Ionic liquids or eutectic solvents? Identifying the best solvents for the extraction of astaxanthin and β-carotene from Phaffia rhodozyma yeast and preparation of biodegradable films†
Cassamo U.
Mussagy
 *ab,
Valéria C.
Santos-Ebinuma
b,
Rondinelli D.
Herculano
bc,
João A. P.
Coutinho
*ab,
Valéria C.
Santos-Ebinuma
b,
Rondinelli D.
Herculano
bc,
João A. P.
Coutinho
 d,
Jorge F. B.
Pereira
d,
Jorge F. B.
Pereira
 *e and
Adalberto
Pessoa
Jr.
a
*e and
Adalberto
Pessoa
Jr.
a
aDepartment of Pharmaceutical-Biochemical Technology, School of Pharmaceutical Sciences, University of São Paulo, São Paulo, Brazil. E-mail: cassamo.mussagy@unesp.br; jfbpereira@eq.uc.pt
bDepartment of Engineering of Bioprocesses and Biotechnology, School of Pharmaceutical Sciences, São Paulo State University (UNESP), Araraquara 14800-903, São Paulo, Brazil
cTerasaki Institute for Biomedical Innovation (TIBI), 11570 West Olympic Boulevard, Los Angeles, CA 90064, USA
dCICECO-Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193, Aveiro, Portugal
eUniv. Coimbra, CIEPQPF, Department of Chemical Engineering, Rua Sílvio Lima, Pólo II – Pinhal de Marrocos, 3030-790, Coimbra, Portugal
First published on 22nd November 2021
Abstract
In order to replace conventional organic solvents with more benign equivalents such as ionic liquids or eutectic solvents, in this work we attempt to develop a simple and ecofriendly process using these alternative solvents for the extraction of astaxanthin and β-carotene from Phaffia rhodozyma biomass, which could simultaneously be used as a colorant and plasticizer agent for the preparation of bioactive starch-based biodegradable films without further purification. The use of cholinium-based eutectic solvents appears to be a promising solution envisioning the development of functionalized carotenoid-rich biofilms.
In nature, the most promising sources of carotenoids are microbes. However, among microorganisms, only microalgae (e.g. Haematococcus pluvialis) and yeast (e.g. Phaffia rhodozyma) demonstrate the ability to biosynthesize high levels of carotenoids with powerful antioxidant properties, such as astaxanthin.1P. rhodozyma NRRL Y-17268, certified as “generally recognized as safe” (GRAS),2 is an aerobic yeast able to convert carbon from agroindustrial residues into natural high value added astaxanthin and β-carotene3–5 (cf. chemical structures in Fig. 1). These carotenoids are two pigments with a large commercial value, being already applied in the feed, food, pharmaceutical and cosmeceutical industries,6,7 in particular, due to their beneficial biological properties such as antioxidant and anticancer activities.8–10 Since astaxanthin and β-carotene are biosynthesized intracellularly by P. rhodozyma, appropriate cell-disruption methodologies are required for their recovery.11,12 Traditionally, intracellular carotenoids are recovered from P. rhodozyma yeast using volatile organic compounds (VOCs), such as dimethyl sulfoxide (DMSO) and acetone, as cell-disrupting agents.13,14 Despite being highly efficient, these VOC-based extraction procedures have been regarded as “dangerous” to human health and “harmful” to the natural ecosystems.15 Coupled with those environmental and health concerns, the use of VOCs not only is highly energy-intensive (requiring high temperatures) but also exhibits low selectivity,8 encouraging a move towards greener and more sustainable alternative platforms,16 in particular, using more biocompatible, renewable (at least partially) and eco-friendly solvents.
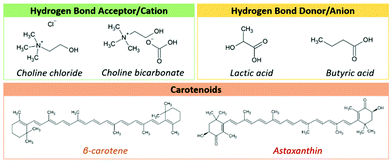 | ||
| Fig. 1 Chemical structures of carotenoids and precursors for the synthesis of ionic liquids and eutectic solvents. | ||
The development of sustainable, efficient, and eco-friendly extraction platforms has become a critical challenge for the scientific community and the industrial sector. As a consequence, over the last two decades, several studies have been proposing the use of ionic liquids (ILs) and eutectic solvents (ESs) as “ideal solvents” for the extraction of compounds from natural matrices, mainly due to their outstanding solvation properties.17–19 Interestingly, the successful use of biocompatible ILs and ESs containing cation/anion or hydrogen bond donor/acceptor (HBA/HBD), respectively, fully (or partially) derived from low-cost and eco-friendly renewable natural sources has been growing.20 Good examples of more biocompatible and less toxic alternative solvents include the cholinium-based ILs/ESs, whose cation or HBA is derived from the same precursor, i.e., choline, a compound that can be found in nature.21,22 Despite advances in using ILs and ESs as prominent “greener” solvents for the recovery of biomolecules from complex matrices,17,23 there is no clear evidence as to which of these two classes are in fact the “best” extraction solvents. Considering the specificity of each matrix, for efficient solvent selection, fundamental key parameters, i.e., the type of microorganism, biomass type, target compound characteristic and the technical-economic analysis, must also be considered.
In the search for designing alternative, greener and efficient extraction platforms, as well as finding the most efficient ones between ILs and ESs, this work provides a comprehensive study regarding the extraction abilities of cholinium-based ILs and equivalent ES counterparts for the selective recovery of astaxanthin and β-carotene from P. rhodozyma biomass and their application in the production of starch-based films. Therefore, we first evaluated the extraction performance of ILs and ESs synthesized with the same precursors (choline chloride/choline bicarbonate, lactic and butyric acid, cf. respective chemical structures in Fig. 1), comparing respective recovery yields with that of DMSO as a control and those of the precursors. Considering the highest recovery yield of ES composed of cholinium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyric acid ([Ch]Cl
butyric acid ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But), subsequently, the extraction performance was maximized as a function of the HBA
But), subsequently, the extraction performance was maximized as a function of the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio, water content and solid–liquid ratio (i.e., solvent-to-biomass ratio or wet cells concentration). Finally, ES extracts containing carotenoids were used as plasticizer agents for the formulation of cornstarch-based biodegradable films, demonstrating the industrial potential of using ESs for creating new functionalized bio-based materials for food packaging and other commercial applications.
HBD molar ratio, water content and solid–liquid ratio (i.e., solvent-to-biomass ratio or wet cells concentration). Finally, ES extracts containing carotenoids were used as plasticizer agents for the formulation of cornstarch-based biodegradable films, demonstrating the industrial potential of using ESs for creating new functionalized bio-based materials for food packaging and other commercial applications.
The performance of aqueous solutions of two ILs, i.e., choline lactate ([Ch][Lac]) and choline butanoate ([Ch][But]), and two ESs, i.e., choline chloride + lactic acid ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac) and choline chloride + butyric acid ([Ch]Cl
Lac) and choline chloride + butyric acid ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But) (in molar ratios of 1
But) (in molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
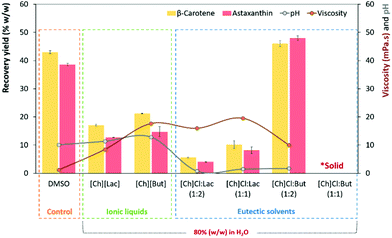
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), in the simultaneous extraction of intracellular astaxanthin and β-carotene from wet biomass of P. rhodozyma (at a concentration of 0.2 g mL−1) was screened after 1 h of stirring at 300 rpm and 65 °C (Fig. 2). All experimental protocols and detailed values are given in the Solid–liquid extraction of carotenoids using ILs and ESs section and Table S3 in the ESI,† respectively. Note that all the results in Fig. 2 and Table S3† are expressed as the extraction recovery yield (% w/w) relative to the initial content of carotenoids in the P. rhodozyma wet biomass, i.e., astaxanthin = 7.9 μg mLwet biomass−1 and β-carotene = 25.3 μg mLwet biomass−1.
2), in the simultaneous extraction of intracellular astaxanthin and β-carotene from wet biomass of P. rhodozyma (at a concentration of 0.2 g mL−1) was screened after 1 h of stirring at 300 rpm and 65 °C (Fig. 2). All experimental protocols and detailed values are given in the Solid–liquid extraction of carotenoids using ILs and ESs section and Table S3 in the ESI,† respectively. Note that all the results in Fig. 2 and Table S3† are expressed as the extraction recovery yield (% w/w) relative to the initial content of carotenoids in the P. rhodozyma wet biomass, i.e., astaxanthin = 7.9 μg mLwet biomass−1 and β-carotene = 25.3 μg mLwet biomass−1.
The results (p ≤ 0.05) depicted in Fig. 2 demonstrate that although the control (DMSO) and aqueous solutions (at 80% w/w) of ILs and ESs allow the recovery, at 65 °C, of at least 10% (w/w) of astaxanthin (red bars) and β-carotene (yellow bars), except for the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution, both carotenoid recovery yields were lower than the control (DMSO). Comparing the performance of ILs and ESs, significant differences (p ≤ 0.05) in the recovery of astaxanthin and β-carotene were found. The highest recovery yields of astaxanthin [47.9 ± 0.8 (w/w)] and β-carotene [46.0 ± 1.0 (w/w)] were achieved using an ES solution of ChCl
1) solution, both carotenoid recovery yields were lower than the control (DMSO). Comparing the performance of ILs and ESs, significant differences (p ≤ 0.05) in the recovery of astaxanthin and β-carotene were found. The highest recovery yields of astaxanthin [47.9 ± 0.8 (w/w)] and β-carotene [46.0 ± 1.0 (w/w)] were achieved using an ES solution of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (at 80 w/w), with increases of ∼10% (w/w) compared to the control.
2) (at 80 w/w), with increases of ∼10% (w/w) compared to the control.
Despite the lower recovery yields of other IL/ES aqueous solutions, there seems to be a relationship between the nature of each solvent and its intrinsic capacity to extract each pigment. Specifically, the extraction of astaxanthin (red bars) using ESs was favoured by [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), while [Ch]Cl
2), while [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac (1
Lac (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1
1/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) always exhibited high selectivity for β-carotene (yellow bars). Both ILs also revealed high selectivity for β-carotene recovery. The change in the carotenoid selectivity using an ES solution of 80% (w/w) of [Ch]Cl
2) always exhibited high selectivity for β-carotene (yellow bars). Both ILs also revealed high selectivity for β-carotene recovery. The change in the carotenoid selectivity using an ES solution of 80% (w/w) of [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But seems to be a result of the increase in the relative hydrophobicity of HBD (log
But seems to be a result of the increase in the relative hydrophobicity of HBD (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of But = 0.78).24 In this case, since the hydroxyl and keto groups present in the astaxanthin molecule give it a complex polar-nonpolar-polar nature,25 the most suitable solvent for the recovery of astaxanthin from wet cells of P. rhodozyma is a mixture of polar (i.e., [Ch]Cl and water) and non-polar (i.e., butyric acid) components, that is, solvents with an “amphiphilic nature” seem to be effective solubilizing agents of wet biomass, as previously demonstrated by our research group.8
Kow of But = 0.78).24 In this case, since the hydroxyl and keto groups present in the astaxanthin molecule give it a complex polar-nonpolar-polar nature,25 the most suitable solvent for the recovery of astaxanthin from wet cells of P. rhodozyma is a mixture of polar (i.e., [Ch]Cl and water) and non-polar (i.e., butyric acid) components, that is, solvents with an “amphiphilic nature” seem to be effective solubilizing agents of wet biomass, as previously demonstrated by our research group.8
Previously some researchers reported that the pH and viscosity of solutions are crucial for the recovery of carotenoids.17,26 To infer these effects, the pH values and viscosities of each IL and ES solution were determined. As depicted in Fig. 2, there is no significant influence (p ≤ 0.05) of the pH parameter in the recovery of astaxanthin and β-carotene. The highest recoveries of both carotenoids were achieved using [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a solution with a pH value of 1.68, pH values similar to or lower than those of IL/ES solutions with a weaker extraction performance (i.e., pH values similar to [Ch]Cl
2), a solution with a pH value of 1.68, pH values similar to or lower than those of IL/ES solutions with a weaker extraction performance (i.e., pH values similar to [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac at 1
Lac at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and lower than those of [Ch][Lac] and [Ch][But] solutions). On the other hand, regarding the viscosity, it is widely recognized that the high viscosity of solutions limits the mass-transfer process, hindering the effect of solvents on the membrane permeation and consequently reducing the recovery of carotenoids from the intracellular environment.17 The results presented in Fig. 2 confirm a viscosity dependence, since the lowest recovery yields were obtained with more viscous ES solutions (i.e., [Ch]Cl
2 and lower than those of [Ch][Lac] and [Ch][But] solutions). On the other hand, regarding the viscosity, it is widely recognized that the high viscosity of solutions limits the mass-transfer process, hindering the effect of solvents on the membrane permeation and consequently reducing the recovery of carotenoids from the intracellular environment.17 The results presented in Fig. 2 confirm a viscosity dependence, since the lowest recovery yields were obtained with more viscous ES solutions (i.e., [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac), while the highest recovery was achieved with [Ch]Cl
Lac), while the highest recovery was achieved with [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But, which has a viscosity of 9.99 mPa s at 65 °C (about half the viscosity of [Ch]But). However, despite some influence of viscosity on recovery abilities, it is important to note that the highest recovery yields are mainly a result of the high relative hydrophobicity of the solvent, i.e., the more hydrophobic ES solutions ([Ch]Cl
But, which has a viscosity of 9.99 mPa s at 65 °C (about half the viscosity of [Ch]But). However, despite some influence of viscosity on recovery abilities, it is important to note that the highest recovery yields are mainly a result of the high relative hydrophobicity of the solvent, i.e., the more hydrophobic ES solutions ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But) (as well as DMSO) have the greatest capacity to recover astaxanthin and β-carotene from P. rhodozyma wet cells (Fig. 2).
But) (as well as DMSO) have the greatest capacity to recover astaxanthin and β-carotene from P. rhodozyma wet cells (Fig. 2).
The importance of HBD as a key parameter to control carotenoid recovery yields was revealed. Thus, to gain a deeper understanding of the relative influence of the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio, the extraction of astaxanthin and β-carotene using concentrated (80% w/w) solutions of different [Ch]Cl
HBD molar ratio, the extraction of astaxanthin and β-carotene using concentrated (80% w/w) solutions of different [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But ESs (molar ratios of 1
But ESs (molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
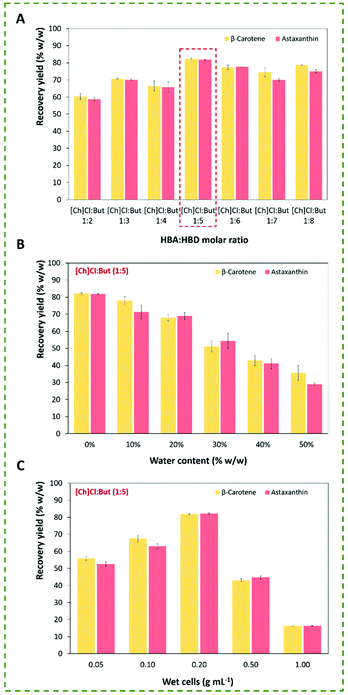
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) was performed. This experimental step was carried out during 1 h of stirring at 300 rpm and 65 °C, using a concentration of 0.2 g mL−1of P. rhodozyma wet biomass (yeast cells). The respective recovery yields (% w/w) of astaxanthin and β-carotene are shown in Fig. 3A and detailed in Table S4 in the ESI.†
8) was performed. This experimental step was carried out during 1 h of stirring at 300 rpm and 65 °C, using a concentration of 0.2 g mL−1of P. rhodozyma wet biomass (yeast cells). The respective recovery yields (% w/w) of astaxanthin and β-carotene are shown in Fig. 3A and detailed in Table S4 in the ESI.†
As shown in Fig. 3A, the effect of the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio of the concentrated [Ch]Cl
HBD molar ratio of the concentrated [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But solution was analysed, specifically by evaluating how the increase in HBD alters the selective recovery of carotenoids. Interestingly, there is a clear effect of the HBA
But solution was analysed, specifically by evaluating how the increase in HBD alters the selective recovery of carotenoids. Interestingly, there is a clear effect of the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio not only on increasing the extraction performance, but also on the selectivity of carotenoids. Regarding the extraction performance, the recovery yield was maximized by increasing the HBA
HBD molar ratio not only on increasing the extraction performance, but also on the selectivity of carotenoids. Regarding the extraction performance, the recovery yield was maximized by increasing the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio up to 1
HBD molar ratio up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, achieving values of 82.2% ± 0.4 and 81.8% ± 0.3 (w/w) for β-carotene and astaxanthin, respectively. Subsequent increases of HBD concentration (i.e., 1
5, achieving values of 82.2% ± 0.4 and 81.8% ± 0.3 (w/w) for β-carotene and astaxanthin, respectively. Subsequent increases of HBD concentration (i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 1
7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) were not favourable, particularly for astaxanthin recovery. Despite similar selectivity performance from 1
8) were not favourable, particularly for astaxanthin recovery. Despite similar selectivity performance from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio (recovery of β-carotene > astaxanthin), Fig. 3-A demonstrates a change in the carotenoid extraction profile at a molar ratio of 1
4 molar ratio (recovery of β-carotene > astaxanthin), Fig. 3-A demonstrates a change in the carotenoid extraction profile at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (recovery of β-carotene = astaxanthin, p ≤ 0.05), from which an increase of HBD (from 1
5 (recovery of β-carotene = astaxanthin, p ≤ 0.05), from which an increase of HBD (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 1
6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratio) turns the ES solution more selective for recovery of β-carotene. Together, these results confirm that the increase in the relative hydrophobicity of ESs favours the extraction of the more hydrophobic carotenoid (β-carotene), while an amphiphilic character of ES is desirable to maximize the recovery yields of both intracellular carotenoids from wet yeast biomass.
8 molar ratio) turns the ES solution more selective for recovery of β-carotene. Together, these results confirm that the increase in the relative hydrophobicity of ESs favours the extraction of the more hydrophobic carotenoid (β-carotene), while an amphiphilic character of ES is desirable to maximize the recovery yields of both intracellular carotenoids from wet yeast biomass.
Concerning water influence (only evaluated for [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But 1
But 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), as shown in Fig. 3B (detailed values in Table S5 in the ESI†), the recovery yields linearly decreased with the increase of water concentration in the [Ch]Cl
5), as shown in Fig. 3B (detailed values in Table S5 in the ESI†), the recovery yields linearly decreased with the increase of water concentration in the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But solution, achieving decreases of 50% in the extraction performance with a solution with 50% of water. As expected, the treatment with concentrated [Ch]Cl
But solution, achieving decreases of 50% in the extraction performance with a solution with 50% of water. As expected, the treatment with concentrated [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But confers a more hydrophobic character to the solvent enhancing the solubilization of the carotenoids and the weakening of the cell structure. At this point, it is important to emphasize the importance of balancing diffusion and solubility, i.e., although more diluted solutions are less viscous benefiting the diffusion of the carotenoids, the negative influence on increasing the solvent polarity confirms the importance of ensuring an adequate hydrophobicity of the solvent for the solubilization of the two carotenoids.
But confers a more hydrophobic character to the solvent enhancing the solubilization of the carotenoids and the weakening of the cell structure. At this point, it is important to emphasize the importance of balancing diffusion and solubility, i.e., although more diluted solutions are less viscous benefiting the diffusion of the carotenoids, the negative influence on increasing the solvent polarity confirms the importance of ensuring an adequate hydrophobicity of the solvent for the solubilization of the two carotenoids.
From an industrial perspective, the solid–liquid ratio (SLR) is also a crucial parameter for designing cell disrupting procedures to recover intracellular molecules from microbial biomass. Therefore, to identify the optimal SLR (i.e., P. rhodozyma wet biomass/ES solution volume), the ES solution with higher extraction abilities ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But at 1
But at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio) at five different concentrations (0.05, 0.10, 0.20, 0.50 and 1.00) were tested to maximize the recovery of astaxanthin and β-carotene. As shown Fig. 3C (detailed values are listed in Table S6 in the ESI†), the concentration of both carotenoids (astaxanthin and β-carotene) increased up to a concentration of 0.2 g mL−1 of P. rhodozyma wet cells, from which the extraction abilities decreased due to the solubility limits of carotenoids in the ES solution.
5 molar ratio) at five different concentrations (0.05, 0.10, 0.20, 0.50 and 1.00) were tested to maximize the recovery of astaxanthin and β-carotene. As shown Fig. 3C (detailed values are listed in Table S6 in the ESI†), the concentration of both carotenoids (astaxanthin and β-carotene) increased up to a concentration of 0.2 g mL−1 of P. rhodozyma wet cells, from which the extraction abilities decreased due to the solubility limits of carotenoids in the ES solution.
A general analysis of Fig. 3 confirms the potential of ILs and ESs (mainly But-based ones) as “greener” solvents for the recovery of intracellular carotenoids from microbial matrices, with clear indications that the use of eutectic mixtures is preferable. In sequence, to obtain a comprehensive understanding of the extraction performance of the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
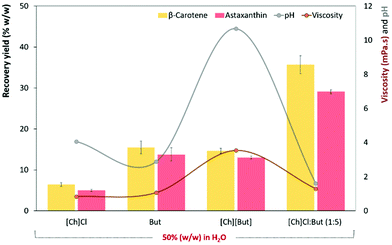
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But ES solution, equivalent IL ([Ch][But]) and respective ES precursor ([Ch]Cl and butyric acid), aqueous solutions of these components were used for the selective recovery of astaxanthin and β-carotene from P. rhodozyma biomass. Note that in this set of experiments, all solutions were prepared at 50% (w/w) due to the solubility limits of [Ch]Cl, while all other conditions were similar to those of previous experiments (a wet cell concentration of 0.2 g mL−1, in 1 h of stirring at 300 rpm, and 65 °C). As shown in Fig. 4 (detailed values are provided in Table S7 in the ESI†), the aqueous solution of [Ch]Cl, the most hydrophilic, has the lowest extraction ability (5.0% ± 0.3 and 6.4% ± 0.5 of astaxanthin and β-carotene, respectively), with intermediate yields (about 15% for both carotenoids) being obtained for butyric acid and [Ch][But] solutions, and the highest astaxanthin and β-carotene recovery yields achieved with the [Ch]Cl
But ES solution, equivalent IL ([Ch][But]) and respective ES precursor ([Ch]Cl and butyric acid), aqueous solutions of these components were used for the selective recovery of astaxanthin and β-carotene from P. rhodozyma biomass. Note that in this set of experiments, all solutions were prepared at 50% (w/w) due to the solubility limits of [Ch]Cl, while all other conditions were similar to those of previous experiments (a wet cell concentration of 0.2 g mL−1, in 1 h of stirring at 300 rpm, and 65 °C). As shown in Fig. 4 (detailed values are provided in Table S7 in the ESI†), the aqueous solution of [Ch]Cl, the most hydrophilic, has the lowest extraction ability (5.0% ± 0.3 and 6.4% ± 0.5 of astaxanthin and β-carotene, respectively), with intermediate yields (about 15% for both carotenoids) being obtained for butyric acid and [Ch][But] solutions, and the highest astaxanthin and β-carotene recovery yields achieved with the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) aqueous solution. These results confirm that more than the influences of pH and viscosity (no relationship of these parameters with recovery yields) is the nature of the [Ch]Cl
5) aqueous solution. These results confirm that more than the influences of pH and viscosity (no relationship of these parameters with recovery yields) is the nature of the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But ES, which is the key for maximizing the recovery of astaxanthin and β-carotene. In fact, the increase in the extraction abilities using ESs in aqueous media seems to be a result of a hydrotropic mechanism (ability to boost the aqueous solubility of hydrophobic solutes23) of [Ch]Cl
But ES, which is the key for maximizing the recovery of astaxanthin and β-carotene. In fact, the increase in the extraction abilities using ESs in aqueous media seems to be a result of a hydrotropic mechanism (ability to boost the aqueous solubility of hydrophobic solutes23) of [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Recent experimental works have confirmed that hydrotropy is a key mechanism behind the outstanding ability of ESs to solubilize/extract hydrophobic molecules.27,28 Furthermore, as microbial biomass is “wet”, the presence of a minimal amount of water is crucial to aid in the extraction of intracellular molecules. Recently, we8,15 demonstrated that the presence of water in solvent mixtures not only facilitates the miscibility of solvents with the wet biomass but also helps in permeabilization/disintegration of the yeast cell wall.
5). Recent experimental works have confirmed that hydrotropy is a key mechanism behind the outstanding ability of ESs to solubilize/extract hydrophobic molecules.27,28 Furthermore, as microbial biomass is “wet”, the presence of a minimal amount of water is crucial to aid in the extraction of intracellular molecules. Recently, we8,15 demonstrated that the presence of water in solvent mixtures not only facilitates the miscibility of solvents with the wet biomass but also helps in permeabilization/disintegration of the yeast cell wall.
As noted above, the results depicted in Fig. 4 reinforce the notion that pH and even viscosity does not have (or have a low) influence (p ≤ 0.05) on the recovery of astaxanthin and β-carotene. For example, aqueous solutions of [Ch]Cl (pH = 4.06 and viscosity = 0.84 mPa s at 65 °C) and But (pH = 2.88 and viscosity = 1.07 mPa s at 65 °C) do not extract the same carotenoids as [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (pH = 1.61 and viscosity = 1.30 mPa s at 65 °C) even with similar acidity values and lower viscosity.
5) (pH = 1.61 and viscosity = 1.30 mPa s at 65 °C) even with similar acidity values and lower viscosity.
To implement a circular and sustainable industrial ES-based extraction platform, the recovery of astaxanthin and β-carotene from [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and solvent recycling arise as the most problematic/challenging issues, mainly due to the low vapour pressure of [Ch]Cl and But.29 Fortunately, there are successful studies for the polishing of carotenoids and recovery of the ES and IL solutions, including, for example, three-phase partition systems induced by precipitation (with antisolvents or strong salting-out salts)17 or using adsorptive membranes.30 However, in this particular case, due to the naturally biocompatible character of ES and excellent biological activities of carotenoids, we propose to combine both as green alternatives to obtain new commercial bioproducts/biomaterials. Therefore, due to the acidic character of the [Ch]Cl
5) and solvent recycling arise as the most problematic/challenging issues, mainly due to the low vapour pressure of [Ch]Cl and But.29 Fortunately, there are successful studies for the polishing of carotenoids and recovery of the ES and IL solutions, including, for example, three-phase partition systems induced by precipitation (with antisolvents or strong salting-out salts)17 or using adsorptive membranes.30 However, in this particular case, due to the naturally biocompatible character of ES and excellent biological activities of carotenoids, we propose to combine both as green alternatives to obtain new commercial bioproducts/biomaterials. Therefore, due to the acidic character of the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But solution rich in carotenoids, it can be used directly (“one-pot”) as a green plasticizer solution for biopolymers, e.g. chitosan or starch, and can produce environment friendly biodegradable active packaging films with high antioxidant activities.31 The process integration of the proposed technology for the recovery of astaxanthin and β-carotene from yeast biomass using [Ch]Cl
But solution rich in carotenoids, it can be used directly (“one-pot”) as a green plasticizer solution for biopolymers, e.g. chitosan or starch, and can produce environment friendly biodegradable active packaging films with high antioxidant activities.31 The process integration of the proposed technology for the recovery of astaxanthin and β-carotene from yeast biomass using [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) with a subsequent formulation of corn-starch biofilms (CS) was here attempted. The [Ch]Cl
5) with a subsequent formulation of corn-starch biofilms (CS) was here attempted. The [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) extract rich in astaxanthin and β-carotene was mixed with corn-starch according to the methodology described in the Preparation of biofilms section in the ESI.†
5) extract rich in astaxanthin and β-carotene was mixed with corn-starch according to the methodology described in the Preparation of biofilms section in the ESI.†
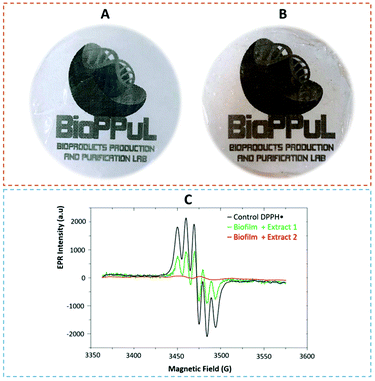
As depicted in Fig. 5A and B, a CS-based thermoplastic formulation was efficiently produced using [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But-rich carotenoids as green plasticizers. The FTIR spectra (Fig. S2 in the ESI†) obtained from the CS/[Ch]Cl
But-rich carotenoids as green plasticizers. The FTIR spectra (Fig. S2 in the ESI†) obtained from the CS/[Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But and CS/[Ch]Cl
But and CS/[Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But-rich carotenoids showed almost the same pattern. The strong peak (broad band at 3304 cm−1) observed in the biofilm spectrum corresponds to the hydrogen bonds formed by the hydroxyl groups of starch and ES. The tensile strength, elasticity modulus (Young's modulus) and elongation at break of the CS-based biofilms were determined as the main mechanical properties and to estimate the resistance to tensile ruptures. The values of the elasticity modulus (2.6 ± 0.1 MPa), tensile strength (1.0 ± 0.2 MPa) and elongation at break (70.0 ± 3.1%) show the material to be less rigid for the CS
But-rich carotenoids showed almost the same pattern. The strong peak (broad band at 3304 cm−1) observed in the biofilm spectrum corresponds to the hydrogen bonds formed by the hydroxyl groups of starch and ES. The tensile strength, elasticity modulus (Young's modulus) and elongation at break of the CS-based biofilms were determined as the main mechanical properties and to estimate the resistance to tensile ruptures. The values of the elasticity modulus (2.6 ± 0.1 MPa), tensile strength (1.0 ± 0.2 MPa) and elongation at break (70.0 ± 3.1%) show the material to be less rigid for the CS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ES ratio tested, supporting the plasticizer character of the solution. These values are in line with those obtained by Ibrahim et al.32 using fructose, sorbitol and urea as green plasticizers for the formulation of CS-based films.
ES ratio tested, supporting the plasticizer character of the solution. These values are in line with those obtained by Ibrahim et al.32 using fructose, sorbitol and urea as green plasticizers for the formulation of CS-based films.
Finally, in order to evaluate if the proposed integrated approach to produce CS-based biofilms is safe and does not affect the biological activity of astaxanthin and β-carotene, the antioxidant activity of the formulated biofilms was evaluated. For that purpose, the DPPH˙ radical scavenging method was performed to estimate the antioxidant activity of biofilms using electron paramagnetic resonance (EPR) spectroscopy, which directly measures the concentration of the DPPH˙ radical. As observed in Fig. 5C, the DPPH˙ solution exhibited a decrease in the EPR signal intensity after the addition of biofilms containing two-different concentrations of carotenoid-rich extracts, confirming that the biofilms preserve the original antioxidant properties of astaxanthin and β-carotene. Biofilms containing carotenoids revealed encouraging results in the DPPH˙ radical scavenging assay, with a percentage inhibition increased with the increase of the concentration of the extracts in biofilms, in which the CS-based biofilm containing 4 μg mL−1 of astaxanthin and 15 μg mL−1 of β-carotene [biofilm + extract 2 (Fig. 5C)] allowed the highest antioxidant activity (99%).
These results confirm that carotenoid-rich biofilms exhibit powerful antioxidant activities, which can be further used in the formulation of biodegradable active packaging films for food, pharmaceutical, nutraceutical and cosmeceutical purposes.
Conclusions
In this work, an integrated sustainable platform for the recovery of astaxanthin and β-carotene from P. rhodozyma biomass using cholinium-based ILs and ESs was evaluated. The results confirmed that an adequate hydrophobicity degree in solvents and the hydrotropic mechanism of ESs are crucial for the solubilization and recovery of the intracellular carotenoids from P. rhodozyma wet biomass (as shown by the highest recovery yields obtained with [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But). This work revealed why ESs can be better solvents than their IL counterparts. The HBA
But). This work revealed why ESs can be better solvents than their IL counterparts. The HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD ratio of ESs influenced the recovery yields and selectivity of carotenoids, with [Ch]Cl
HBD ratio of ESs influenced the recovery yields and selectivity of carotenoids, with [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But at 1
But at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio being the “best” for the simultaneous extraction of astaxanthin and β-carotene. Furthermore, this work shows the potential of using the [Ch]Cl
5 molar ratio being the “best” for the simultaneous extraction of astaxanthin and β-carotene. Furthermore, this work shows the potential of using the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But (1
But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) extract rich in carotenoids directly as a plasticizer agent for the preparation of corn-starch biodegradable active biofilms. The antioxidant potential of biofilms confirmed that this extraction–formulation integrative [Ch]Cl
5) extract rich in carotenoids directly as a plasticizer agent for the preparation of corn-starch biodegradable active biofilms. The antioxidant potential of biofilms confirmed that this extraction–formulation integrative [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) But-based platform is “pigment-friendly” (i.e., without negative effects on their biological properties). These findings demonstrate that acidic ES extracts containing bioactive molecules can be directly used for the development of new commercial products without separation/polishing of pigments from extractant agents, also opening a new environmentally friendly and biocompatible alternative for the cost-effective and efficient production of new biomaterials.
But-based platform is “pigment-friendly” (i.e., without negative effects on their biological properties). These findings demonstrate that acidic ES extracts containing bioactive molecules can be directly used for the development of new commercial products without separation/polishing of pigments from extractant agents, also opening a new environmentally friendly and biocompatible alternative for the cost-effective and efficient production of new biomaterials.
Author contributions
Cassamo U. Mussagy: conceptualization, investigation, formal analysis, data curation, writing – original draft preparation, writing – review & editing, and visualization. Valéria C. Santos-Ebinuma: conceptualization and writing – review & editing. Rondinelli D. Herculano: conceptualization and writing – review & editing. João A. P. Coutinho: conceptualization and writing – review & editing. Jorge F. B. Pereira: conceptualization, writing – review & editing, and visualization. Adalberto Pessoa Jr.: writing – review & editing, visualization, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by grants from the FAPESP (São Paulo Research Foundation Brazil) through the projects, 2020/08655-0, 2019/15493-9, 2018/06908-8 and 2015/11759-3, and by projects CIEPQPF (UIDB/EQU/00102/2020 and UIDP/EQU/00102/2020) and the CICECO-Aveiro Institute of Materials (UIDB/50011/2020 and UIDP/50011/2020), financed by national funds through the Portuguese Foundation for Science and Technology/MCTES.References
- C. U. Mussagy, J. F. B. Pereira, L. Dufossé, V. Raghavan, V. C. Santos-Ebinuma and A. Pessoa, Crit. Rev. Food Sci. Nutr., 2021a, 1–15 Search PubMed.
- FDA, U.S Food & Drug Administration, https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices, (accessed 28 July 2021).
- J. Zhang, Q. Li, J. Liu, Y. Lu, Y. Wang and Y. Wang, Bioresour. Technol., 2020, 311, 123525 CrossRef CAS.
- A. R. Domínguez-Bocanegra, T. Ponce-Noyola and J. A. Torres-Muñoz, Appl. Microbiol. Biotechnol., 2007, 75, 783–791 CrossRef.
- A. J. Nathan and A. Scobell, Foreign Aff., 2012, 91, 1–49 Search PubMed.
- C. Mussagy, S. Khan and A. M. Kot, Crit. Rev. Food Sci. Nutr., 2021b, 1–15 Search PubMed.
- C. Mussagy, J. Winterburn, V. C. Santos-Ebinuma and J. F. B. Pereira, Appl. Microbiol. Biotechnol., 2019, 103, 1095–1114 CrossRef CAS PubMed.
- C. Mussagy, D. Remonatto, A. V. Paula, R. D. Herculano, V. C. Santos-Ebinuma, J. A. P. Coutinho and J. F. B. Pereira, Sep. Purif. Technol., 2021, 266, 118548 CrossRef CAS.
- K. S. Liao, C. L. Wei, J. C. Chen, H. Y. Zheng, W. C. Chen, C. H. Wu, T. J. Wang, Y. S. Peng, P. Y. Chang and Y. W. Lin, Regul. Toxicol. Pharmacol., 2016, 81, 353–361 CrossRef CAS PubMed.
- X. Ao and I. H. Kim, Poult. Sci., 2019, 98, 4954–4960 CrossRef CAS PubMed.
- A. H. Batghare, N. Singh and V. S. Moholkar, Bioresour. Technol., 2018, 254, 166–173 CrossRef CAS PubMed.
- H. Schewe, A. Kreutzer, I. Schmidt, C. Schubert and J. Schrader, Biotechnol. Bioprocess Eng., 2017, 22, 319–326 CrossRef CAS.
- T. Gervasi, A. Santini, P. Daliu, A. Z. M. Salem, C. Gervasi, V. Pellizzeri, L. Barrega, P. De Pasquale, G. Dugo and N. Cicero, Agrofor. Syst., 2020, 94, 1229–1234 CrossRef.
- Y. Zhuang, G.-L. Jiang and M.-J. Zhu, Process Biochem., 2020, 91, 330–338 CrossRef CAS.
- C. U. Mussagy, V. C. Santos-Ebinuma, K. A. Kurnia, A. C. R. V. Dias, P. Carvalho, J. A. P. Coutinho and J. F. B. Pereira, Green Chem., 2020, 22, 8478–8494 RSC.
- M. González-Miquel and I. Díaz, Curr. Opin. Green Sustainable Chem., 2021, 29, 100469 CrossRef.
- C. U. Mussagy, V. C. Santos-Ebinuma, M. Gonzalez-Miquel, J. A. P. Coutinho and J. F. B. Pereira, ACS Sustainable Chem. Eng., 2019, 7, 16765–16776 CrossRef CAS.
- C. A. Suarez Ruiz, M. Martins, J. A. P. Coutinho, R. H. Wijffels, M. H. M. Eppink, C. van den Berg and S. P. M. Ventura, Chem. Eng. J., 2020, 399, 125683 CrossRef CAS.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860 RSC.
- H. Vanda, Y. Dai, E. G. Wilson, R. Verpoorte and Y. H. Choi, C. R. Chim., 2018, 21, 628–638 CrossRef CAS.
- D. J. S. Patinha, L. C. Tomé, C. Florindo, H. R. Soares, A. S. Coroadinha and I. M. Marrucho, ACS Sustainable Chem. Eng., 2016, 4, 2670–2679 CrossRef CAS.
- J. I. Santos, A. M. M. Gonçalves, J. L. Pereira, B. F. H. T. Figueiredo, F. A. e. Silva, J. A. P. Coutinho, S. P. M. Ventura and F. Gonçalves, Green Chem., 2015, 17, 4657–4668 RSC.
- E. S. Morais, P. V. Mendonça, J. F. J. Coelho, M. G. Freire, C. S. R. Freire, J. A. P. Coutinho and A. J. D. Silvestre, ChemSusChem, 2018, 11, 753–762 CrossRef CAS PubMed.
- Chemspider, http://www.chemspider.com/, (accessed 29 July 2021).
- I. Schmidt, H. Schewe, S. Gassel, C. Jin, J. Buckingham, M. Hümbelin, G. Sandmann and J. Schrader, Appl. Microbiol. Biotechnol., 2011, 89, 555–571 CrossRef CAS PubMed.
- F. Qu, N. Gong, S. Wang, Y. Gao, C. Sun, W. Fang and Z. Men, Dyes Pigm., 2020, 173, 107975 CrossRef CAS.
- D. O. Abranches, L. P. Silva, M. A. R. Martins and J. A. P. Coutinho, J. Chem. Phys., 2021, 155, 034501 CrossRef CAS.
- J. P. Wojeicchowski, D. O. Abranches, A. M. Ferreira, M. R. Mafra and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2021, 9, 10240–10249 CrossRef CAS.
- S.-H. Wu, A. R. Caparanga, R. B. Leron and M.-H. Li, Thermochim. Acta, 2012, 544, 1–5 CrossRef CAS.
- L. Wang, J. Hu, W. Lv, W. Lu, D. Pei, Y. Lv, W. Wang, M. Zhang, R. Ding and M. Lv, Food Chem., 2021, 363, 130369 CrossRef CAS PubMed.
- V. Chandra Roy, T. C. Ho, H.-J. Lee, J.-S. Park, S. Y. Nam, H. Lee, A. T. Getachew and B.-S. Chun, J. Cleaner Prod., 2021, 284, 125417 CrossRef CAS.
- M. I. J. Ibrahim, S. M. Sapuan, E. S. Zainudin and M. Y. M. Zuhri, Int. J. Food Prop., 2019, 22, 925–941 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03521e |
| This journal is © The Royal Society of Chemistry 2022 |