introduced into the plasma is known. Therefore, all values needed for the on-line ID approach are accessible. Furthermore, by using the ToF mass analyzer more than one isotope system can be investigated. Also, the possibility to investigate and characterize more than one element in nanoparticles allows for the differentiation between natural and engineered nanoparticles.19
The suitability of on-line ID for nanoparticles has already been reported by Telgmann et al.20 and Sötebier et al.21 for silver nanoparticles in recent years. Since for these studies scanning mass analyzers were used, it was not possible to determine both silver isotopes (107Ag/109Ag) in single particles. Furthermore, this limitation does not allow for a simultaneous mass bias correction. By using a mass bias correction, the correctness of the data may be improved, which may affect the result and therefore the determined diameter. In addition, both studies rely on the correct determination of the TE and the stability of this value during the measurements. This lowers the precision and reproducibility of these approaches.
Within our study, the use of the MDG as sample introduction system is being proposed for the direct mass quantification within single nanoparticles via on-line ID. With this approach, the TE is not needed, and a direct calibration via liquid standards is enabled. Furthermore, by using an ICP-ToF-MS detector, the isotope ratio can be determined for each individual particle; for calibration as well as mass bias correction a second isotope system is applicable.
Experimental
For the on-line ID MDG-sp-ICP-ToF-MS measurements, a combination of an ICP-ToF-MS system (2R, TOFWERK, Switzerland) and a microdroplet generator introduction, control and autosampler system (TOFWERK) equipped with a 50 μm nozzle microdroplet generator (microdrop Technologies GmbH, Germany) was used. The sp-ICP-ToF-MS analysis was carried out with the standard sample introduction system that consists of a concentric nebulizer and a cyclonic spray chamber. The sp-ICP-ToF-MS parameters were tuned daily to optimize the signal intensity. The on-line ID MDG-sp-ICP-ToF-MS approach was optimized daily to ensure the introduction of all generated droplets into the plasma and a high sensitivity. The helium flow rate used for the MDG was kept at 0.5 L min−1 and the droplet frequency at 50 Hz. Also, the RF power for all experiments was set at 1550 W.The platinum and gold nanoparticles used were obtained from nanoComposix (50 nm and 70 nm platinum NPs and 100 nm Gold NPs, USA). Further TEM characterized platinum particles were provided by division 6.5 (Federal Institute for Materials Research and Testing (BAM), Germany). For the analysis with the sp-ICP-ToF-MS and the MDG-sp-ICP-ToF-MS approaches, all samples were prepared in precleaned PP-vials (DigiTUBEs 15 mL and 50 mL, SCP Science Corp., USA) leached with diluted sub-boiled nitric acid (ϕ = 1.3%) in ultra-pure water for at least one week. The particles were diluted according to the different methods to achieve a sufficient number of investigated particles. For external calibration, gold and platinum standard solutions were used. For the on-line ID-MDG-sp-ICP-ToF-MS approach, Pb and W standard solutions were used for the mass bias correction; a Cs standard solution was used as internal standard for droplet monitoring (all standard solutions: 1000 mg L−1 Certipur, Merck KGaA, Germany). During measurements the three elements (i.e., Cs, W, Pb) were diluted to a mass concentration of 10 μg L−1 each. For the isotope spike solution, the reference material ERM-AE141 (w(Pt) = 19.90 mg kg; n(194Pt)/n(Pt) = 0.9143; n(195Pt)/n(Pt) = 0.0676; BAM) was used. The data treatment, calculations and fits were done in Origin 2021 Pro (OriginLab Corporation, USA). All ICP-ToF-MS measurements were carried out in triplicates.
Results and discussion
Isotope dilution is one of the most precise methods available for mass fraction determination. However, the exact knowledge of the spike's mass fraction as well as the isotope ratio of the spike and the absolute amount of spike solution introduced into the system is required to provide correct results. For the determination of the mass fraction of interest in single particles with a microdroplet generator (MDG) as sample introduction system, the following equation (eqn (1)) was derived and applied. Since the volume of a nanoparticle (about 10−8 pL) is negligible (compared to the volume of the droplets, which is about 102 pL), it is excluded from the calculations: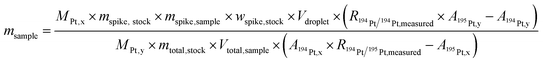 | (1) |
Within eqn (1)M is the natural (Mx) and spiked (My) molar mass of the analyte, mspike,stock is the mass of the spike solution used (ERM-AE-141) to prepare the stock solution, mtotal,stock is the total mass of the stock solution prepared, mspike,sample is the mass of the stock solution added to the sample and msample the obtained sample mass; wspike,stock is the mass fraction of the platinum in the spike solution (ERM-AE-141); Vdroplet is the volume of the generated droplet and Vtotal,sample the volume of the final sample solution; R is the ratio of the isotopic system used (“measured” refers to the uncorrected ratio and xy refers to the corrected ratio) and A (x refers to the sample and y refers to the spike) the abundance of the isotopes taken for calculation. By using a reference material, only the 194Pt/195Pt isotope ratio in the sample and Vdroplet must be determined, which makes the calculation fast and easy. The values regarding the isotopic abundances and atomic mass are provided by the CIAAW22 (for the natural platinum isotopic composition) and the reference material certificate (for the platinum isotopic composition and the concentration of the spike solution). The masses and volumes used in the calculation are determined prior to the analysis either volumetrically or gravimetrically.
V droplet is automatically calculated via the diameter and provided by the MDG-System software. To ensure the correct droplet volume, the build-in microscope and the software used were size calibrated by means of a 50 μm pin provided by TOFWERK. Since Vdroplet [pL] is calculated by the software, also the final volume Vtotal,sample was measured volumetrically to avoid conversion errors and to keep the equation consistent. The advantage of the ICP-ToF-MS lies in the quasi-simultaneous detection of (nearly) all elements as well as isotopes, all time in short transient signals – thus, all information needed for the on-line ID MDG-sp-ICP-ToF-MS approach is obtained within one single run.
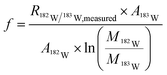
To cope with potential mass bias effects a second isotopic system was monitored simultaneously for correction. For platinum, two close systems with a similar isotopic pattern were tested for this purpose. Since the chance that Pb in the sample is higher, the 182W/183W (natural abundance 26.3%/30.6%) isotope ratio was used, which has a similar isotopic pattern and mass in comparison to the 194Pt/195Pt (natural abundance 32.9%/33.5%) isotope ratio. For the platinum 194Pt/195Pt isotope ratio correction with the 182W/183W isotopic ratio, the following equations (eqn (2) and (3)) were used. In these equations f is the mass bias correction factor.
 | (2) |
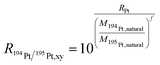 | (3) |
Taking eqn (3) into account, a modified version of eqn (1) (eqn (4)) can be derived, taking the correction factor into account. Eqn (4) was applied for all further calculations upon on-line MDG-sp-ICP-ToF-MS analyses:
 | (4) |
The nanoparticles' diameter (dNP) can then be obtained using msample and the density of platinum particles (ρPt), supposing a spherical shape for the particles (verified by TEM) and assuming that the particle consists of Pt only (100%) (eqn (5)).
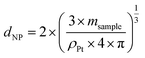 | (5) |
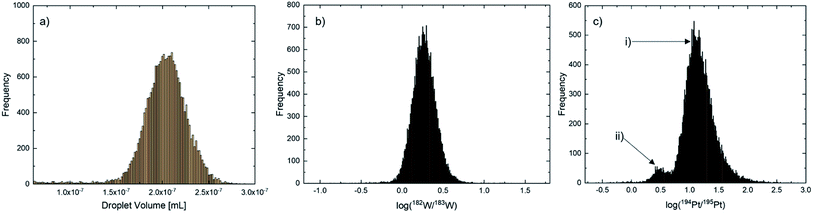
With the high number of droplets measured using the MDG-sp-ICP-ToF-MS approach, a Gaussian distribution was observed for all particles/droplets. Since the fluctuation from droplet to droplet was too high for the determination of the values needed for msample calculation based on a droplet-to-droplet basis the center of Gaussian fits were applied. Histograms were calculated via Origin 2021 Pro. The droplet volume (Fig. 1(a)) and the 182W/183W isotopic ratio (Fig. 1(b)) are not affected by the added isotope spike and therefore for these two a monomodal distribution is expected. In contrast, for the 194Pt/195Pt ratio, a bimodal distribution should occur – (i) the isotope ratio of the spike solution within a single droplet (Fig. 1c(i)) and (ii) the mixed isotope ratio of the spike solution and a Pt-nanoparticle (Fig. 1c(ii)).
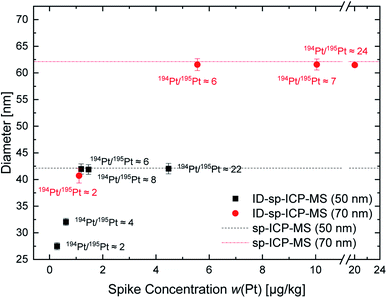
For the on-line isotope dilution analysis, the shifts in the isotopic ratios are crucial. Therefore, at first, different spike concentrations were used to identify an appropriate working range for the on-line ID-MDG-sp-ICP-ToF-MS approach. To derive an appropriate isotope ratio of the spike solution, a threshold was set: once a distinction of droplets from the MDG with and without Pt-particles included using the Origin software package is not possible anymore, an upper spike concentration limit is obtained. Thus, the optimal spike concentration/spike isotope ratio lies below this threshold. For isotopic spike optimization two different commercially available platinum nanoparticle suspensions (nominal size 50 nm and 70 nm) were investigated: five different spike isotope ratios for the 50 nm and four different spike isotope ratios for the 70 nm platinum nanoparticles were tested. The results obtained by the on-line ID-MDG-sp-ICP-ToF-MS approach in comparison to classical sp-ICP-ToF-MS are shown in Fig. 2. To determine the mass fraction with the classical sp-ICP-ToF-MS approach, the calibration strategy by Pace et al.23 was applied.
Fig. 2 illustrates the correlation of the Pt nanoparticle's diameter and the applied spike concentration as well as the resulting isotope ratio of the blend. For both nanoparticle sizes a threshold for the Pt isotopic ratio of the blend of 6 was observed. With lower values an underestimation of the diameter occurs. This is mainly due to “underspiking” and the resulting error magnification, since the spike concentration and spike isotope ratio do not sufficiently change the “natural” Pt isotope ratio of the particles.24,25 Once the 194Pt/195Pt isotope ratio reaches an appropriate value (approx. 194Pt/195Pt = 6), a reproducible diameter of 42 nm ± 1 nm and 62 nm ± 1 nm is achieved. Due to the high discrepancy between the given size according to the given and the calculated size of the nanoparticles, a “classical” sp-ICP-ToF-MS approach was also applied to verify the obtained values. This approach provided identical values for the particles' diameter of 42 nm ± 1 nm and 62 nm ± 1 nm respectively. Also, the “classical” sp-ICP-ToF-MS approach delivered the same particle diameter, lower than the values provided by the manufacturer. Thus, it was concluded that the nanoparticles' size had changed, most probably during the storage time. To verify this assumption, a third nanoparticle sample was investigated. For this purpose, a synthesized and well characterized platinum nanoparticle sample was used. For these particles, a mean diameter of 63 nm was determined using TEM. For the on-line ID-MDG-sp-ICP-ToF-MS measurements, a Pt isotope ratio (194Pt/195Pt) above 6 was adjusted; the on-line ID-MDG-sp-ICP-ToF-MS approach delivered a diameter of 64 nm ± 1 nm. Thus, a perfect agreement between TEM and on-line ID-MDG-sp-ICP-ToF-MS results could be shown. The results are within the same range as the “classical” sp-ICP-MS approach, but with less effort and steps and therefore this method is less prone to errors. Also, the stability, identity, and size of nanoparticles, which are applied for calibration of the transport efficiency, are difficult to check and control, since usually another technique and/or instrument (e.g., TEM) is needed. Whereas the concentration and isotope ratio of the spike solution can be determined relatively easily.
To test the matrix robustness of the proposed method sodium chloride was added to samples containing the spike solution and platinum nanoparticles with the nominal diameter of 50 nm. A mixed 194Pt/195Pt isotopic ratio of the natural platinum nanoparticles and the platinum spike of a ratio of about 10 was adjusted in accordance with the previous findings. The calculated diameter for the samples were 42 nm ± 1 nm for the sample without added sodium chloride and 42 nm ± 1 nm for the test solutions with added sodium chloride at three different concentration levels: 1 mg L−1, 10 mg L−1 and 100 mg L−1. The determined diameter of the Pt nanoparticles was constant even at the highest sodium chloride concentration. However, the formation of sodium chloride crystals at the tip of the nozzle of the MDG was observed, therefore higher sodium chloride concentrations were not tested. The obtained results illustrate the matrix robustness of the proposed method within the tested range. As the results suggest, the on-line ID-MDG-sp-ICP-ToF-MS approach is suitable to characterize the size of nanoparticles also in heavy (environmental) matrices, with little to no sample preparation.
Conclusion
The combination of on-line ID, MDG and sp-ICP-ToF-MS is fit for purpose for nanoparticle size characterization. With higher reproducibility and better characterization capabilities of the droplets produced by the MDG the precise determination of the nanoparticles' size is enabled by on-line ID. With on-line ID for the size determination of nanoparticles, TE as well as calibration curve determination becomes redundant. Our new approach is highly reproducible and robust against matrix effects – the latter is of particular interest for environmental samples, i.e., diluted sea water. In addition, the ToF mass analyzer has no limit in the number of simultaneously detectable isotopes/elements, thus, more than one type of nanoparticles can be characterized simultaneously – provided that the element under investigation comprises at least two interference free and stable isotopes. In addition, this work lays the foundation for the characterization of multi-element NPs as well as for uncertainty calculations, which would allow this technique as complementary tool for the certification of candidate materials.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors want to thank Andreas F. Thünemann and Tina Rybak (Federal Institute for Materials Research and Testing (BAM), Division 6.5 – Synthesis and Scattering of Nanostructured Materials) for providing the platinum nanoparticles. Furthermore, the authors want to thank the German Federal Ministry for Economic Affairs and Climate Action (BMWK) for the financial support.References
- Q. Bai, Y. Yin, Y. Liu, H. Jiang, M. Wu, W. Wang, Z. Tan, J. Liu, M. H. Moon and B. Xing, Appl. Spectrosc. Rev., 2021, 1–22 CrossRef.
- R. D. Handy and B. J. Shaw, Health Risk Soc., 2007, 9, 125–144 CrossRef.
- R. D. Handy, F. Von der Kammer, J. R. Lead, M. Hassellöv, R. Owen and M. Crane, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS PubMed.
- European Commission, Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU), 2011 Search PubMed.
- S. Fernández-Trujillo, M. Jiménez-Moreno, Á. Ríos and R. d. C. R. Martín-Doimeadios, J. Anal. At. Spectrom., 2021, 36, 528–534 RSC.
- M. D. Montano, J. W. Olesik, A. G. Barber, K. Challis and J. F. Ranville, Anal. Bioanal. Chem., 2016, 408, 5053–5074 CrossRef CAS PubMed.
- C. Degueldre and P. Y. Favarger, Colloids Surf., A, 2003, 217, 137–142 CrossRef CAS.
- T. Nomizu, S. Kaneco, T. Tanaka, T. Yamamoto and H. Kawaguchi, Anal. Sci., 1993, 9, 843–846 CrossRef CAS.
- F. Laborda, A. C. Gimenez-Ingalaturre and E. Bolea, in Comprehensive Analytical Chemistry, Elsevier, 2021, vol. 93, pp. 35–67 Search PubMed.
- S. Gschwind, L. Flamigni, J. Koch, O. Borovinskaya, S. Groh, K. Niemax and D. Günther, J. Anal. At. Spectrom., 2011, 26, 1166–1174 RSC.
- K. Mehrabi, R. Kaegi, D. Günther and A. Gundlach-Graham, Environ. Sci.: Nano, 2021, 8, 1211–1225 RSC.
- L. Hendriks, A. Gundlach-Graham and D. Günther, J. Anal. At. Spectrom., 2019, 34, 1900–1909 RSC.
- L. Hendriks, A. Gundlach-Graham and D. Günther, Chimia, 2018, 72, 221–226 CrossRef CAS PubMed.
- O. Borovinskaya, S. Gschwind, B. Hattendorf, M. Tanner and D. Günther, Anal. Chem., 2014, 86, 8142–8148 CrossRef CAS PubMed.
- J. W. Olesik and S. E. Hobbs, Anal. Chem., 1994, 66, 3371–3378 CrossRef CAS.
- M. P. Dziewatkoski, L. B. Daniels and J. W. Olesik, Anal. Chem., 1996, 68, 1101–1109 CrossRef CAS PubMed.
- M. von der Au, O. Borovinskaya, L. Flamigni, K. Kuhlmeier, C. Büchel and B. Meermann, Algal Res., 2020, 49, 101964 CrossRef.
- S. Faßbender, M. von der Au, M. Koenig, J. Pelzer, C. Piechotta, J. Vogl and B. Meermann, Anal. Bioanal. Chem., 2021, 413, 5279–5289 CrossRef PubMed.
- A. Praetorius, A. Gundlach-Graham, E. Goldberg, W. Fabienke, J. Navratilova, A. Gondikas, R. Kaegi, D. Günther, T. Hofmann and F. von der Kammer, Environ. Sci.: Nano, 2017, 4, 307–314 RSC.
- L. Telgmann, C. Metcalfe and H. Hintelmann, J. Anal. At. Spectrom., 2014, 29, 1265–1272 RSC.
- C. A. Sötebier, D. J. Kutscher, L. Rottmann, N. Jakubowski, U. Panne and J. Bettmer, J. Anal. At. Spectrom., 2016, 31, 2045–2052 RSC.
- IUPAC Commission on Isotopic Abundances and Atomic Weights, (accessed February 15th 2022), https://ciaaw.org Search PubMed.
- H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, C. P. Higgins and J. F. Ranville, Anal. Chem., 2011, 83, 9361–9369 CrossRef CAS PubMed.
- J. D. Fassett and P. J. Paulsen, Anal. Chem., 1989, 61, 643A–[649A] CrossRef CAS.
- J. Vogl, J. Anal. At. Spectrom., 2007, 22, 475–492 RSC.
| This journal is © The Royal Society of Chemistry 2022 |