 Open Access Article
Open Access ArticleThe gut barrier and the fate of engineered nanomaterials: a view from comparative physiology†
Meike
van der Zande
 *a,
Anita
Jemec Kokalj
*a,
Anita
Jemec Kokalj
 b,
David J.
Spurgeon
b,
David J.
Spurgeon
 c,
Susana
Loureiro
c,
Susana
Loureiro
 d,
Patrícia V.
Silva
d,
Zahra
Khodaparast
d,
Damjana
Drobne
b,
Nathaniel J.
Clark
d,
Patrícia V.
Silva
d,
Zahra
Khodaparast
d,
Damjana
Drobne
b,
Nathaniel J.
Clark
 e,
Nico W.
van den Brink
e,
Nico W.
van den Brink
 f,
Marta
Baccaro
f,
Marta
Baccaro
 f,
Cornelis A. M.
van Gestel
f,
Cornelis A. M.
van Gestel
 g,
Hans
Bouwmeester
af and
Richard D.
Handy
g,
Hans
Bouwmeester
af and
Richard D.
Handy
 *e
*e
aWageningen Food Safety Research part of Wageningen University & Research, Akkermaalsbos 2, 6708 WB, Wageningen, The Netherlands. E-mail: meike.vanderzande@wur.nl
bUniversity of Ljubljana, Biotechnical Faculty, Jamnikarjeva 101, 1000 Ljubljana, Slovenia
cUK Centre for Ecology and Hydrology, MacLean Building, Benson Lane, Wallingford, Oxon Ox10 8BB, UK
dUniversity of Aveiro, Department of Biology and CESAM- Centre for Environmental and Marine Studies, 3830-193 Aveiro, Portugal
eSchool of Biological and Marine Sciences, University of Plymouth, UK. E-mail: rhandy@plymouth.ac.uk
fDivision of Toxicology, Wageningen University, Stippeneng 4, 6708 WE, Wageningen, The Netherlands
gDepartment of Ecological Science, Vrije Universiteit, De Boelelaan 1085, 1018 HV Amsterdam, The Netherlands
First published on 27th April 2020
Abstract
The structure of the gut barrier and luminal chemistry in non-mammalian vertebrates and invertebrates has been given little attention with respect to the dietary uptake of engineered nanomaterials (ENMs). This review compares the diversity of gut anatomy in selected species used for regulatory toxicity testing, especially in relation to gut lumen chemistry and the behaviour of ENMs, and the gut as a barrier to ENMs. High ionic strength, the presence of divalent ions and organic matter promote particle aggregation in the lumen. The redox chemistry of the gut offers reducing conditions for ENM transformation, and corona formation will depend on the gut contents. Areas of low pH in the gut lumen in several species will promote the dissolution of metallic ENMs. There is a protective unstirred layer over the surface of the epithelium that may concentrate ENMs. Some organisms, especially vertebrates, can slough mucus to remove this adsorbed nanomaterial and lower bioavailability. Invertebrates also have protective layers of cuticle or peritrophic membranes that will modulate ENM uptake. Paracellular uptake of ENMs is unlikely. Transcellular uptake via vesicular-dependent pathways remains the most likely route across the gut epithelium. Most species have receptor-mediated endocytosis pathways and/or macropinocytosis in the gut epithelium. Crucially, many invertebrates have another potential pathway via ‘intracellular digestion’ uptake routes leading into the gut epithelium, and with gut associated immune cells being a potential route for ENM translocation across the epithelium. The basal lamina provides another barrier prior to the internal compartments of many animals. The features of the gut lumen and epithelium can limit the uptake of ENMs across the gut barrier in vivo, although some ENMs are detected in the tissues. Invertebrates also have the ability for biogenic mineral formation at the nano scale inside tissues. In conclusion, despite the diverse structural anatomies of the gut barrier of animals, some common features in the gut lumen chemistry tend to promote particle aggregation and settling onto the gut surface. The functional anatomy ensures the gut remains a formidable barrier to ENMs, and with some potential novel uptake processes in invertebrates that are not present in vertebrate animals.
Environmental significanceThe bioaccumulation of engineered nanomaterials (ENMs) through aquatic or terrestrial food webs is a concern. However, the diverse structure of the gut barrier and luminal chemistry in the animal kingdom has been given little attention with respect to ENMs. A few key factors such as luminal pH, redox chemistry, ionic strength, and the organic matter in the gut enable some cross-species consideration of the hazard of ENMs. Differences in gut structure also achieve a functional physiology where the gut is likely to be a barrier to ENMs. Most species have receptor-mediated endocytosis pathways and/or macropinocytosis in the gut epithelium, but invertebrates are also at extra risk due to additional uptake routes involving cells that are used for ‘intracellular digestion.’ |
1. Introduction
The use of engineered nanomaterials (ENMs) has increased exponentially in the last decade, with the materials finding new applications in a wide variety of industrial sectors. Inevitably, ENMs are predicted to be released into the environment and exposure modelling suggests that ENMs and/or their transformation products will be found in all the major environmental compartments (i.e., air, water and soil).1 The predicted environmental concentrations in surface waters in Europe are around the μg L−1 level or less,2–4 and at μg kg−1 concentrations in soils,5 especially where sludge disposal to agricultural land occurs. Recent measurements in surface waters, at least for a few ENMs so far, are broadly consistent with the predicted concentrations.3,6 Thus, it is expected that biota will routinely be exposed to parts per million (microgram) concentrations of ENMs in the long term.In the environment, ENMs are unlikely to remain in their pristine ‘as produced’ state, but will usually be subject to both chemical and physical transformations. These transformations can include alteration of the particle's composition, including the surface of the particle core, or any surface coatings on the ENMs; with subsequent effects on the agglomeration and aggregation behaviours of the materials, and/or dissolution of the particles, as well as changes in their chemical reactivity.3,7,8 Data suggests that these nanomaterial transformation processes are determined by both the physicochemical properties of the ENMs and the characteristics of their environment that are important in the behaviour of colloids (e.g., pH, ionic strength, the presence of natural organic matter).7,9,10 So, as is the case for traditional chemicals, it is expected that the environmental conditions will influence the fate and behaviour of ENMs in the ecosystem of concern. It has also been suggested that the chemical reactivity and colloid behaviours of ENMs in the environment will also influence their bioavailability to organisms, especially at critical external barriers such as the gills of fishes,11 or the human lung.12,13
However, the gut as a barrier has been given less attention, despite concerns that dietary exposure is likely a main route of exposure to wildlife. Aquatic mesocosm studies have shown that ENMs are deposited in sediments and biofilms resulting in contamination of the base of the food web.14,15 The trophic transfer of ENMs from primary producers to aquatic invertebrates has also been demonstrated in the laboratory.16,17 Fishes will also eat food contaminated with ENMs (TiO2,18 Ag,19,20 ZnO, carbon nanotubes,21). Similarly, in terrestrial ecosystems, invertebrates such as earthworms will ingest soil contaminated with ENMs,22–24 and potentially initiate a food chain hazard to predators.25 However, studies on the ecotoxicity and bioaccumulation of ENMs have been criticised for focussing on a few organisms that are used in regulatory toxicity tests.26,27 In contrast, the essence of legislation on environmental protection has always been to ‘protect most of the organisms most of the time’, and so some consideration of the vast biodiversity of wildlife is needed with respect to ENMs. There are far too many taxonomic groups and species to address concerns organism by organism. Instead, from the viewpoint of comparative physiology, biodiversity can be rationalised into a handful of basic body designs where form (structure) and biological function (physiology) are in harmony. The concept of ‘form and function’ is readily extended to toxicology, where adverse alterations in structure (pathology) and function (pathophysiology) informs on the hazard.
The overall aim of the current review is to focus on the importance of the gut as a main biological barrier to ENM uptake by wildlife, and to address those concerns for a wide variety of organisms by also considering the ‘body plan’ which in traditional comparative physiology refers to the ‘structure’ of the body and the arrangement of any tissues or organs therein, and how the structure of the organism relates to function. The specific objectives are to: (i) explore bioavailability in the gut lumen from the view point of diverse luminal chemistries of organisms and colloid theory, (ii) highlight the concerns for ENM uptake across the gut epithelium for different designs of gut anatomy, (iii) consider the fate after crossing the gut barrier with respect to the different body plans of animals and the internal organs. The ‘gut barrier’ is often considered in terms of the physical barrier of the intact epithelium that separates the external environment from the underlying tissue and the internal body fluids or equivalent serosal compartment. Here we also take a physiological approach, where, functionally, the various extracellular matrices that form layers in the lumen (e.g., the cuticle of invertebrates, mucus secretions of vertebrates) contribute to the overall barrier properties and the characteristics of uptake of substances across the gut.
2. Gut lumen chemistry and the bioavailability of engineered nanomaterials
A prerequisite to uptake or toxicity is that the external surfaces of the organism are first exposed to the hazardous substance. This notion has been applied to ENMs at the surface of fish gills,11,28 the human respiratory epithelium,13 and to some extent to the mammalian or human gastrointestinal tract.29,30 However, the bioaccessible fraction(s) of ENMs in the diverse gut chemistry of biota, and subsequent bioavailability to the tissue, has been given much less attention. The key steps in the uptake of an ENM across gut epithelium include the: (i) movement of the ENM by diffusion and other behaviours from the bulk of the luminal fluid into the unstirred layer (USL) on the surface of the gut epithelium, (ii) interaction of the ENM with the unstirred layer and the associated mucus, or any other extracellular matrix that may constitute a barrier close to or on the epithelium, (iii) binding to the surface of apical membrane of the gut epithelial cells, then uptake by various membrane transport pathways into the cells, (iv) intracellular trafficking, and (v) active export against the electrochemical gradient from the cell into the serosal compartment (Fig. 1).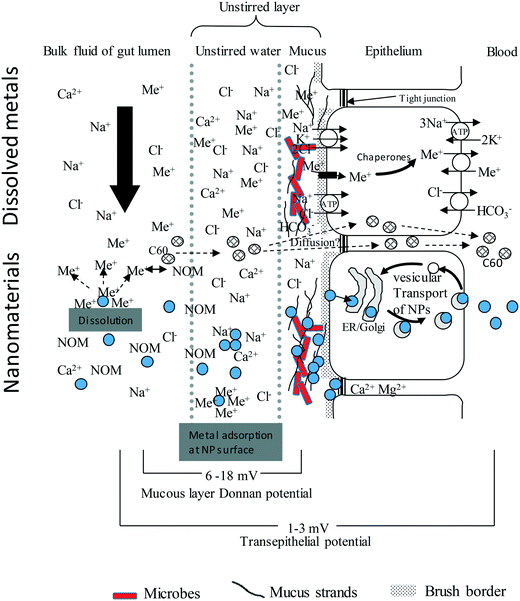 | ||
| Fig. 1 An idealised diagram of the vertebrate gut epithelium showing the mechanisms of uptake for electrolytes, toxic metal ions (Me+), compared to nanoparticles (NPs, filled circles). Modified from Handy and Eddy (2004).102 The substances in the luminal fluid diffuse into an unstirred layer (USL) comprising of water/mucus secretions, prior to transfer across the gut epithelium. The upper portion shows this for solutes. Cations bind to strands of polyanionic mucus where the exclusion of free anions like Cl− contributes to the Donnan potential at the cell surface. Electrolytes and toxic metal ions move through the cell using ion transport pathways. The lower portion of the diagram is for nanomaterials. The NPs will diffuse into the USL, albeit at a slower rate than solutes, and may be influenced by natural organic matter (NOM), pH, NaCl and divalent ions in the media. NPs will bind to strands of mucus, either by electrostatic attraction and/or become entangled in the mucoproteins (steric hindrance). Nanomaterials are taken up by endocytosis-related mechanisms and trafficked through the cell. Lipophilic ENMs such as C60 might diffuse through the lipid bilayer. The Ca2+ and Mg2+ rich environment in the tight junctions suggest that NPs would aggregate rather than diffuse through the paracellular route. For clarity the serosal processing of ENMs in the blood is not shown. | ||
The first few steps leading up to the delivery of the ENM to the apical membrane of the gut epithelial cells (i.e., adsorption to any external protective layers and bioaccessibility to the cell surface) will be considered here. First consider the importance of colloid theory to the behaviour of ENMs in the gut lumen. Engineered nanomaterials generally form suspended dispersions or emulsions in water. They are not truly dissolved in the aqueous phase, except where atoms or ions are released from the particle surface by dissolution (see reviews by Handy et al.11,31). It is possible to make seemingly ‘stable’ dispersions of ENMs in liquid for applications in the food or drinks sector, dentistry and oral medicines. For example, the dispersion of colloidal silver products in sodium citrate solution. However, while such a liquid may appear homogeneous, it is still a dispersion, not a solution of ENMs. Consequently, in principle, the behaviour of the ENM in the liquid environment of the gut lumen will be partly explained by colloid chemistry as outlined in extended DLVO theory [after Derjaguin and Landau (1941), and Verwey and Overbeek (1948)].31–33 Engineered nanomaterials will move in the liquid phase by Brownian motion (i.e., diffusion) and periodically collide with each other in the dispersion. If there is enough energy in the dispersion, the particles may then separate and stay dispersed. Alternatively, if the attractive forces (e.g., van der Waals forces) acting on the particles are stronger than the repulsive forces (e.g., the surface charge of the particles), then the particles will tend to agglomerate, or aggregate and eventually settle out of the liquid. In colloid theory, agglomeration is considered a somewhat reversible process where the addition of some energy may re-disperse the material, while aggregation relates to stronger attraction between the particles that leads to settling (see Handy et al.31 for details of DLVO and the forces involved). Aggregation and settling can occur if the particles in the dispersion are all the same (homo-aggregation), or where the ENM is aggregating with other colloids in the dispersion (hetero-aggregation). For ENMs in the gut lumen hetero-agglomeration and hetero-aggregation with proteins, food particles, etc., is therefore the most likely processes in vivo.
According to DLVO theory, conditions in the gut lumen such as altered pH, high ionic strength, the presence of divalent ions, and the type and concentration of organic matter will play a crucial role in any aggregation behaviours. Table 1 shows the pH, ionic strength, divalent ion concentrations and likely natural organic matter of content and composition in the gut lumens of a range of invertebrate and vertebrate animals that are used in ecotoxicity testing. The phylogeny of the organisms, and the regulatory tests they are used in, is shown in ESI† Fig. S1. Inevitably, there are species differences in gut lumen chemistry that also vary along the digestive tract (Table 1). The complexity of those changes will depend on the anatomy of each organism, the feeding strategy and the type of digestion. The presence and types of extracellular matrices (e.g., mucus or other secretions), and the innate permeability and tightness of the gut epithelium (i.e., passive properties of the barrier), according to the anatomy of the animals might also affect the uptake of ENMs across the epithelium. However, knowledge on the bioavailability and mechanisms of uptake of ENMs has, so far, mostly been elucidated using vertebrate animal models and mammalian cell lines, and so some consideration of invertebrate species is warranted.
| Organism (species) | Chemistry aspect critical in DLVO theory | |||
|---|---|---|---|---|
| pH | Ionic strength | Divalent ions | NOM/other colloids | |
| ND, no data available, either not reported in the literature, or not measured in a quantitative way with appropriate concentration units. FW, freshwater. SW, seawater. | ||||
|
Earthworms
(Lumbricus terrestris, Eisenia fetida, E. andrei) |
6.3–7.3 (ref. 173) | NaCl, ND |
Ca2+, 4 ≥ 0.5 mg g−1 (ref. 174)
Simulated gut fluid, 3.6 mmol L−1 Ca2+ (ref. 65). |
NOM from ingested soil. Also mucus, monosaccharides and glycoproteins.175 |
|
Aquatic polychaete worms
(Lumbriculus variegatus, Arenicola marina) |
Stomach: 5.4–6.0
Oesophagus: 6.5 Intestinal content: 7.0 (ref. 150) |
NaCl, ND |
Ca2+, ND
Mg2+, ND |
The gut content is 70–85% algae.176
Possible algal exudates and colloids from ingested water. Concentration of high molecular amino acids 58–215 mmol L−1, and high molecular organics, 85–233 mmol L−1 (ref. 177). Concentration of surfactants ∼13.3 mM (ref. 178). |
|
Roundworms
(Caenorhabditis elegans) |
Anterior pharynx, 5.96 ± 0.31;
posterior intestine, 3.59 ± 0.09 (ref. 179) |
NaCl, ND |
Ca2+, ND
Mg2+, ND |
ND |
|
Freshwater snails
(Physa acuta, Lymnea stagnalis) |
Oesophagus, 6.9–7.2;
gizzard/crop, 6.4; pylorus, 6.6; intestine, 7.1 (ref. 66) |
Surrogate artificial alimentary solution,66
NaCl, 35 mmol L−1. |
Surrogate artificial alimentary solution,66
Ca2+, 2.7 mmol L−1. |
Mucus and associated glycoproteins. |
|
Terrestrial isopod
(Porcellio scaber) |
Mid-gut, 6
Hindgut anterior part, 5.5–6.0; hindgut posterior part 6.0–6.5 (ref. 46 and 67) |
Fore- and mid-gut, 244.5 ± 6.1 mmol L−1 NaCl (ref. 180). |
Ca2+, ND
Mg2+, ND |
Around 10 mmol L−1 concentrations of surfactants in the hindgut.87 |
|
Water flea
(Daphnia magna) |
6.8–7.2 (ref. 181) | NaCl, ND |
Ca2+, ND
Mg2+, ND |
Filter feeder on algae. Possible algal exudates and colloids from ingested water. |
|
Springtail
(Folsomia candida) |
Anterior midgut and hindgut, 5.4–6.4
posterior midgut, 8.2–8.8 (ref. 182) |
NaCl, ND |
Ca2+, ND
Mg2+, ND |
Soil organism that grazes on fungal hyphae. Possible colloids from the food and soil in the gut. |
|
Mites
(Oppia nitens) |
Ventriculus and caeca, 5.4–6
Colon, 5.9–7.4 Post-colon, 6.5–8. In acaridid mites pH 4–7 from anterior to posterior.183 |
Large hindgut (in mmol L−1):
Na+, 35, K+, 98 Cl−, 146 (ref. 61). |
Ca2+, ND
Mg2+, ND |
Colloids from the liquid food (e.g., blood) of prey items. |
|
Honeybee
(Apis mellifera) |
Anterior, middle and posterior ventriculus: pH 6.0, 5.7 and 5.6 respectively.184
Large hindgut, 6.0–8.0 (ref. 61). |
No data on bees. Other insects (lepidopteran larvae)185
Na+, 1–1.3 mmol L−1 K+, 145–200 mmol L−1 |
No data on bees. Other insects (lepidopteran larvae).185
Mg2+, 8.6–27.4 mmol L−1 Ca2+, 11–19.6 mmol L−1 |
Numerous surfactants present in insect digestive fluid.62 |
|
Rainbow trout
(Oncorhynchus mykiss) |
Stomach; pH 2–5 (ref. 186)
Intestinal fluid187: pH 8.5 (FW), pH 8.1 (SW) |
Stomach (FW) in mmol L−1 (ref. 188):
Na+, 140–170 Cl−, 190–225 K+, 55–7 Intestinal fluid in mmol L−1 (ref. 187): Na+, 170 (FW), 20 (SW) K+, 4 (FW), 1 (SW) Cl−, 70 (FW), 50 (SW) |
Stomach (FW) in mmol L−1 (ref. 37):
Ca2+, 7–50 Mg2+, 12–40 Intestinal fluid in mmol L−1 (ref. 187): Mg2+, <1 (FW), 110 (SW) Ca2+, 2.1 (FW), 2.2 (SW) SO42−, <1 (FW), 110 (SW) |
Secreted mucus and organic matter from ingested food. |
|
Rat
(Rattus norvegicus) |
Stomach, pH 2.6–5.0 (ref. 189 and 190)
Intestine, pH 6.5–7.8 (ref. 189 and 190) |
Stomach in mmol L−1 (ref. 190 and 191):
Na+, 30.0–52.0 K+, 18.0–29.0 Cl−, 82.0–96.0 Small intestine in mmol L−1 (ref. 190 and 191): Na+, 113.0–153.0 K+, 6.0–52.0 Cl−, 60.0–100.0 |
Stomach in mmol L−1 (ref. 190 and 191):
Ca2+, 1.5–2.0 PO43−, 3.0 Small intestine in mmol L−1 (ref. 190 and 191): Ca2+, 0.25–8.0 PO43−, 23.0–24.0 SO42−, 3.4 |
Secreted mucus, and organic matter from ingested food. |
|
Chicken
(Gallus gallus domesticus) |
Proventriculus in chicken pH 2.1–3.8 (ref. 35 and 39)
Intestine of chicken, pH 6.4–7.7 (ref. 192) |
Intestine in mmol L−1 (ref. 192):
Na+, 67–83 K+, 19–27 |
Intestine in mmol L−1 (ref. 193):
filterable Ca2+, 17–11 |
In the intestine, the secreted mucus and organic matter from ingested food. |
|
Human
(Homo sapiens) |
Stomach: Fasted, pH 1.5–2; fed pH 3–7 (ref. 194)
Small intestines: Fasted, pH 4–8, typical value 6.5 in the upper small intestine.195 Fed, pH 3–7, typical value pH 5 in the upper small intestine.195 |
Simulated gastric fluid based on human in vivo data196 In mmol L−1:
Na+, 72.2 Cl−, 70.2 K+, 7.8 Simulated intestinal fluid based on human in vivo data.196 In mmol L−1: Na+, 123.4 Cl−, 55.5 K+, 7.6 |
Simulated gastric fluid based on human in vivo data.196 In mmol L−1:
Mg2+, 0.1 Ca2+, 0.15 Simulated intestinal fluid based on human in vivo data.196 In mmol L−1: Mg2+, 0.33 Ca2+, 0.6 |
Secreted mucus and organic matter from ingested food. |
2.1. Effect of pH in gut lumen on the behaviour of engineered nanomaterials
First, consider the effects of gut lumen pH on the propensity of ENMs to aggregate according to DLVO theory, and/or to dissolve. The effect of luminal pH on the behaviour of the ENM will depend especially on the chemical reactivity and the point of zero charge (PZC) of the material. With respect to acidity, the concerns are that the very acidic conditions will either degrade or modify the surface of the particles, resulting in changes in the behaviour of the material. For example, low pH can promote the dissolution of metal ENMs by acid hydrolysis of the metal surface (e.g., Cu NPs34). For the latter, the particles may shrink (smaller diameter) due to dissolution, or could completely dissolve over time. For ENMs deliberately manufactured with an organic coating (e.g., carboxylated polypeptide side chains attached to a metal core, lipid coating, etc.), the acidity may modify, or even completely degrade the organic coating. If the coating is polar or charged to give the ENM the ability to disperse in biological media (like charges will repel to aid dispersion31), then this functionality would be lost and the material would precipitate onto the gut surface.The pH values in the stomach of carnivorous vertebrate animals with acid digestion can be as low as pH 2–3 (Table 1). Although, in the terrestrial vertebrate animals, the precise pH might vary with the age of the animals and type of food (e.g., in birds35,36). In contrast, for the terrestrial invertebrate animals such as earthworms, snails, isopods and honey bees, the gut lumen is less acidic with the lowest pH values around pH 5; with the possible exception of Caenorhabditis elegans where the posterior intestine can be around pH 3.5 (Table 1). Dissolution and/or the degradation of any surface coating on the ENM will also depend on the residence time of the ingested material in the acidic part of the gut. Gut transit time is influenced by many factors including body size, ration size, the type of food eaten, and body temperature.
The residence times of food in the stomach is typically a few hours in vertebrate animals (fish, <8 hours;37 rats, 6–9 hours;38 chicken, 0.5–1 hour in the proventiculus;39 humans, 0.5–5 hours40). Some species, like geese have relatively short gut transition times (several hours), with relatively low digestion efficiency,41 while vultures for instance may have gut residence times of >24 h.42 In contrast, gut transit times in invertebrate species are much less. Indeed, for invertebrate species the entire gut transit time can be from minutes (e.g., < 30 minutes in Daphnia,43 ∼35 min for springtails44) to several hours (e.g., in earthworms,45 isopods,46 aquatic worms47). At neutral pH in saline conditions the maximum dissolution rates of metal NPs are typically at the μg min−1 level,48 and even if acidity increased this one hundred fold, this would still only represent around milligram amounts of dissolved metal in the gut lumen. For nutritionally required metals such as Zn or Cu, where a few mg per day are needed for animal health, such releases would be of no consequence. However, in the case of a trace metals that are known to bioaccumulate or are toxic, such as Cd released from CdTe quantum dots,48 then a repeated dose of a few μg with each meal could present a long-term hazard. In contrast, some metal oxides are resistant to acidity and tend to show low dissolution in gut salines (e.g., TiO2,49). The higher temperature of the mammalian gut compared to cold blooded animals may also enhance dissolution of the ENMs, since in simple dispersions increased rates of dissolution with rising temperature can be demonstrated.50
Regardless of the dissolution mechanism, this highlights that the luminal chemistry may lead to a dissolved metal fraction that is available for uptake on solute transporters in the gut lumen (Fig. 1). For dissolved metals, bioavailability depends on the chemical speciation of the metal and the presence of competing cations in the media such as H+,51 Ca2+ or Mg2+ (e.g., as water hardness,52) and Na+ (ionic strength or salinity,53). These ideas have culminated in the biotic ligand model (BLM) which predicts metal exposure to the fish gill,54 and also to aquatic invertebrates.55 A gut BLM is not yet available and metal sources from ENMs are not currently included in the aquatic BLM model.
Alkaline pH values are also found in the intestines of fish and mammals, as well as parts of the gut in invertebrate species, with the intestinal fluid in fish reaching pH 8.5, and in posterior midgut of springtails reaching pH 8.8 (Table 1). Such alkaline conditions may preserve at least metal ENMs, since strong alkaline digestion methods are used to extract ‘intact’ ENMs from tissue (e.g., Ag NPs from fish liver,56). However, the extreme ranges of pH from acid to alkaline would suggest that at some point during the transit through the gut, the ENM will be at a pH value close to its zero point of charge, where particle settling due to aggregation may occur. Metal ENMs are often designed so that they disperse at pH 7 in water, and so for example, Ag NPs that have a point of zero charge around pH 3,57 might be expected to aggregate in the stomach of vertebrate animals.
2.2. The effect of luminal ionic strength and divalent ions
The ionic strength (NaCl) and divalent ion concentrations in the gut lumen of different animals are shown in Table 1. In the gut of vertebrate animals, the Na+ concentration is typically more than 100 mmol L−1 and the Cl− concentrations tens of mmol L−1. According to DLVO theory, tens of millimoles of ionic strength will readily promote particle settling by aggregation and this has been demonstrated in gut salines used for vertebrate animals. For example, Al-Jubory and Handy49 showed rapid particle settling of TiO2 ENMs in the gut salines used for intestinal perfusions in trout, such that most of the particles had settled from the gut saline within 4 h, leading to exposure of the underlying tissue. In humans, the high ionic strength in combination with the low pH in simulated in vitro stomach fluids has been reported to lead to agglomeration of nanomaterials (i.e., Fe3O4, Ag, and SiO2 nanomaterials in Di Silvio et al., Walzack et al., and Peters et al. respectively),58–60 whereas the following in vitro intestinal environment led to de-agglomeration of the particles.59,60 The electrolyte concentrations in the gut lumens of invertebrate species are more varied, and they can be much higher than in mammals. For example, NaCl concentrations exceed 200 mmol L−1 in isopods (Table 1), but values of tens of millimoles of Na+ or Cl− are typical of invertebrates such as mites61 and some insects.62 However, for most of the invertebrate species investigated in this study, data in molar concentrations or similar relevant units for the gut ionic strength could not be found. Nevertheless, the threshold for particle settling due to NaCl concentrations is typically around 10 mmol L−1 or more, so some particle settling is expect in the gut of most animals.The divalent ions in the gut lumen include Ca2+, Mg2+, SO42− and PO43− (Table 1). In birds, mammals, and freshwater fish, the dissolved Ca2+ and Mg2+ concentrations in the gut lumen are generally a few millimoles, but much higher in seawater adapted fish that drink the surrounding medium (Table 1). Some animals precipitate calcium and magnesium carbonates in the lumen of the intestine as a means of removing secreted HCO3− from the gut lumen as part of the animal's acid–base balance strategy (fish,63), or sometimes as phosphates (cows,64). Consequently, some caution is needed when interpreting calcium and magnesium measurements in the gut lumen as ‘dissolved’ metal. For invertebrates, not many data are available specifically for divalent ions. For earthworms65 and snails66 values of 3.6 mmol L−1 and 2.6 mmol L−1 Ca2+ respectively, were used to simulate gut fluid. Nonetheless, millimolar concentrations of other cations have been reported for some invertebrates (e.g. K+ in isopods;67 NH4+ in earthworms65) (Table 1), and this would at least contribute to particle aggregation along with the NaCl present. The enhanced charge screening of ENMs due to divalent cations is well known in DLVO theory (see Handy et al.31) and for metal ion adsorption to epithelial surfaces.54 On an equimolar basis, the higher charge density of divalent cations relative to monovalent ions such as Na+, will drive adsorption to the fixed negative charge of the particle, or the cell membrane in the case of epithelia. In reality, the ionic activity of all the competing cations in solution should be considered, and the mobility in water of the divalent metals relative to H+ which is the fastest diffusing ion in solution.53 As the mobile cations are attracted to the surface of fixed negative charge on the particle, the diffusible anions are excluded, and this contributes to the surface potential or zeta potential of the particles. Similarly, diffusible anion exclusion also contributes to the measurable Donnan potential (the voltage arising from the passive distribution of ions) on the surface of the gut mucosa (Fig. 1). In theory, these processes should also apply to divalent anions in the gut lumen being attracted to the surface of a material that has been manufactured with a positive coating or surface charge. So, one might expect phosphates and sulphates in the gut lumen to influence the behaviour of positively charged particles, leading to charge screening and eventually aggregation. However, these effects of anions appear not to have been investigated in biota for ENMs.
2.3. Dissolved organic matter and other colloids in the gut lumen
The presence of natural or ‘dissolved’ organic matter in freshwater is known to influence the agglomeration and aggregation behaviours of ENMs. There are many possibilities according to DLVO theory.3,68 For example, the addition of humic acid can stabilise dispersions of ENMs, while the presence of larger colloids might cause particles to be ‘trapped’ in the colloid matrix by steric hindrance or electrostatic attraction (e.g., iron particles,69). With respect to the gut lumen, the type of organic matter present will inevitably vary with the type of food item ingested and the feeding habits of the animal. The secretion of digestive juices is also a critical function of the gut and the types of secretions vary depending on the anatomical region of the digestive tract or stage of digestion. However, there are some features that are common to most animals. For example, most organisms will secrete enzymes to start the digestion of the proteins, fats and/or carbohydrates in food. The enzymes therefore typically might include proteinases, trypsin, carboxypeptidases, etc., to break down proteins and peptides, lipases for fats, and amylases for starch, and so on. Such enzymes are secreted into the gut lumens of most organisms, for example in daphnids,70 isopods,46 earthworms,71–73 marine polychaete worms,74 fish,75,76 rats77 and other small mammals.78 These enzymes, proteins and other macromolecules might be considered as colloids that will be involved in agglomeration or hetero-aggregation of ENMs. However, these interactions have yet to be studied for individual enzymes. In theory, the digestive enzymes might even be able to degrade organic components of the manufactured surface coatings of ENMs, for example, peptidases attacking polypeptide coatings, or lipases in the case of ENMs coated in membrane lipids. However, this has not been demonstrated in organisms yet for most materials, although it is known that the type of coating on a particle can influence the uptake of metal into intestinal cells (Ag NPs with Caco-2 cells,79).In vitro studies have also shown that the composition of the media in which nanomaterials are suspended will affect the composition of the ‘biomolecular corona' that spontaneously forms on the surface of the ENM, and subsequently how the nanomaterial interacts with the cell membrane to enable cellular (i.e. epithelial) uptake of the material.58,80–84 However, with so many colloids from the food and gut secretions present in the gut lumen, it is not yet possible to model how the corona could be modified as the ENM moves along the gut tract. However, empirical results on the behaviour of ENMs in the human digestive tract in vitro vary with the type of material, with the gut lumen conditions either increasing85 or decreasing86 the translocation of nanomaterials over the epithelial cell layer.
The presence of food during digestion in the gut has also been suggested to reduce agglomeration of the nanoparticles, perhaps by stabilising the nanomaterial dispersion with organic matter through corona acquisition and steric hinderance.86 Surfactants are also secreted into the guts of many animals to prevent the digestive enzymes from precipitating, or to improve the digestion of lipophilic nutrients.62,87 These are natural dispersing agents that might also improve the dispersion of certain types of ENMs in the gut lumen. For humans at least, there is some evidence that bile salts might influence the aggregation behaviour and dispersion of ENMs in the gut lumen,29 but almost nothing is known of these processes with ENMs in the gut of wildlife. Given the small size of invertebrate animals, sometimes only a few microliters of luminal fluid can be collected, albeit with uncertainty about contamination of the sample with sloughed cells, mucus, etc. Consequently, most of the information on the enzymes and proteins secreted by the gut of small invertebrates is derived by semi-quantitative methods such as immunohistochemistry of the gut epithelium. Nonetheless, it is likely that the luminal protein concentrations, digestive enzymes, presence of salts, divalent ions, etc., together exceed by far the critical concentrations for particle settling according to DLVO theory. The gut, by its very nature, will contain high levels of solid phase food components such as fibre that may adsorb ENMs, as is known for other chemicals, but alternatively, dissolved organic carbon throughout the digestive tract might aid dispersion. Further research is needed to resolve these issues for most ENMs and species of animals.
2.4. Redox chemistry and the intestinal microbiome
Another factor that may influence the fate and behaviour of nanomaterials during gut transit are the prevailing redox conditions. The chemical composition of the ENM will impart aspects of its chemical reactivity, and ENMs, depending on their composition will undergo a range of reactions including oxidation, reduction, sulfidation, etc.3,8 From the type of microorganisms present in the lumen and redox potential measurements in the gut lumen, it is possible to deduce whether the gut is oxic or anoxic. In insects such as cockroaches,88 and also invertebrates that feed on poorly oxygenated sediment,89 the gastrointestinal tract has mainly anoxic conditions; and even animals in well aerated conditions can have regions or areas within the gut which are mainly anoxic, as in case of isopods.67 Similarly, based on the presence of fermenting and/or obligate anaerobes, the distal parts of fish intestine,90 and the human colon,91 can be anaerobic. Anoxic conditions will favour reducing reactions, such as the reduction of silver nanoparticles to transform them to silver sulphide-containing particles.92,93 In the case of silver at least, this leads to a stable persistent form of Ag2S particle, which has lower bioavailability to the gut than Ag NPs.94Limited research has been performed on how interactions between the gut microbiome and ENMs affects the ENM transformation, and thus bioaccessibility of the ENMs from the gut lumen matrix. The gut microbes are as a consortium of organisms involved in the digestion of the food and other processes. They can use carbon sources in the food directly, or the redox energy in the gut environment, to fuel their own energy metabolism. This might also include using the organic coatings on some ENMs as a carbon source (i.e., microbial degradation of the coating). Regardless, the microbial activity in the gut has the potential to alter the gut lumen chemistry, and certainly gut function.95 While the collection of data on the interaction between ENMs and gut microbes is at an early stage, there are some suggestions that ENMs might change the microbial community structure.30,96,97 Merrifield et al. demonstrated that the microbiome in the zebrafish gut following dietary Ag or Cu NP exposure varied, suggesting some effect relating to the chemical substance itself.98 However, the study also demonstrated that dietary CuSO4 resulted in a different microbial biodiversity to that of Cu NPs; indicating a ‘nano effect’ on the microbiome for the form of Cu presented to the fish. Whether or not such changes in the microbiome ultimately alter ENM bioavailability as well is currently unknown. The toxicological or nutrition consequences of such changing microbiology of the gut are also not clear. Important aspects, such as recolonization of the gut, or the evolution of a new steady state in the microbiome following ENM exposure have not been investigated.
2.5. Mucus and the unstirred layer
The fluid in the gut lumen faces the unstirred layer (USL) which forms over the epithelium (Fig. 1). The USL is a film of mucous liquid formed on the epithelium by the sol–gel properties of mucus and the surrounding media. It is typically a few micrometers in thickness.99 The USL is well-defined in vertebrate tissues and has not been studied in as much detail in the invertebrates most likely because of their small size and the difficulty of measuring ion activities, etc., without disturbing the USL. However, it is a fundamental physico-chemical phenomenon found on a wide variety of epithelia and other biological surfaces. Crucially, the microenvironment in the USL of the gut can be markedly different to the chemistry of the bulk luminal fluid.100 For example, the pH in the USL at the cell surface can remain neutral despite a lower or higher luminal pH.101 The USL generally has slower diffusion and therefore tends to concentrate solutes more than the bulk of the lumen. In the case of electrolytes, cations are drawn into the USL and anions, such as Cl−, tend to be excluded in favour of fixed negative charges of the mucus (see below) and the polyanionic ligands (glycocalyx) of cell surface. This leads to a Donnan potential of around 6–18 mV (Fig. 1, see Handy and Eddy for details on USL chemistry in aquatic species102). Studies on the movement of ENMs into the USL of the gut are presently lacking, but for example, shear forces at the luminal fluid-USL interface might drive peri-kinetic aggregation,31 so that ENMs concentrate in the USL. The effects of particle size, shape and surface charge have yet to be determined, but the viscosity of the USL might also tend to trap particles at the epithelial surface.The mucus layer secreted by the epithelium is integral to the USL and its sol–gel properties. The hydrated mucus of vertebrate animals is typically around 97% water and contains mucoproteins, which are made of a peptide backbone with numerous polysaccharide side chains. The composition of mucus is very highly conserved across species, with the mucus produced by diverse organisms such as anemones, jellyfishes, molluscs, rainbow trout skin, pig and human gut, showing similarity in structure (see review on mucus103). From the viewpoint of metal ions, mucus is a polyanionic matrix that attracts metals according to their charge density and ionic mobility in solution relative to H+.53 Thus trivalent metals such as Al3+ are attracted into mucus more than say Cu2+ ions, and divalent ions much more than Na+ on an equimolar basis. Engineered nanomaterials that have a net positive surface charge, would in theory, be electrostatically attracted to the USL and the mucoproteins therein. Negatively charged particles might show some exclusion. In mammalian mucus at least, neutral or slightly negatively charged ENMs have been shown to interact minimally with the mucous layer, enabling quick access to the intestinal cells.104
Alternatively, since ENMs are much larger than solutes, they may physically tangle with the strands of mucus (steric hindrance). Decoration of nanomaterials with longer surface chains has been shown to decrease mucous penetration, most likely caused by entanglement of the surface groups in the mucous mesh. The average pore size of the mucous mesh in mammalian preparations is 100 nm,105–107 indicating potential access to the cell layer for nanomaterials <100 nm, the size cut-off value is suggested to lie around 500 nm.108 Whatever the mechanism, the precipitation of mucus with ENMs in gut preparations has been observed (TiO2,49 Ag NPs,94), such that typically two thirds of the exposure dose is sloughed from the epithelium. When this is coupled with aggregation of ENMs in the gut saline, the bioavailable fraction that is taken up into the tissue is often only a few percent of the initial dose in vivo.19 It is of course, one of the functions of the gut mucus to protect the underlying epithelium from chemical insult in the gut lumen, and this would seem to be the case also for ENMs.
3. Structural diversity of the gut barrier in animals and the uptake of ENMs
Once the ENMs are in the USL and in close contact with the extracellular matrix on the cell surface, then uptake at the apical membrane of the epithelial cells can potentially occur (Fig. 1). However, the precise route, and the overall permeability of the gut barrier for ENMs will depend on the anatomy of digestive system (Fig. 2). Arguably, the gut barrier has evolved from a single layer of tissue in the simplest invertebrates to the complex multi-layered structure found in vertebrate animals. However, the gut of many organisms shows facets that are relevant to uptake of ENMs.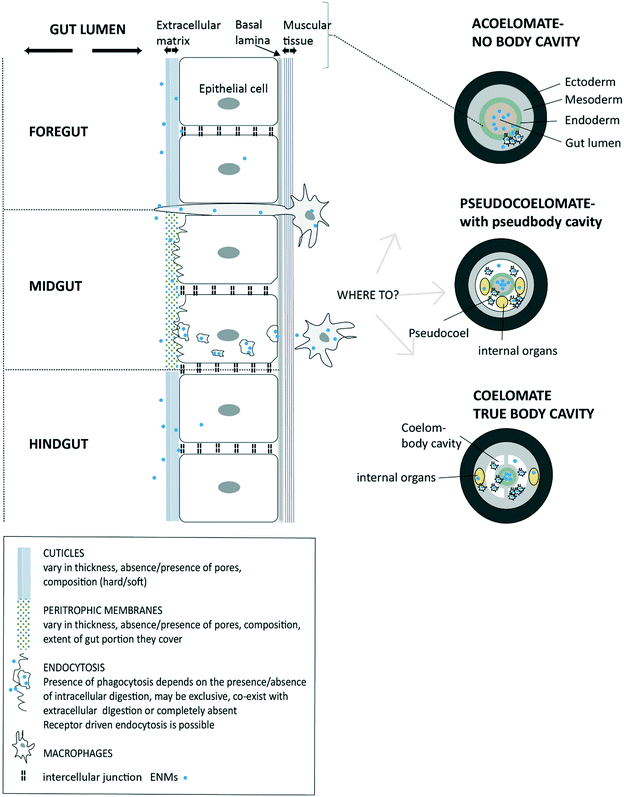 | ||
| Fig. 2 A diagram of invertebrate gut barriers showing different types of extracellular matrix on the apical side (facing gut lumen) of gut epithelium and with the external cuticles and/or peritrophic membranes. Mucus secretion into the lumen is possible in some species (not shown). The basal lamina and muscle layer on basal side of the epithelial cell provide some protection from uptake into the internal body compartment. Invertebrate gut is typically composed of three regions (fore-, mid-, and hind-gut) each with distinct functions and type of extracellular matrix. Potential uptake across epithelium is shown via receptor-driven endocytosis, phagocytosis or pinocytosis. Also, the protrusion of phagocytic cells to the gut lumen as part of the immune function is possible and being a potential pathway for the uptake of ENMs. The subsequent fate of ENMs after passing the epithelial barrier is shown depending on the absence of a body cavity (acoelomates), and presence of a pseudo cavity (pseudocoelomates) and with a true body cavity (coelomates) (after Sadava et al.228) | ||
3.1. The gut barrier in invertebrate species
Some key features of the gut barriers of invertebrates, and those particularly relevant for ENM uptake or particle processing, are shown in Table 2. In its simplest form in more primitive organisms like coelenterates (marine hydras, jellyfish, etc.), the gut epithelium is one cell layer thick and with a limited extracellular matrix. In this case, the gut is a relatively poor barrier to solutes, and possibly to ENMs, although permeability for ENMs has not been measured in these anatomically simple animals. However, other invertebrates have evolved complex extracellular matrices such as a layer of cuticle which lines the apical surface of the enterocytes (Table 2). In some cases, only minor parts of the gut are protected by a cuticle (Table 2). For example, the stomach in gastropod snails is covered by a cuticle called the gastric shield, however the rest of the gut is lined with mucus,109 which is a physical and chemical barrier that limits access to epithelium.110 Mucus can be mainly secreted by mucocytes located in the gut epithelium, but some invertebrates additionally have salivary glands that produce mucous secretions. Alternatively, in many invertebrates both the fore- and hindgut epithelium are protected from the gut contents by the cuticle (Table 2). The composition of the cuticle varies among different phylogenetic groups (Fig. 2; Table 2). The cuticle which covers the foregut and hindgut may be either: (i) a thin layer of sclerotized proteins without chitin, as found in the Annelida species; (ii) a multi-layered cuticle of proteins, highly cross-linked collagens and specialised insoluble proteins (called ‘cuticlins’), glycoproteins and lipids, as found in nematode worms, or (iii) a multi-layered mineralised cuticle made mainly of chitin as found in arthropods (Table 2, see Ruppert et al.;111 Brusca et al.112).| Organism and plan of the gut | Structures in the gut barrier | Evidence of endocytosis-related mechanisms in the gut epithelium | Biomineralisation of metal granules | |||
|---|---|---|---|---|---|---|
| Peritrophic membranes | Cuticle | Mucus secretion | Epithelium and underlying tissue | |||
| Earthworms (Eisenia fetida and Lumbriculus sp). Foregut, midgut and hindgut. The foregut contains the buccal chamber, pharynx and anterior portion of oesophagus. | Over the midgut. The peritrophic membrane in some species also contain chitin.197,198 | The foregut and hindgut are lined with non-chitinous cuticle.197 | The pharyngeal glands secrete mucus.199 | Epithelial lining of glandular cells, and non-glandular ciliated cells.199 Muscular contractions of the gizzard mechanically grind up the food. Circular and longitudinal smooth muscle under the epithelium. | Digestion mostly extracellular with no evidence of phagocytosis or pinocytosis.151 | The calciferous glands have type D granules composed of Ca. Chloragocytes contain two types of metal granules; the ‘chloragosomes’ which are type A granules containing P, Ca, Zn, Mg, Fe., and ‘debris vesicles’ which contain S.200 |
| Aquatic polychaete worms (Arenicola marina): Basic annelid plan of foregut, midgut and hindgut, similar to earthworms. | Absent in the midgut, but in Arenicola, a peritrophic membrane-like structure is formed around the faeces.198 | The foregut and hindgut are lined with a cuticle of sclerotized proteins without chitin.112 | Oesophageal pouches secrete mucus destined for the stomach, goblet cells and mucus in the intestines.150 | Epithelial cells are ciliated in parts of the gut. Circular and longitudinal smooth muscle under the epithelium. The buccal cavity and foregut secrete enzymes involved in digestion. The mid- and hindgut are restricted to absorption. | In the stomach, some epithelial cells take up food particles by phagocytosis. Some food components are digested by wandering amoebocytes.150 | Ca and Mg rich phosphate granules in the proventriculus and muscle tissue.201 |
| Roundworms the gut of Caenorhabditis elegans comprises pharynx and buccal cavity, and a long tube of intestine, and short hindgut/rectum.202 | Active glycocalyx protects the intestine, rather than peritrophic membranes.202 | The lumen of the pharynx and buccal cavity are lined with cuticle,203 and is composed of cross-linked collagens, cuticle proteins or ‘cuticlins’, glycoproteins and lipids.204 | Mucus is secreted in the pharynx.203 | The intestine has epithelial cells with a brush border and specialization cells involved in digestion. The pharynx is lined with muscle cells, but elsewhere the serosal surface is less protected.203 | Phagocytosis occurs in the gut epithelial cells.205 Intracellular digestion is present in nematodes.141 | Formation of Pb phosphate granules during metal exposures, crystalline pyromorphite.206 |
| Freshwater snails: in Lymnaea stagnalis comprises of mouth and pharynx (buccal mass), the oesophagus, stomach, digestive gland, intestine, and anus. | A mucous and viscous material surrounds the fecal string in the gut appearing arranged like a peritrophic membrane which has no chitin and no microfibrils.198 | Mollusc lack hard chitinous cuticle. It was only found in the buccal cavity and in some cases the epithelium of the stomach in gastropods is covered by a cuticle called the gastric shield.109 | Mucus secreting cells in the stomach.207 | The entire digestive system, with the exception of the gizzard and parts of the buccal cavity, is ciliated A muscularis supports the epithelium. Food is ground with sand in the gizzard by muscular movements in the stomach.66 Sorting of food particles takes place in a complex system of ciliated passages in the pylorus. | Amebocytes were found in digestive gland.208 Only particles with a diameter of less than 400 nm enter in the digestive gland, where intracellular digestion via phagocytosis occurs.208 | At least three types of metal storage granules are observed for metal exposure.209 |
| Terrestrial isopod: In Porcellio scaber, a straight tube consisting of a small foregut (oesophagus, and stomach or proventriculus), a junction with two pairs of tubular midgut glands, and a large hindgut (80–90% of the total length). | Fine mesh filters (mesh size 40–50 nm) prevents access of particles to digestive gland lumen.114 | The foregut and hindgut are lined with a thick chitinous cuticle which is permeable to digestive products up to 1.9 nm.210 | No evidence of mucus production in gut. | Food is mechanically degraded in the foregut and digested in the hindgut. Adsorption of nutrients takes place in midgut digestive gland. | No evidence of phagocytosis in terrestrial isopods. Pinocytotic vesicles were found in digestive gland of predatory marine isopod,211 and in hindgut of terrestrial isopod Armadillidium vulgare.212 | Cu-, sulfur- and Fe- rich granules in cells of the digestive gland (type B and C granules).200 |
| Water flea (Daphnia magna). The alimentary canal of Daphnia consists of a tube-shaped foregut, a midgut, and a short hindgut. | The tubular peritrophic membrane surrounds the food and extends through the midgut and hindgut.213 The peritrophic membrane is typically 280 nm thick, made of chitin containing microfibrils, embedded in a matrix of proteins, glycoproteins and proteoglycans. The peritrophic membranes are permeable to 31 and 130 nm nanoparticles but not to 327 nm (ref. 214). | The foregut and hindgut are lined with a thick cuticle (up to 1–2 μm in D. pulex).215,216 | No evidence of mucus production in gut. | The midgut consists of three parts: one dedicated to produce peritrophic membranes, a pair of small diverticula or hepatic caeca for secretion of digestive enzymes, and the third part for adsorption of substances. The inner surface of the midgut is folded and densely covered with numerous microvilli.213 The thin gut muscularis encircles the entire length of the midgut and caeca. | No evidence of phagocytosis. Pinocytotic vesicles were described in epithelial cells of other crustaceans.141 | In case of cadmium exposure calcium-contain granules are formed in the midgut.217 |
| Springtail: (Folsomia candida) a foregut, an enlarged sac-like midgut, and a small tubular hindgut. | The midgut is protected by peritrophic membranes. Polysaccharides, glycoproteins and carbohydrate components have been demonstrated on the surface of microvilli.218 | The fore- and hindgut are lined by a cuticle. | No evidence of mucus production in gut. | Nutrients are absorbed from the lumen by the midgut epithelium that consists of a single layer of simple columnar or cuboidal cells which bear numerous filament-like microvilli.219 | No evidence of phagocytosis or pinocytosis. | Midgut cells contain numerous ‘type A' granules with Ca, Mg and K.200 Mineral aggregates (spherites) in the gut epithelium during metal exposure.219 |
| Mites (Oppia nitens): a foregut consisting of pharynx and oesophagus, a midgut composed of ventriculus, paired caeca, colon, intercolon and postcolon, and a hindgut or anal atrium.117 | The midgut in several Acari species is lined by a peritrophic membrane.198 Peritrophic membrane can be up to 1 μm thick.117 | The foregut and hindgut are lined by a cuticle.117 The foregut cuticle thickness is ∼500 nm thick, hindgut up to up to 3 μm. Foregut cuticle it is perforated by numerous pore canals (70–150 nm). | No evidence of mucus production in gut. | Digestion begins externally using enzymes that are pumped into the body of prey or a plant cell, and continues in the midgut and gastric caeca.220 In most mite species, the caeca and ventriculus compose a large portion of the gut, and therefore probably play a key role in food digestion.183,221 The gut epithelium is as simple squamous epithelium, or cuboid in places, and a digestive cells have numerous microvilli.117 The midgut is surrounded by two layers of muscles. | Some mites that feed on decomposed prey have intracellular digestion processes implying phagocytosis.149Pinocytotic vesicles were detected in digestive glands of mite Acarus siro no specific data for Oppia sp. | There are granules in the cells of the caeca granules and seem to play a role in the storage of Ca and metals.222 |
| Honey bee (Apis mellifera): a tube containing a foregut (a mouth cavity, long thin oesophagus and the crop or ‘honey stomach’), a midgut (ventriculus or stomach), and a hindgut followed by rectum).223 | The midgut secretes the peritrophic envelope or membrane,.61,113,224 Made of proteins (peritrophins) and chitin. There is a particle diameter limitation to pass the envelope in insects, ranging from 4.5–35 nm depending on species.61,113 | The foregut and hindgut are lined by a cuticle (e.g., 10 μm thick in cockroaches)61,224 cuticle is impermeable to polysaccharides, and inulin (3 nm).225 | No evidence of mucus production in gut. | The gut is lined with an epithelium consisting of a single layer of cells with microvilli. | No evidence of phagocytosis. Generally, the digestion of insects is considered exclusively extracellular.226 | Midgut and fat body cells contain granules with Fe, P, Ca, K and also other metals.227 |
The midgut epithelium is the absorptive epithelium and lacks a cuticle, instead it is separated from the lumen contents by peritrophic membranes (Table 2). While these peritrophic membranes have no associated cuticle, they may be with or without chitin (in Arthropoda and some annelids and nematodes, respectively). In some cases the peritrophic membrane may be permeated by microscopic pores (for example up to 35 nm for honey bee, up to 130 nm for daphnids).61,113 In Crustacea, the peritrophic membrane is present in some species (e.g., Daphnia magna), but completely absent in others (terrestrial isopods e.g. Porcellio scaber). In the latter organisms, access of particles to the midgut cells is likely limited by a fine mesh filter positioned at the entrance to digestive glands. The mesh size of these filters was described as 40–50 nm,114 but, wolfram oxide fiber-like ENMs (mean diameter below 100 nm, their length was on the millimetre scale) were found inside the digestive gland lumen and attached on the cells.115
Clearly, the cuticle and/or any associated peritrophic membranes will vary in composition, thickness and the size of pores; and these factors are likely to be important in the physical access of ENMs to the apical surface of the gut epithelial cells. The structure of the cuticle also varies significantly in different regions of the gut. The isopod cuticle can be 1.5–3 μm thick and allows the passage of 0.7–1.9 nm particles,116 while 70–150 nm pore canals were found in the cuticle of some gut regions in mites with the cuticle thickness up to 0.8–2.5 μm.117 Beneath the epithelial cells typically lays the basal lamina, which can have a thickness from 100–300 nm in isopods (being regarded as outstandingly thick for invertebrates).116 The basal lamina is supposed to act like a charged sieve, in which the passage of macromolecules depends on its charge and porosity. In insects, for example, it has been shown that the basal lamina of the midgut prevented the passage of 6–15 nm gold nanoparticles.118
3.2. The gut barrier of vertebrate animals
The gut barrier of vertebrate animals usually consists of: (i) a mucous layer over the epithelium; (ii) the gut epithelium which is responsible for absorbing nutrients from the gut lumen; (iii) a sub-mucosa of connective tissue that incorporates the essential vasculature needed to transport nutrients away from the absorptive epithelium (i.e. lymphatic drainage and capillary networks); (iv) the muscularis externa (inner circular muscle, outer longitudinal muscles) which is responsible for gut motility, and (v) an outer serosa that lubricates and protects the organ system from abrasion or other mechanical injuries during the movements of the gut. In most vertebrates, the gut epithelial cells of the intestine have microvilli to increase their surface area, with an oligosaccharide matrix on the surface (the apical or mucosal surface is often called the brush border). The multi-layered anatomy of the vertebrate gut provides a modest passive permeability for solutes and is regarded as a reasonably ‘tight’ epithelium with respect to solutes in the gut lumen. This general design of the layers constituting the vertebrate gut is reasonably well conserved within the vertebrates.11 However, within the classes of vertebrate animals there are also some differences in the functional anatomy of the gastro-intestinal tract and the accessory organs (liver, pancreas, etc.) due to the diverse feeding habits of the animals.Teleost fishes can have six major anatomical sections to the digestive tract.119,120 These are the buccal cavity (mouth), oesophagus, stomach, pyloris (anterior intestine), mid and hind intestine. However, with some 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 species of fishes it is perhaps no surprise that there are some very diverse anatomies.121 In some herbivores (e.g. carp species) the stomach pouch may be less distinct or absent with the stomach being simply a continuous tube with the rest of the intestine. In carnivores that use acid digestion (e.g. trout), the stomach is usually well defined, while omnivores may show an intermediate anatomy. Notably, the volume of lymphatic drainage in fishes and some amphibians far exceeds anything that a mammal could achieve. These former animals may therefore take up dispersed materials faster than anticipated by their body temperature/metabolic rate. In many lower vertebrates, the gut anatomy can be transient or seasonal with the food supply. Only birds and mammals tend to maintain the gut anatomy in a constant state of readiness to absorb substances from the gut lumen. Thus, feeding status will be a critical factor in the passive gut permeability of some fishes, amphibians and especially reptiles.
000 species of fishes it is perhaps no surprise that there are some very diverse anatomies.121 In some herbivores (e.g. carp species) the stomach pouch may be less distinct or absent with the stomach being simply a continuous tube with the rest of the intestine. In carnivores that use acid digestion (e.g. trout), the stomach is usually well defined, while omnivores may show an intermediate anatomy. Notably, the volume of lymphatic drainage in fishes and some amphibians far exceeds anything that a mammal could achieve. These former animals may therefore take up dispersed materials faster than anticipated by their body temperature/metabolic rate. In many lower vertebrates, the gut anatomy can be transient or seasonal with the food supply. Only birds and mammals tend to maintain the gut anatomy in a constant state of readiness to absorb substances from the gut lumen. Thus, feeding status will be a critical factor in the passive gut permeability of some fishes, amphibians and especially reptiles.
With respect to mammals, the laboratory rat has been used as a model for the dietary uptake of pollutants for many years (e.g. metals122 and organic chemicals123). The gross anatomy is similar to human anatomy with the mouth, oesophagus, stomach, an absorptive small intestine (duodenum, jejunum, ileum), the large intestine (caecum leading to colon), and the rectum. However, there are many functional differences in the rat gut physiology compared to humans.124 The topography and spatial arrangement of the vertebrate gut can influence the transit time of particulate materials; but in the small intestine, solutes and particulate materials may be absorbed. There is some controversy on the total surface area of the small intestine in humans, as it is estimated at 250–300 m2, but more recent at ∼30 m2.125 Microfold cells (commonly referred to as M-cells) are associated with the Peyers' patches in humans and rats.126 M-cells are involved in the active transport of ENMs over the gut barrier.127–129 Mammals as terrestrial animals must conserve water by reabsorbing any fluids secreted into the lumen during digestion as well as osmotically drying the faeces. There is generally a net secretion of watery fluid into the lumen of the stomach and small intestine of mammals, but it is removed in the large intestine. Consequently, like freshwater fishes, the prospect of passive net uptake of nanomaterials by solvent drag processes in the small intestine of mammals is not likely. However, solvent drag would be theoretically possible in the large intestine where water is reabsorbed.
Birds comprise a wide range of species and their digestive tract is very adjustable, changing according to food availability,130 but also, for instance, in relation to wintering conditions,131 migration132 and moulting.133 Thus the readiness of the absorptive epithelium to take up ENMs will likely vary with these conditions, although it has not been specifically investigated. The digestive tract of birds consists of different regions, with specific functions: oesophagus, crop, proventriculus, ventriculus or gizzard, duodenum, jejunum, ileum, caeca and colon. It is known that the grain size of the food particles can alter the nutritional performance of poultry.134 However, the absorption efficiency of ENMs or how much they are taken up in the different regions of the gut in birds has not been investigated. The oesophagus contains a fold of tissue called the crop where food can be temporarily stored, before being released onwards to the stomach or gizzard. Whether or not ENMs incidentally stored in the crop are modified is unknown. Mucus is also secreted into the oesophagus in order to transport the food to the gizzard, and the concerns for ENMs in mucus (above) also apply to the gut of birds. The oesophagus widens into the proventriculus which secretes enzymes and acids for the digestion of the food. Following this, the food will go to the ventriculus/gizzard which is the part of the stomach that will mechanically grind and mix the food. This grinding can be relatively harsh in order to break up the hard grains of wheat seeds, etc., that birds may eat and so there is a prospect also of mechanical and chemical erosion of ENMs in this stage of the gut. In the small intestines (duodenum, jejunum and ileum) further digestion takes place of the food. In the duodenum digestive enzymes are being released and the bile duct also enters the gastrointestinal tract here. The caeca are blind pouches at the end of the small intestines where further digestion of the food can take place, and where water is reabsorbed.135 Similar to mammals, the net fluid secretion into the small intestine would prevent the passive uptake of ENMs by solvent drag, but in the lower reaches of the intestine and colon some absorption might be possible. The colon, which can be relatively short in birds, is where further water is reabsorbed. The net fluid influx would promote ENM movement onto the epithelium. Unfortunately, dietary studies on birds, so far, have tended not to characterise the ENMs in the food, or include metal salt or bulk material controls in the study design, so drawing nano-specific conclusions is problematic. Nonetheless, there are some tentative suggestions that some ingested ENMs might alter the weight gain and food conversion ratio of young poultry (e.g., chromium-containing ENMs,136).
3.3. Uptake of engineered nanomaterials into the gut epithelial cells
Regardless of the species of organism, there are fundamentally two potential routes to cross the gut epithelium; the paracellular route between the cells, or the transcellular route going through the cells. First consider paracellular uptake. In a healthy gut epithelium, paracellular diffusion of ENMs in between the epithelial cells is extremely unlikely because the tight junctions between gut epithelial cells in most organisms contain high concentrations of Ca2+ and Mg2+ ions that would cause aggregation (see discussion in Handy et al.11), and in any case the intercellular space within tight junctions is narrow (e.g., 6–7 nm in rat intestine,137). Some studies have reported that transepithelial electrical resistance (TEER) declines during the exposure of confluent monolayers of gut cells to ENMs (e.g., gold NPs on Caco-2 cells,138) and take this as evidence that the tight junctions are permeable to ENMs. However, this is often erroneous thinking. Firstly, because the innate permeability of any epithelium can only be shown after measuring the resistance and voltage under short circuit conditions (see the original paper on frog skin by Ussing and Zerahn,139). These conditions are often not met or demonstrated in routine cell culture. Secondly, the dissipation of any apparent TEER is more likely to do with solvent drag and water permeability of epithelium (see review,140), not the movement of the ENM per se. In perfused intestines net water flux through the tissue can alter during ENM exposure and be in the opposite direction to the measured uptake of total metal from metal-containing ENMs (TiO2,49), so changes in TEER should be interpreted more carefully.If paracellular uptake is excluded or limited, then uptake through the gut epithelial cells is the most likely route to the serosal compartment. In vertebrates, extracellular digestion is prevalent where secreted enzymes degrade food particles in the gut lumen and smaller molecules (sugars, amino acids, etc.,) are then taken up by cells. Therefore, vertebrate enterocytes are regarded as ‘non-phagocytic’.141 Nonetheless, the possible active mechanisms of vesicular uptake of ENMs by the gut of vertebrate epithelial cells includes macropinocytosis, clathrin-mediated endocytosis and caveolae-mediated endocytosis (reviews,107,142). In some special cases, such as bacterial infection in the gut, immune dendritic cells can protrude to the apical surface of the gut between two adjacent enterocytes as demonstrated with monolayers of Caco-2 cells.143 While the extent of this latter phenomena is unclear for vertebrate animals, the phagocytosis of ENMs is theoretically possible via this route, and especially in some invertebrates (see below).
Uptake of ENMs on solute transporters in the cell membrane of gut epithelial cells is excluded because the ENMs are far too big.11 For spherical lipophilic materials, such as pristine C60 particles with a size of ∼0.7 nm, it has been shown that they translocate through the artificial lipid bilayer in a manner consistent with diffusion.144 However, pristine C60 might be a special case, and for example, engineered liposomes (∼100–200 nm) are often designed to fuse with the apical membrane to release their contents, rather than be transported as intact liposomes. Regardless, caution is needed when extrapolating the idea of lipophilic diffusion to other materials, or from in vitro models with artificial media to the in vivo situation. However, most of the effort on mechanisms of ENM uptake comes from studies on rodent gut,145 fish gut,49 or gut cell lines such as the Caco-2 cell culture and co-culture variants of this model.146,147 For example, nystatin, a putative caveolae-mediated endocytosis inhibitor, blocks Ti uptake during TiO2 exposures in perfused intestine preparations from trout.49 Similar pharmacological studies have been done with Caco-2 cells along with electron microscopy to capture vesical formation at the cell membrane.146 However, one should remember that most of the mammalian cell lines are derived from carcinoma tissue,147 and may not show ‘comparable’ endocytosis processes to primary cell cultures or in vivo. Nonetheless, endocytosis is an active, energy-dependent, receptor-driven process and is implicated in the uptake of different ENMs (reviewed in detail by des Rieux et al.).148 Albeit, with some uncertainty about how ENMs might interact with the membrane receptors involved.
In contrast to vertebrates, there are few pharmacological or kinetic studies on the gut uptake mechanisms of ENMs in invertebrate species. This is partly because their small size makes techniques such as gut perfusions challenging, but also because cell cultures of gut cells from invertebrate species are not routinely available. Nonetheless, several invertebrate species possess phagocytosis or pinocytosis in the gut epithelium (Table 2). These organisms can also have ‘intracellular digestion’ where food material is only partly digested in the gut lumen and the resulting food particles are taken up by phagocytosis or pinocytosis and digested intracellularly by cells in the lumen. Intracellular digestion primarily occurs in primitive unicellular animals (like Protozoa, e.g., Amoeba) as well as some organisms without a true gut (Porifera, e.g., sponges).149 From the viewpoint of evolution, intracellular digestion was first assisted by amoeboid cells in the gut lumen, then gradually evolved to be entirely replaced by extracellular digestion in the gut lumen. Some invertebrate groups, however, have retained some mechanisms of intracellular digestion in co-existence with extracellular digestion. The cells involved in intracellular digestion can be “wandering” amoeboid cells,150 or gut epithelial cells that phagocytose food.112,151 In a recent review on the phagocytosis in enteric cells, Hartenstein et al.141 refers to two types of phagocytic cells differing in terms of the size of particles they take up: (i) cells that perform phagocytosis of large particles (even particles almost the size of a cell) that are sequestered in large phagosomes, and (ii) cells that are involved mainly in uptake of smaller particles ending up in endocytic vesicles. The intracellular digestion has been related most to the type of food collected, being more commonly observed in organisms that have fine sorting systems of food and those that suck partly-digested food. Some examples of organisms with evident presence of phagocytosis were found in Rotifera (e.g., Brachionus), Platyhelminthes (e.g., planarians), many classes of Mollusca (especially Bivalvia and Gastropoda), Cnidaria (e.g., jellyfish), Ctenophora (e.g., comb jellies), and some blood sucking Nematoda species.112,149,152,153 Annelids predominately have extracellular digestion, but some members like marine polychaetes phagocytose food particles.150,151 In the majority of Arthropoda (crustaceans, insects), except for Arachnida (spiders, mites), the digestion is considered predominately extracellular but also here some evidence of pinocytosis does exist. Clearly, where phagocytosis or pinocytosis mechanisms exist in the gut of invertebrates, ENMs may follow the same route of intracellular uptake as food particles. The extent to which this is possible will depend on the type of phagocytosis and the predominant type of digestion. However, this has yet to be demonstrated in the gut of most invertebrates for ENMs.
4. Export from the gut to the internal organs
While the uptake of ENMs into gut epithelial cells likely occurs by endocytosis and related mechanisms, this step either deposits the ENM inside the epithelium, and/or ENMs could continue through the cell (transcytosis,154) to the other side (i.e., the serosal compartment of vertebrates, or through basal membrane of invertebrates). Export from the gut epithelial cells to blood or lymphatic vessels of the serosal compartment in case of vertebrates, or directly to the extracellular fluid of invertebrates, is indicated by the presence of ENMs in internal organs in a range of animals (e.g., fish,56 rats,155 humans,156). The conventional thinking for solutes is that export from the epithelial cells to the blood side is against the electrochemical gradient, and is often an energy-dependent and rate limiting step in the uptake of chemicals.11 This also applies to vesicular trafficking to the serosal compartment (Fig. 1), or basal membrane, where energy would be required to fuse and empty the contents of any intracellular vesicle into extracellular fluid and/or surrounding tissues (e.g., loose connective tissue) beneath the epithelium. Indeed, energy-dependent vesicular trafficking of metals such as Cu is long established.157 The only requirement for ENM uptake is that the ENM can also access the endogenous trafficking systems of gut epithelial cells, and this seems to be the case.For the invertebrates, when the ENMs have transversed the epithelial cells via endocytosis, or similar mechanisms, they most probably do not move freely in the extracellular fluid, but are taken up again by cells (e.g., haemocytes) responsible for recognition of ‘non-self material.’ These cells are broadly analogous in function to the macrophages in the immune system of vertebrate animals. This has been evidenced in vivo in the case of haemocytes in insect larvae158 and mussels.159 There are also numerous in vitro data reporting ENM uptake by the haemocytes of invertebrates.160,161 The fate of ENMs in extracellular fluid will partly depend on the local immune response, and any congestion of haemocytes (or similar cells) in the tissue to remove foreign material. Similar arguments could be applied to vertebrate animals when the ENMs arrive in the blood supply or lymphatics, since, for example, macrophages can engulf ENMs. A detailed discussion of immune systems is beyond the scope here, but there are substantial differences in the components that make up the immune systems of vertebrates and invertebrates. For example, invertebrates do not have the range of functions found in the white blood cells of mammals, and they do not have antibodies per se.162 The immune response of different organisms to chemicals is also dependent on this phylogeny of the immune system functions (e.g., with pesticides163), but the importance of differences in how the immune systems of animals enables ENM uptake across the gut and distribution in the tissues requires further investigation.
The concerns raised regarding pH, ionic strength, divalent ions and organic matter in the gut lumen (Table 1), also apply to the extracellular fluid or haemolymph. The pH values of extracellular fluids are usually in the neutral range (e.g., pH 7.4 in humans, pH 7.8 in freshwater trout and crayfish), and therefore less likely for any organic surface coating of ENMs to be ionised, i.e., perhaps close to the point of zero charge where aggregation can occur. For invertebrates, the NaCl concentrations typically exceed 100 mmol L−1 (i.e., high ionic strength), except insects which have high KCl concentrations in their body fluids, and with millimolar concentrations of divalent ions or higher also present.164 Similarly, vertebrate blood has around 140 mmol L−1 as NaCl concentrations. Consequently, ENMs, when present, are likely to aggregate in the extracellular fluid of both invertebrate and vertebrate animals.
The distribution of ENMs around the body and to the different internal organs will depend on the body design of the animal and the presence and/or type of circulatory system. Some groups (acoelomate, for example flatworms and sponges) lack an enclosed fluid-filled body cavity and do not have a circulatory system. In this case ENMs that potentially cross the gut barrier are more probably retained locally at the site of entry and entrapped in phagocytic cells, such as in the macrophage-like cells and granule-containing cells of sponges.165 On the other hand, in organisms with a pseudo body cavity (pseudocoelomate, e.g., roundworms) and true body cavity (coelomate, e.g., earthworms), the extracellular fluid could transmit cells with ENMs to other sites internally (Fig. 2). For animals with open circulatory systems (e.g., in molluscs and arthropods) the ENM is likely to be moved around more rapidly by the bulk transport of the body fluids. In animals with closed circulations, where the arterial and venous systems are joined in a continuous circuit (as in earthworms, squid, octopus and vertebrate animals), it implies that the blood vessels provide an additional barrier. However, the volume of distribution for animals exposed in vivo to ENMs has mostly not been measured. Engineered nanomaterials can cause damage to the vascular system in fish,166 or accumulate at the site of injection in dosimetry investigations,167 but there are no large scale defects in cardiovascular functions reported so far. There is also a concern that the aggregation of ENMs in the body fluids will result in aggregates that adsorb to the surface of the blood cells, with the latter subsequently controlling the fate of ENMs in the circulation. However, the precise fraction of ‘free’ ENMs compared to adsorbed ENMs in the body fluids has not been determined in vivo in most animals. Also, any damaged blood cells with ENMs adsorbed to their surface might be removed by the spleen of vertebrate animals (e.g., Cu NPs in fish168), and perhaps by melanisation and formation of nodules in invertebrates. Thus, the distribution of ENMs to the internal organs may be a limited portion of the original exposure dose.
Furthermore, many invertebrate species have the ability to store dissolved metals as granules by biomineralisation processes (Table 2). Fish can sometimes also make metal storage granules in the liver.169 However, these granules should not be misinterpreted as stored internalised ENMs. In the case where the ENMs dissolve to ions (e.g., in the acidic stomach pH) and the metal is subsequently taken up in the ionic form, it is expected that these ions might be mineralised and stored, especially by the invertebrates.170 Interestingly, in the case of at least one ENM where there was no dissolution (and therefore no re-mineralisation), the particles were not retained in the tissues in vivo in terrestrial isopods (CoFe2O4 NPs171). A review of the uptake and excretion kinetics of ENMs in invertebrates is reported elsewhere (see van den Brink et al.172).
5. Conclusions
The gut lumen chemistry of animals inevitably plays a critical role in the behaviour of ENMs in the gastrointestinal tract. From the viewpoint of DLVO theory, despite the diverse structural anatomies of the gut barrier of animals, there are some common features in the gut lumen chemistry including; high ionic strength, the presence of divalent ions and natural organic matter from food and other colloids, that together will mainly promote particle aggregation and settling in the luminal contents as well as onto the USL on the surface of the gut epithelium. The low pH in the stomach of vertebrates and other equivalent low pH regions of invertebrate gut will promote the dissolution of some metallic ENMs, with the possibility of both dissolved and particulate metal uptake in the gastrointestinal tract. The redox chemistry of the gut tends to promote reducing conditions where ENMs could be transformed (e.g., by reduction with sulphide) and the diverse organic matter in the gut will contribute in, as yet, unquantified ways to a modified corona on the ENMs. However with regard data gaps on gut lumen chemistry, the molar concentrations of NaCl, Ca2+ and Mg2+ in the gut lumen of terrestrial invertebrates are not well reported (Table 1); partly because the small size of the animals presents a challenge for the reliable collection of the luminal fluid. It is also surprising that information on the gut lumen chemistry is limited for Daphnia magna as one of the most widely used organism for aquatic toxicity tests. Data on dissolution rates of ENMs in physiological salines that mimic the gut lumen of invertebrate species are also needed.The USL has the potential to concentrate ENMs, but it is also a protective layer. In animals that produce mucus, the sloughing of mucous secretions protects the underlying epithelium, and together with particle settling in the gut lumen, the overall effect is to reduce the bioavailability of most EMNs to the epithelium to a fraction of the ingested dose. Apart from a few studies on fish or rats, more measurements of the gut surface-associated fraction of ENMs is needed in order to understand how much of the potentially labile pool of surface adsorbed ENMs is available for true uptake. The effects of ENMs on mucus secretion rates and intestinal sloughing is also poorly understood. The various extracellular matrices, such as the cuticle of invertebrates, also likely serve to limit the access of ENMs to the underlying epithelial cells. However, more measurements of particle interactions with cuticle, and peritrophic membranes are needed, especially with respect to the integrity and apparent permeability of these matrices in the presence of ENMs.
The mechanisms of ENM uptake by gut epithelial cells is most well-known for mammalian models and these include active uptake by receptor-mediated endocytosis pathways and/or macropinocytosis. While these mechanisms also exist in the gut of invertebrates, it is also crucial to understand that many invertebrates have another potential pathway – the uptake of ENMs via the ‘intracellular digestion’ process including phagocytosis and pinocytosis by cells in the gut lumen, and subsequent delivery to, and across, the epithelium. The importance of this pathway for invertebrates requires further research. Rarely, for a few pristine very small lipophilic materials such as C60, diffusional uptake across the gut epithelium may occur, but this phenomenon, which has mostly been demonstrated with artificial lipid bilayers, needs to be explored in vivo in the complex chemical matrix of the gut lumen. Care also needs to be taken with electrophysiology and its interpretation. Paracellular uptake of ENMs is not likely in a healthy epithelium, and TEER measurements alone that relate to electrolyte/water flux do not inform on the integrity of the paracellular route for ENMs. Transcellular uptake via vesicular-dependent pathways remains the most likely route to cross the gut epithelium of animals. The basal lamina acts as a barrier to the serosal compartment in many animals, and in invertebrates where the gut epithelium is in direct contact with underlying loose connective tissue or muscle fibres, there are immune phagocytic cells that will remove ENMs from the extracellular fluid. Such housekeeping roles of the immune system and the propensity of particle settling in the high ionic strength of extracellular fluid will limit the distribution of ENMs in animals. The role of all the blood cells for the incidental transport and distribution of ENMs to the internal organs requires more investigation. However, at least some measurements such as single particle inductively coupled mass spectrometry (spICP-MS) are detecting ENMs inside some internal organs or tissues. Invertebrates especially, have the ability for biogenic mineral formation and any nano scale granules present in the tissue may not be the original ENMs.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
All the authors were funded by the EU H2020 project Nano-FASE (Nanomaterial Fate and Speciation in the Environment; grant no. 646002). SL, PVS and ZK received additional financial support from FCT/MCTES, through national funds, to CESAM (UIDP/50017/2020+UIDB/50017/2020); PVS was awarded with a PhD grant by FCT (SFRH/BD/51571/2014). MvdZ received additional financial support from the Dutch Ministry of Agriculture, Nature and Food Quality (project KB-23-002-005). RH was partly supported by the EU H2020 Nanoharmony project (grant no. 8859231). AJK was funded by Slovenian Research Agency through program P1-0184 Integrative zoology and speleobiology.References
- F. Gottschalk, T. Y. Sun and B. Nowack, Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS.
- B. Giese, F. Klaessig, B. Park, R. Kaegi, M. Steinfeldt, H. Wigger, A. von Gleich and F. Gottschalk, Risks, release and concentrations of engineered nanomaterial in the environment, Sci. Rep., 2018, 8, 1–18 CrossRef CAS.
- J. R. Lead, G. E. Batley, P. J. Alvarez, M. N. Croteau, R. D. Handy, M. J. McLaughlin, J. D. Judy and K. Schirmer, Nanomaterials in the environment: behavior, fate, bioavailability, and effects—an updated review, Environ. Toxicol. Chem., 2018, 37, 2029–2063 CrossRef CAS PubMed.
- K. L. Garner, S. Suh and A. A. Keller, Assessing the risk of engineered nanomaterials in the environment: development and application of the nanoFate model, Environ. Sci. Technol., 2017, 51, 5541–5551 CrossRef CAS PubMed.
- F. Gottschalk, E. Kost and B. Nowack, Engineered nanomaterials in water and soils: a risk quantification based on probabilistic exposure and effect modeling, Environ. Toxicol. Chem., 2013, 32, 1278–1287 CrossRef CAS PubMed.
- R. J. Peters, G. van Bemmel, N. B. Milani, G. C. den Hertog, A. K. Undas, M. van der Lee and H. Bouwmeester, Detection of nanoparticles in Dutch surface waters, Sci. Total Environ., 2018, 621, 210–218 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- S. J. Klaine, P. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Nanomaterials in the environment: behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- S. H. Joo and D. Y. Zhao, Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review, J. Hazard. Mater., 2017, 322, 29–47 CrossRef CAS.
- E. M. Hotze, T. Phenrat and G. V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment, J. Environ. Qual., 2010, 39, 1909–1924 CrossRef CAS.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Manufactured nanoparticles: their uptake and effects on fish-a mechanistic analysis, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS.
- W. Yang, J. I. Peters and R. O. Williams III, Inhaled nanoparticles— a current review, Int. J. Pharm., 2008, 356, 239–247 CrossRef CAS PubMed.
- M. Riediker, D. Zink, W. Kreyling, G. Oberdörster, A. Elder, U. Graham, I. Lynch, A. Duschl, G. Ichihara, S. Ichihara, T. Kobayashi, N. Hisanaga, M. Umezawa, T.-J. Cheng, R. Handy, M. Gulumian, S. Tinkle and F. Cassee, Particle toxicology and health-where are we?, Part. Fibre Toxicol., 2019, 16, 19 CrossRef PubMed.
- J. L. Ferry, P. Craig, C. Hexel, P. Sisco, R. Frey, P. L. Pennington, M. H. Fulton, I. G. Scott, A. W. Decho and S. Kashiwada, Transfer of gold nanoparticles from the water column to the estuarine food web, Nat. Nanotechnol., 2009, 4, 441 CrossRef CAS PubMed.
- M. Tella, M. Auffan, L. Brousset, J. Issartel, I. Kieffer, C. Pailles, E. Morel, C. Santaella, B. Angeletti and E. Artells, Transfer, transformation, and impacts of ceria nanomaterials in aquatic mesocosms simulating a pond ecosystem, Environ. Sci. Technol., 2014, 48, 9004–9013 CrossRef PubMed.
- R. D. Holbrook, K. E. Murphy, J. B. Morrow and K. D. Cole, Trophic transfer of nanoparticles in a simplified invertebrate food web, Nat. Nanotechnol., 2008, 3, 352–355 CrossRef CAS PubMed.
- X. Zhu, J. Wang, X. Zhang, Y. Chang and Y. Chen, Trophic transfer of TiO2 nanoparticles from Daphnia to zebrafish in a simplified freshwater food chain, Chemosphere, 2010, 79, 928–933 CrossRef CAS PubMed.
- C. S. Ramsden, T. J. Smith, B. J. Shaw and R. D. Handy, Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain, Ecotoxicology, 2009, 18, 939–951 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle, B. P. Eynon and R. D. Handy, Dietary exposure to silver nitrate compared to two forms of silver nanoparticles in rainbow trout: bioaccumulation potential with minimal physiological effects, Environ. Sci.: Nano, 2019, 6, 1393–1405 RSC.
- M. Connolly, M. Fernández, E. Conde, F. Torrent, J. M. Navas and M. L. Fernández-Cruz, Tissue distribution of zinc and subtle oxidative stress effects after dietary administration of ZnO nanoparticles to rainbow trout, Sci. Total Environ., 2016, 551, 334–343 CrossRef PubMed.
- T. W. K. Fraser, H. C. Reinardy, B. J. Shaw, T. B. Henry and R. D. Handy, Dietary toxicity of single-walled carbon nanotubes and fullerenes (C-60) in rainbow trout (Oncorhynchus mykiss), Nanotoxicology, 2011, 5, 98–108 CrossRef CAS PubMed.
- C. Coutris, T. Hertel-Aas, E. Lapied, E. J. Joner and D. H. Oughton, Bioavailability of cobalt and silver nanoparticles to the earthworm Eisenia fetida, Nanotoxicology, 2012, 6, 186–195 CrossRef CAS PubMed.
- M. Diez-Ortiz, E. Lahive, P. Kille, K. Powell, A. J. Morgan, K. Jurkschat, C. A. M. Van Gestel, J. F. W. Mosselmans, C. Svendsen and D. J. Spurgeon, Uptake routes and toxicokinetics of silver nanoparticles and silver ions in the earthworm Lumbricus rubellus, Environ. Toxicol. Chem., 2015, 34, 2263–2270 CrossRef CAS PubMed.
- K. Tatsi, B. J. Shaw, T. H. Hutchinson and R. D. Handy, Copper accumulation and toxicity in earthworms exposed to CuO nanomaterials: Effects of particle coating and soil ageing, Ecotoxicol. Environ. Saf., 2018, 166, 462–473 CrossRef CAS PubMed.
- J. M. Unrine, W. A. Shoults-Wilson, O. Zhurbich, P. M. Bertsch and O. V. Tsyusko, Trophic transfer of Au nanoparticles from soil along a simulated terrestrial food chain, Environ. Sci. Technol., 2012, 46, 9753–9760 CrossRef CAS PubMed.
- R. D. Handy, J. Ahtiainen, J. M. Navas, G. Goss, E. A. Bleeker and F. von der Kammer, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046 RSC.
- H. Selck, R. D. Handy, T. F. Fernandes, S. J. Klaine and E. J. Petersen, Nanomaterials in the aquatic environment: A European Union–United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead, Environ. Toxicol. Chem., 2016, 35, 1055–1067 CrossRef CAS PubMed.
- B. J. Shaw and R. D. Handy, Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions, Environ. Int., 2011, 37, 1083–1097 CrossRef CAS PubMed.
- S. Bellmann, D. Carlander, A. Fasano, D. Momcilovic, J. A. Scimeca, W. J. Waldman, L. Gombau, L. Tsytsikova, R. Canady and D. I. Pereira, Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 609–622 CAS.
- H. Bouwmeester, M. van der Zande and M. A. Jepson, Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2018, 10, e1481 Search PubMed.
- R. D. Handy, F. von der Kammer, J. R. Lead, M. Hassellov, R. Owen and M. Crane, The ecotoxicology and chemistry of manufactured nanoparticles, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS PubMed.
- B. Derjaguin and L. Landau, Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes, Acta Physicochim. URSS, 1941, 14, 733–762 Search PubMed.
- E. Verwey and J. Overbeek, Theory of the stability of lyophobic colloids: the interaction of sol particles having an electric double layer, Elsevier, New York, 1948 Search PubMed.
- G. A. Al-Bairuty, D. Boyle, T. B. Henry and R. D. Handy, Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: physiology and metal accumulation, Aquat. Toxicol., 2016, 174, 188–198 CrossRef CAS PubMed.
- N. D. Dono, N. H. Sparks and O. A. Olukosi, Association between digesta pH, body weight, and nutrient utilization in chickens of different body weights and at different ages, J. Poultry Sci., 2014, 51, 180–184 CAS.
- Q. J. Wu, Y. M. Zhou, Y. N. Wu and T. Wang, Intestinal development and function of broiler chickens on diets supplemented with clinoptilolite, Asian-Australas. J. Anim. Sci., 2013, 26, 987–994 CrossRef CAS PubMed.
- C. Bucking and C. M. Wood, Gastrointestinal transport of Ca2+ and Mg2+ during the digestion of a single meal in the freshwater rainbow trout, J. Comp. Physiol., B, 2007, 177, 349–360 CrossRef CAS PubMed.
- C. Tuleu, C. Andrieux, P. Boy and J. C. Chaumeil, Gastrointestinal transit of pellets in rats: effect of size and density, Int. J. Pharm., 1999, 180, 123–131 CrossRef CAS PubMed.
- B. Svihus, Function of the digestive system, J. Appl. Poult. Res., 2014, 23, 306–314 CrossRef CAS.
- U. B. Kompella and V. H. L. Lee, Delivery systems for penetration enhancement of peptide and protein drugs: design considerations, Adv. Drug Delivery Rev., 2001, 46, 211–245 CrossRef CAS PubMed.
- S. J. Moore, Food breakdown in an avian herbivore: who needs teeth?, Aust. J. Zool., 1999, 47, 625–632 CrossRef.
- D. C. Houston and J. A. Copsey, Bone digestion and intestinal morphology of the Bearded Vulture, J. Raptor Res., 1994, 28, 73–78 Search PubMed.
- P. A. Murtaugh, The influence of food concentration and feeding rate on the gut residence time of Daphnia, J. Plankton Res., 1985, 7, 415–420 CrossRef.
- E. Joosse and H. Verhoef, in Advances in Ecological Research, ed. A. Macfadyen and E. Ford, Academic Press, London, 1987, vol. 16, pp. 175–248 Search PubMed.
- D. J. D. Cosín, M. P. Ruiz, M. H. Garvín, M. Ramajo and D. Trigo, Gut load and transit time in Hormogaster elisae (Oligochaeta, Hormogastridae) in laboratory cultures, Eur. J. Soil Biol., 2002, 38, 43–46 CrossRef.
- M. Zimmer, Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach, Biol. Rev., 2002, 77, 455–493 CrossRef PubMed.
- S. R. Gelder, Diet and histophysiology of the alimentary canal of Lumbricillus-lineatus (Oligochaeta, Enchytraeidae), Hydrobiologia, 1984, 115, 71–81 CrossRef.
- J. Vassallo, A. Besinis, R. Boden and R. Handy, The minimum inhibitory concentration (MIC) assay with Escherichia coli: An early tier in the environmental hazard assessment of nanomaterials?, Ecotoxicol. Environ. Saf., 2018, 162, 633–646 CrossRef CAS PubMed.
- A. R. Al-Jubory and R. D. Handy, Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: nystatin, vanadate and novel CO2-sensitive components, Nanotoxicology, 2013, 7, 1282–1301 CrossRef CAS PubMed.
- A. G. Schultz, D. Boyle, D. Chamot, K. J. Ong, K. J. Wilkinson, J. C. McGeer, G. Sunahara and G. G. Goss, Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing, Environ. Chem., 2014, 11, 207–226 CrossRef CAS.
- R. F. Cusimano, D. F. Brakke and G. A. Chapman, Effects of pH on the toxicities of cadmium, copper, and zinc to steelhead trout (Salmo gairdneri), Can. J. Fish. Aquat. Sci., 1986, 43, 1497–1503 CrossRef CAS.
- L. Hollis, L. Muench and R. Playle, Influence of dissolved organic matter on copper binding, and calcium on cadmium binding, by gills of rainbow trout, J. Fish Biol., 1997, 50, 703–720 CrossRef CAS.
- R. Handy and F. Eddy, Effects of inorganic cations on Na+ adsorption to the gill and body surface of rainbow trout, Oncorhynchus mykiss, in dilute solutions, Can. J. Fish. Aquat. Sci., 1991, 48, 1829–1837 CrossRef CAS.
- P. R. Paquin, J. W. Gorsuch, S. Apte, G. E. Batley, K. C. Bowles, P. G. C. Campbell, C. G. Delos, D. M. Di Toro, R. L. Dwyer, F. Galvez, R. W. Gensemer, G. G. Goss, C. Hogstrand, C. R. Janssen, J. C. McGee, R. B. Naddy, R. C. Playle, R. C. Santore, U. Schneider, W. A. Stubblefield, C. M. Wood and K. B. Wu, The biotic ligand model: a historical overview, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 3–35 Search PubMed.
- K. A. de Schamphelaere and C. R. Janssen, A biotic ligand model predicting acute copper toxicity for Daphnia magna: the effects of calcium, magnesium, sodium, potassium, and pH, Environ. Sci. Technol., 2002, 36, 48–54 CrossRef CAS PubMed.
- N. J. Clark, R. Clough, D. Boyle and R. D. Handy, Development of a suitable detection method for silver nanoparticles in fish tissue using single particle ICP-MS, Environ. Sci.: Nano, 2019, 6, 3388–3400 RSC.
- F. Piccapietra, L. Sigg and R. Behra, Colloidal stability of carbonate-coated silver nanoparticles in synthetic and natural freshwater, Environ. Sci. Technol., 2011, 46, 818–825 CrossRef PubMed.
- D. Di Silvio, N. Rigby, B. Bajka, A. Mackie and F. B. Bombelli, Effect of protein corona magnetite nanoparticles derived from bread in vitro digestion on Caco-2 cells morphology and uptake, Int. J. Biochem. Cell Biol., 2016, 75, 212–222 CrossRef CAS PubMed.
- A. P. Walczak, R. Fokkink, R. Peters, P. Tromp, Z. E. H. Rivera, I. M. C. M. Rietjens, P. J. M. Hendriksen and H. Bouwmeester, Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model, Nanotoxicology, 2013, 7, 1198–1210 CrossRef CAS PubMed.
- R. Peters, E. Kramer, A. G. Oomen, Z. E. H. Rivera, G. Oegema, P. C. Tromp, R. Fokkink, A. Rietveld, H. J. P. Marvin, S. Weigel, A. A. C. M. Peijnenburg and H. Bouwmeester, Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive, ACS Nano, 2012, 6, 2441–2451 CrossRef CAS PubMed.
- R. F. Chapman, The Insects: Structure and Function, Cambridge University Press, United Kingdom, 1998 Search PubMed.
- M. M. Martin and J. S. Martin, Surfactants: their role in preventing the precipitation of proteins by tannins in insect guts, Oecologia, 1984, 61, 342–345 CrossRef PubMed.
- R. W. Wilson and M. Grosell, Intestinal bicarbonate secretion in marine teleost fish—source of bicarbonate, pH sensitivity, and consequences for whole animal acid–base and calcium homeostasis, Biochim. Biophys. Acta, Biomembr., 2003, 1618, 163–174 CrossRef CAS PubMed.
- R. Smith and A. McAllan, Binding of magnesium and calcium in the contents of the small intestine of the calf, Br. J. Nutr., 1966, 20, 703–718 CrossRef CAS PubMed.
- W. K. Ma, B. A. Smith, G. L. Stephenson and S. D. Siciliano, Development of a simulated earthworm gut for determining bioaccessible arsenic, copper, and zinc from soil, Environ. Toxicol. Chem., 2009, 28, 1439–1446 CrossRef CAS PubMed.
- M. Carriker, Observations on the functioning of the alimentary system of the snail Lymnaea stagnalis appressa Say, Biol. Bull., 1946, 91, 88–111 CrossRef CAS PubMed.
- M. Zimmer and A. Brune, Physiological properties of the gut lumen of terrestrial isopods (Isopoda: Oniscidea): adaptive to digesting lignocellulose?, J. Comp. Physiol., B, 2005, 175, 275–283 CrossRef CAS PubMed.
- J. R. Lead and K. J. Wilkinson, Aquatic colloids and nanoparticles: Current knowledge and future trends, Environ. Chem., 2006, 3, 159–171 CrossRef CAS.
- M. Baalousha, A. Manciulea, S. Cumberland, K. Kendall and J. R. Lead, Aggregation and surface properties of iron oxide nanoparticles: influence of pH and natural organic matter, Environ. Toxicol. Chem., 2008, 27, 1875–1882 CrossRef CAS PubMed.
- E. von Elert, M. K. Agrawal, C. Gebauer, H. Jaensch, U. Bauer and A. Zitt, Protease activity in gut Daphnia magna: evidence for trypsin and chymotrypsin enzymes, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2004, 137, 287–296 CrossRef PubMed.
- K. A. Tweeten and A. Reiner, Characterization of serine proteases of Lumbriculus variegatus and their role in regeneration, Invertebr Biol., 2012, 131, 11 CrossRef.
- P. Procházková, V. Šustr, J. Dvořák, R. Roubalová, F. Škanta, V. Pižl and M. Bilej, Correlation between the activity of digestive enzymes and nonself recognition in the gut of Eisenia andrei earthworms, J. Invertebr. Pathol., 2013, 114, 217–221 CrossRef PubMed.
- M. L. Prabha, I. A. Jayaraaj, R. Jeyaraaj and S. Rao, Comparative studies on the digestive enzymes in the gut of earthworms, Eudrilus eugeniae and Eisenia fetida, Indian J. Biotechnol., 2007, 6, 567–569 CAS.
- M. Longbottom, Distribution of the digestive enzymes in the gut of Arenicola marina, J. Mar. Biol. Assoc. U. K., 1970, 50, 121–128 CrossRef CAS.
- S. Deguara, K. Jauncey and C. Agius, Enzyme activities and pH variations in the digestive tract of gilthead sea bream, J. Fish Biol., 2003, 62, 1033–1043 CrossRef CAS.
- V. V. Kuzmina, Influence of age on digestive enzyme activity in some freshwater teleosts, Aquaculture, 1996, 148, 25–37 CrossRef CAS.
- M. Saito, E. Murakami, T. Nishida, Y. Fujisawa and M. Suda, Circadian rhythms in digestive enzymes in the small intestine of rats. I. Patterns of the rhythms in various regions of the small intestine, J. Biochem., 1975, 78, 475–480 CrossRef CAS PubMed.
- V. Oleinik, Distribution of digestive enzyme activities along intestine in blue fox, mink, ferret and rat, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1995, 112, 55–58 CrossRef CAS PubMed.
- A. Abdelkhaliq, M. van der Zande, A. K. Undas, R. J. Peters and H. Bouwmeester, Impact of in vitro digestion on gastrointestinal fate and uptake of silver nanoparticles with different surface modifications, Nanotoxicology, 2020, 14, 111–126 CrossRef CAS PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- I. Lynch, T. Cedervall, M. Lundqvist, C. Cabaleiro-Lago, S. Linse and K. A. Dawson, The nanoparticle - protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century, Adv. Colloid Interface Sci., 2007, 134–135, 167–174 CrossRef CAS PubMed.
- M. P. Monopoli, C. Aberg, A. Salvati and K. A. Dawson, Biomolecular coronas provide the biological identity of nanosized materials, Nat. Nanotechnol., 2012, 7, 779–786 CrossRef CAS PubMed.
- S. J. Soenen, W. J. Parak, J. Rejman and B. Manshian, (Intra)Cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications, Chem. Rev., 2015, 115, 2109–2135 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, F. Y. Song, R. Liu, H. B. Guo, D. W. H. Olsen, Y. Cohen, A. Emili and W. C. W. Chan, Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles, ACS Nano, 2014, 8, 2439–2455 CrossRef CAS PubMed.
- A. P. Walczak, E. Kramer, P. J. M. Hendriksen, R. Helsdingen, M. van der Zande, I. M. C. M. Rietjens and H. Bouwmeester, In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model, Nanotoxicology, 2015, 9, 886–894 CrossRef PubMed.
- D. Lichtenstein, J. Ebmeyer, P. Knappe, S. Juling, L. Bohmert, S. Selve, B. Niemann, A. Braeuning, A. F. Thunemann and A. Lampen, Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells, Biol. Chem., 2015, 396, 1255–1264 CAS.
- T. Romih, K. Kogej and D. Drobne, A novel approach to the measurement of surfactant parameters in arthropod digestive juices, J. Insect Physiol., 2016, 88, 48–54 CrossRef CAS PubMed.
- E. Bauer, N. Lampert, A. Mikaelyan, T. Köhler, K. Maekawa and A. Brune, Physicochemical conditions, metabolites and community structure of the bacterial microbiota in the gut of wood-feeding cockroaches (Blaberidae: Panesthiinae), FEMS Microbiol. Ecol., 2015, 91, 1–14 CrossRef PubMed.
- C. Plante and P. Jumars, The microbial environment of marine deposit-feeder guts characterized via microelectrodes, Microb. Ecol., 1992, 23, 257–277 CrossRef CAS PubMed.
- B. Spanggaard, I. Huber, J. Nielsen, T. Nielsen, K. Appel and L. Gram, The microflora of rainbow trout intestine: a comparison of traditional and molecular identification, Aquaculture, 2000, 182, 1–15 CrossRef CAS.
- M. G. Espey, Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota, Free Radical Biol. Med., 2013, 55, 130–140 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner and G. V. Lowry, Sulfidation of silver nanoparticles: Natural antidote to their toxicity, Environ. Sci. Technol., 2013, 47, 13440–13448 CrossRef CAS PubMed.
- J. P. Stegemeier, F. Schwab, B. P. Colman, S. M. Webb, M. Newville, A. Lanzirotte, C. Winkler, M. R. Wiesner and G. V. Lowry, Speciation matters: Bioavailability of silver and silver sulfide nanoparticles to alfalfa (Medicago sativa), Environ. Sci. Technol., 2015, 49, 8451–8460 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle and R. D. Handy, An assessment of the dietary bioavailability of silver nanomaterials in rainbow trout using an ex vivo gut sac technique, Environ. Sci.: Nano, 2019, 6, 646–660 RSC.
- A. Parker, M. A. E. Lawson, L. Vaux and C. Pin, Host-microbe interaction in the gastrointestinal tract, Environ. Microbiol., 2018, 20, 2337–2353 CrossRef PubMed.
- A. Pietroiusti, A. Magrini and L. Campagnolo, New frontiers in nanotoxicology: Gut microbiota/microbiome-mediated effects of engineered nanomaterials, Toxicol. Appl. Pharmacol., 2016, 299, 90–95 CrossRef CAS PubMed.
- R. H. Stauber, S. Siemer, S. Becker, G.-B. Ding, S. Strieth and S. K. Knauer, Small meets smaller: effects of nanomaterials on microbial biology, pathology, and ecology, ACS Nano, 2018, 12, 6351–6359 CrossRef CAS PubMed.
- D. L. Merrifield and O. Carnevali, Probiotic modulation of the gut microbiota of fish, in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, ed. D. Merrifield and E. Ringø, John Wiley & Sons, Ltd., Chichester, UK, 2014 Search PubMed.
- T. Pedley, Calculation of unstirred layer thickness in membrane transport experiments: a survey, Q. Rev. Biophys., 1983, 16, 115–150 CrossRef CAS PubMed.
- P. H. Barry and J. M. Diamond, Effects of unstirred layers on membrane phenomena, Physiol. Rev., 1984, 64, 763–872 CrossRef CAS PubMed.
- I. N. Ross, H. M. Bahari and L. A. Turnberg, The pH gradient across mucus adherent to rat fundic mucosa in vivo and the effect of potential damaging agents, Gastroenterology, 1981, 81, 713–718 CrossRef CAS.
- R. Handy and F. Eddy, in Physicochemical Kinetics and Transport at Chemical-Biological Interphases, ed. H. van Leeuwen and W. Köster, John Wiley, Chichester, 2004, pp. 337–356 Search PubMed.
- R. Handy and R. Maunder, in Osmoregulation and Ion Transport: Integrating Physiological, Molecular and Environmental Aspects, ed. R. Handy, N. Bury and G. Flik, Society for Experimental Biology Press, London, 2009, vol. 1, pp. 203–235 Search PubMed.
- S. S. Olmsted, J. L. Padgett, A. I. Yudin, K. J. Whaley, T. R. Moench and R. A. Cone, Diffusion of macromolecules and virus-like particles in human cervical mucus, Biophys. J., 2001, 81, 1930–1937 CrossRef CAS PubMed.
- S. K. Lai, Y. Y. Wang and J. Hanes, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues, Adv. Drug Delivery Rev., 2009, 61, 158–171 CrossRef CAS PubMed.
- S. K. Lai, Y. Y. Wang, K. Hida, R. Cone and J. Hanes, Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 598–603 CrossRef CAS PubMed.
- P. Lundquist and P. Artursson, Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues, Adv. Drug Delivery Rev., 2016, 106, 256–276 CrossRef CAS PubMed.
- D. A. Norris, N. Puri and P. J. Sinko, The effect of physical barriers and properties on the oral absorption of particulates, Adv. Drug Delivery Rev., 1998, 34, 135–154 CrossRef CAS PubMed.
- N. Millot, IV - On the morphology of the alimentary canal, process of feeding, and physiology of digestion of the nudibranch mollusc Forunna tomentosa (Cuvier), Philos. Trans. R. Soc., B, 1937, 228, 173–217 Search PubMed.
- M. W. Deny, Invertebrate mucous secretions: functional alternatives to vertebrate paradigms, Symp. Soc. Exp. Biol., 1989, 43, 337–366 Search PubMed.
- E. E. Ruppert, R. D. Barnes and R. S. Fox, Invertebrate Zoology: A Functional Evolutionary Approach, Brooks/Cole - Thompson Learning, Belmont, USA, 2004 Search PubMed.
- R. Brusca, W. Moore and M. Schuster, Invertebrates, Sinauer Associates, Inc, Publishers, Massachusetts, 2016 Search PubMed.
- V. H. C. Resh and R. T. Carde, Encyclopedia of Insects, Elsevier Science, USA, 2003 Search PubMed.
- C. A. C. Hames and S. P. Hopkin, The structure and function of the digestive-system of terrestrial isopods, J. Zool., 1989, 217, 599–627 CrossRef.
- S. Novak, D. Drobne, L. Vaccari, M. Kiskinova, P. Ferraris, G. Birarda, M. Remskar and M. Hocevar, Effect of ingested tungsten oxide (WOx) nanofibers on digestive gland tissue of Porcellio scaber (Isopoda, Crustacea): fourier transform infrared (FTIR) imaging, Environ. Sci. Technol., 2013, 47, 11284–11292 CrossRef CAS PubMed.
- U. Bogataj, M. Praznik, P. Mrak, J. Štrus, M. Tušek-Žnidarič and N. Žnidaršič, Comparative ultrastructure of cells and cuticle in the anterior chamber and papillate region of Porcellio scaber (Crustacea, Isopoda) hindgut, ZooKeys, 2018, 427 CrossRef PubMed.
- J. Šobotník, G. Alberti, F. Weyda and J. Hubert, Ultrastructure of the digestive tract in Acarus siro (Acari : Acaridida), J. Morphol., 2008, 269, 54–71 CrossRef PubMed.
- J. Reddy and M. Locke, The size limited penetration of gold particles through insect basal laminae, J. Insect Physiol., 1990, 36, 397–407 CrossRef CAS.
- S. J. H. Clearwater, R. D. Handy and C. Hogstrand, in Toxicity of Dietborne Metals to Aquatic Organisms, ed. J. S. Meyer, W. J. Adams, K. V. Brix, S. N. Luoma, D. R. Mount, W. A. Stubblefield and C. M. Wood, SETAC Press, Pensacola, USA, 2005, pp. 205–225 Search PubMed.
- J. M. A. C. Wilson and L. F. C. Castro, in The Multifunctional Gut of Fish, ed. M. F. Grosell, A. P. Farrell and C. J. Brauner, Fish Physiology series, Academic Press, London, vol. 30, 2011 Search PubMed.
- F. B. Eddy and R. D. Handy, Ecological and Environmental Physiology of Fishes, Oxford University Press, 2012 Search PubMed.
- E. C. Foulkes and D. M. McMullen, Kinetics of trans-epithelial movement of heavy-metals in rat jejunum, Am. J. Physiol., 1987, 253, G134–G138 CAS.
- T. J. Cook and S. S. Shenoy, Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat, Toxicology, 2003, 184, 125–133 CrossRef CAS PubMed.
- T. T. Kararli, Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals, Biopharm. Drug Dispos., 1995, 16, 351–380 CrossRef CAS PubMed.
- H. F. Helander and L. Fandriks, Surface area of the digestive tract - revisited, Scand. J. Gastroenterol., 2014, 49, 681–689 CrossRef PubMed.
- P. C. Tyrer, A. R. Foxwell, J. M. Kyd, D. C. Otczyk and A. W. Cripps, Receptor mediated targeting of M-cells, Vaccine, 2007, 25, 3204–3209 CrossRef CAS PubMed.
- E. Gullberg, M. Leonard, J. Karlsson, A. M. Hopkins, D. Brayden, A. W. Baird and P. Artursson, Expression of specific markers and particle transport in a new human intestinal M-cell model, Biochem. Biophys. Res. Commun., 2000, 279, 808–813 CrossRef CAS PubMed.
- E. Gullberg, A. V. Keita, S. Y. Salim, M. Andersson, K. D. Caldwell, J. D. Soderholm and P. Artursson, Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the Peyer's patches, J. Pharmacol. Exp. Ther., 2006, 319, 632–639 CrossRef CAS PubMed.
- J. Soni, A. W. Baird, L. M. O'Brien, M. McElroy, J. J. Callanan, H. F. Bassett, D. Campion and D. J. Brayden, Rat, ovine and bovine Peyer's patches mounted in horizontal diffusion chambers display sampling function, J. Controlled Release, 2006, 115, 68–77 CrossRef CAS PubMed.
- W. H. Karasov, Digestive adaptations in avian omnivores, in Nutrition in a Sustainable Environment, ed. M. L. Wahlqvist, Smith-Gordon, Edinburgh, UK, 1994, pp. 494–497 Search PubMed.
- R. E. Olsen, R. R. Cox, A. D. Afton and C. D. Ankney, Diet and gut morphology of male mallards during winter in North Dakota, Waterbirds, 2011, 34, 59–69 CrossRef.
- P. F. Battley, M. W. Dietz, T. Piersma, A. Dekinga, S. X. Tang and K. Hulsman, Is long-distance bird flight equivalent to a high-energy fast? Body composition changes in freely migrating and captive fasting great knots, Physiol. Biochem. Zool., 2001, 74, 435–449 CrossRef CAS PubMed.
- J. R. Jehl, Cyclical changes in body composition in the annual cycle and migration of the eared Grebe Podiceps nigricollis, J. Avian Biol., 1997, 28, 132–142 CrossRef.
- A. Amerah, V. Ravindran, R. Lentle and D. Thomas, Feed particle size: Implications on the digestion and performance of poultry, World's Poult. Sci. J., 2007, 63, 439–455 CrossRef.
- M. H. Clench and J. R. Mathias, The avian cecum - a review, Wilson Bull., 1995, 107, 93–121 Search PubMed.
- N. Sirirat, J.-J. Lu, A. T.-Y. Hung, S.-Y. Chen and T.-F. Lien, Effects different levels of nanoparticles chromium picolinate supplementation on growth performance, mineral retention, and immune responses in broiler chickens, J. Agric. Sci., 2012, 4, 48 Search PubMed.
- L. Staehelin, Further observations on the fine structure of freeze-cleaved tight junctions, J. Cell Sci., 1973, 13, 763–786 CAS.
- I.-C. Lin, M. Liang, T.-Y. Liu, Z. M. Ziora, M. J. Monteiro and I. Toth, Interaction of densely polymer-coated gold nanoparticles with epithelial Caco-2 monolayers, Biomacromolecules, 2011, 12, 1339–1348 CrossRef CAS PubMed.
- H. H. Ussing and K. Zerahn, Active transport of sodium as the source of electric current in the short-circuited isolated frog skin, Acta Physiol. Scand., 1951, 23, 110–127 CrossRef CAS PubMed.
- E. H. Larsen, Hans H. Ussing—scientific work: contemporary significance and perspectives, Biochim. Biophys. Acta, Biomembr., 2002, 1566, 2–15 CrossRef CAS.
- V. Hartenstein and P. Martinez, Phagocytosis in cellular defense and nutrition: a food-centered approach to the evolution of macrophages, Cell Tissue Res., 2019, 377, 527–547 CrossRef CAS PubMed.
- F. Zhao, Y. Zhao, Y. Liu, X. Chang, C. Chen and Y. Zhao, Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials, Small, 2011, 7, 1322–1337 CrossRef CAS PubMed.
- M. Rescigno, M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J.-P. Kraehenbuhl and P. Ricciardi-Castagnoli, Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria, Nat. Immunol., 2001, 2, 361–367 CrossRef CAS PubMed.
- R. Qiao, A. P. Roberts, A. S. Mount, S. J. Klaine and P. C. Ke, Translocation of C60 and its derivatives across a lipid bilayer, Nano Lett., 2007, 7, 614–619 CrossRef CAS PubMed.
- M. Ishii, Y. Fukuoka, S. Deguchi, H. Otake, T. Tanino and N. Nagai, Energy-dependent endocytosis Is involved in the absorption of indomethacin nanoparticles in the small intestine, Int. J. Mol. Sci., 2019, 20, 476 CrossRef CAS PubMed.
- C. Gitrowski, A. R. Al-Jubory and R. D. Handy, Uptake of different crystal structures of TiO2 nanoparticles by Caco-2 intestinal cells, Toxicol. Lett., 2014, 226, 264–276 CrossRef CAS PubMed.
- D. E. Lefebvre, K. Venema, L. Gombau, L. G. Valerio Jr, J. Raju, G. S. Bondy, H. Bouwmeester, R. P. Singh, A. J. Clippinger and E.-M. Collnot, Utility of models of the gastrointestinal tract for assessment of the digestion and absorption of engineered nanomaterials released from food matrices, Nanotoxicology, 2015, 9, 523–542 CrossRef CAS PubMed.
- A. D. Rieux, V. Pourcelle, P. D. Cani, J. Marchand-Brynaert and V. Preat, Targeted nanoparticles with novel non-peptidic ligands for oral delivery, Adv. Drug Delivery Rev., 2013, 65, 833–844 CrossRef PubMed.
- C. M. Yonge, Evolution and adaptation in the digestive system of the Metazoa, Biol. Rev., 1937, 12, 87–114 CrossRef.
- D. M. Kermace, The anatomy and physiology of the gut of the polychaete Arenicola marina (L.), Proc. Zool. Soc. London, 1955, 125, 347–381 CrossRef.
- C. Jeuniaux, Nutrition and digestion, Chemical zoology. Volume IV: Annelida, Echiura, and Sipuncula, 1969, pp. 69–91 Search PubMed.
- W.-X. Wang, N. S. Fisher and S. N. Luoma, Assimilation of trace elements ingested by the mussel Mytilus edulis: effects of algal food abundance, Mar. Ecol.: Prog. Ser., 1995, 129, 165–176 CrossRef CAS.
- J. Riley, Histochemical and ultrastructural observations on digestion in Tetrameres fissispina Diesing, 1861 (Nematoda: Spiruridea) with special reference to intracellular digestion, Int. J. Parasitol., 1973, 3, 157–164 CrossRef CAS.
- M. D. Garcia-Castillo, D. J.-F. Chinnapen and W. I. Lencer, Membrane transport across polarized epithelia, Cold Spring Harbor Perspect. Biol., 2017, 9, a027912 CrossRef PubMed.
- A. P. Walczak, P. J. M. Hendriksen, R. A. Woutersen, M. van der Zande, A. K. Undas, R. Helsdingen, H. H. J. van den Berg, I. M. C. M. Rietjens and H. Bouwmeester, Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats, J. Nanopart. Res., 2015, 17, 231 CrossRef PubMed.
- M. Heringa, R. Peters, R. Bleys, M. Van Der Lee, P. Tromp, P. Van Kesteren, J. van Eijkeren, A. Undas, A. Oomen and H. Bouwmeester, Detection of titanium particles in human liver and spleen and possible health implications, Part. Fibre Toxicol., 2018, 15, 15 CrossRef CAS PubMed.
- R. D. Handy, M. M. Musonda, C. Phillips and S. J. Falla, Mechanisms of gastrointestinal copper absorption in the African walking catfish: Copper dose-effects and a novel anion-dependent pathway in the intestine, J. Exp. Biol., 2000, 203, 2365–2377 CAS.
- M. Alaraby, A. Hernández, B. Annangi, E. Demir, J. Bach, L. Rubio, A. Creus and R. Marcos, Antioxidant and antigenotoxic properties of CeO2 NPs and cerium sulphate: studies with Drosophila melanogaster as a promising in vivo model, Nanotoxicology, 2015, 9, 749–759 CrossRef CAS PubMed.
- N. Couleau, D. Techer, C. Pagnout, S. Jomini, L. Foucaud, P. Laval-Gilly, J. Falla and A. Bennasroune, Hemocyte responses of Dreissena polymorpha following a short-term in vivo exposure to titanium dioxide nanoparticles: preliminary investigations, Sci. Total Environ., 2012, 438, 490–497 CrossRef CAS PubMed.
- L. Canesi, C. Ciacci, M. Betti, R. Fabbri, B. Canonico, A. Fantinati, A. Marcomini and G. Pojana, Immunotoxicity of carbon black nanoparticles to blue mussel hemocytes, Environ. Int., 2008, 34, 1114–1119 CrossRef CAS PubMed.
- A. Katsumiti, D. Berhanu, K. T. Howard, I. Arostegui, M. Oron, P. Reip, E. Valsami-Jones and M. P. Cajaraville, Cytotoxicity of TiO2 nanoparticles to mussel hemocytes and gill cells in vitro: Influence of synthesis method, crystalline structure, size and additive, Nanotoxicology, 2015, 9, 543–553 CrossRef CAS PubMed.
- E. L. Cooper, Immune diversity throughout the animal kingdom, BioScience, 1990, 40, 720–722 CrossRef.
- T. Galloway and R. Handy, Immunotoxicity of organophosphorous pesticides, Ecotoxicology, 2003, 12, 345–363 CrossRef CAS PubMed.
- C. L. Prosser and F. A. Brown, Comparative Animal Physiology, W. B. Saunders Company, Philadelphia, USA, 2nd edn, 1961 Search PubMed.
- A. V. Ereskovsky, D. V. Lavrov, N. Boury-Esnault and J. Vacelet, Molecular and morphological description of a new species of Halisarca (Demospongiae: Halisarcida) from Mediterranean Sea and a redescription of the type species Halisarca dujardini, Zootaxa, 2011, 2768, 5–31 CrossRef.
- C. J. Smith, B. J. Shaw and R. D. Handy, Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects, Aquat. Toxicol., 2007, 82, 94–109 CrossRef CAS PubMed.
- D. Boyle, G. A. Al-Bairuty, T. B. Henry and R. D. Handy, Critical comparison of intravenous injection of TiO2 nanoparticles with waterborne and dietary exposures concludes minimal environmentally-relevant toxicity in juvenile rainbow trout Oncorhynchus mykiss, Environ. Pollut., 2013, 182, 70–79 CrossRef CAS PubMed.
- R. Handy and G. Al-Bairuty, Effects of nanomaterials on the body systems of fishes - An overview from target organ pathology, in Ecotoxicology of Nanoparticles in Aquatic Systems, ed. J. Blasco and I. Corsi, CRC Press, Boca Raton, Florida, 2019, pp. 156–168 Search PubMed.
- R. P. Lanno, B. Hicks and J. W. Hilton, Histological observations on intrahepatocytic copper-containing granules in rainbow trout reared on diets containing elevated levels of copper, Aquat. Toxicol., 1987, 10, 251–263 CrossRef CAS.
- B. E. Brown, The form and fuction of metal-containing 'granules' in invertebrate tissues, Biol. Rev., 1982, 57, 621–667 CrossRef CAS.
- S. Novak, D. Drobne, M. Golobič, J. Zupanc, T. Romih, A. Gianoncelli, M. Kiskinova, B. Kaulich, P. Pelicon and P. Vavpetič, Cellular internalization of dissolved cobalt ions from ingested CoFe2O4 nanoparticles: in vivo experimental evidence, Environ. Sci. Technol., 2013, 47, 5400–5408 CrossRef CAS PubMed.
- N. W. Van den Brink, A. Jeme Kokalj, P. V. Silva, E. Lahive, K. Norrfors, M. Baccaro, Z. Khodaparast, S. Loureiro, D. Drobne, G. Cornelis, S. Lofts, R. D. Handy, C. Svendsen, D. Spurgeon and C. A. M. Van Gestel, Tools and rules for modelling uptake and bioaccumulation of nanomaterials in invertebrate organisms, Environ. Sci.: Nano, 2019, 6, 1985–2001 RSC.
- M. S. Laverack, The Physiology of Earthworms, The Macmillan Company, New York, 1963 Search PubMed.
- E. K. Tillinghast, R. O'Donnell, D. Eves, E. Calvert and J. Taylor, Water-soluble luminal contents of the gut of the earthworm Lumbricus terrestris L. and their physiological significance, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2001, 129, 345–353 CrossRef CAS.
- P. K. Wust, M. A. Horn and H. L. Drake, In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris, Appl. Environ. Microbiol., 2009, 75, 1852–1859 CrossRef PubMed.
- J. W. Moore, Importance of algae in diet of oligochaetes Lumbriculus variegatus (Muller) and Rhyacodrilus sodalis (Eisen), Oecologia, 1978, 35, 357–363 CrossRef PubMed.
- L. M. Mayer, L. L. Schick, R. F. Self, P. A. Jumars, R. H. Findlay, Z. Chen and S. Sampson, Digestive environments of benthic macroinvertebrate guts: enzymes, surfactants and dissolved organic matter, J. Mar. Res., 1997, 55, 785–812 CrossRef CAS.
- I. M. Voparil and L. M. Mayer, Commercially available chemicals that mimic a deposit feeder's (Arenicola marina) digestive solubilization of lipids, Environ. Sci. Technol., 2004, 38, 4334–4339 CrossRef CAS PubMed.
- J. D. McGhee, The C. elegans intestine, WormBook, ed. The C. elegans Research Community, 2007, DOI:10.1895/wormbook.1.133.1, http://www.wormbook.org.
- O. V. Lindqvist and G. Fitzgerald, Osmotic interrelationship between blood and gut fluid in the isopod Porcellio scaber Latr. (Crustacea), Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1976, 53, 3 Search PubMed.
- A. D. Hasler, The physiology of digestion of plankton crustacea: I some digestive enzymes of Daphnia, Biol. Bull., 1935, 68, 207–214 CrossRef CAS.
- A. F. MacFadyen and E. D. Ford, Advances in Ecological Research, Academic Press, 1987 Search PubMed.
- T. H. Erban and J. Hubert, Digestive physiology of synanthropic mites (Acari: Acaridida), SOAJ Entomological Studies, 2012, vol. 1, pp. 1–32 Search PubMed.
- W. R. Terra and C. Ferriera, Insect digestive enzymes: properties, compartmentalization and function, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1994, 109, 1–62 CrossRef.
- M. M. Martin, D. C. Rockholm and J. S. Martin, Effects of surfactants, pH, and certain cations on precipitation of proteins by tannins, J. Chem. Ecol., 1985, 11, 485–494 CrossRef CAS PubMed.
- C. Bucking and C. M. Wood, The effect of postprandial changes in pH along the gastrointestinal tract on the distribution of ions between the solid and fluid phases of chime in rainbow trout, Aquacult. Nutr., 2009, 15, 282–296 CrossRef CAS.
- Z. H. Shehadeh and M. S. Gordon, The role of the intestine in salinity adaptation of the rainbow trout Salmo gairdneri, Comp. Biochem. Physiol., 1969, 30, 397–418 CAS.
- C. Bucking and C. M. Wood, Gastrointestinal processing of Na+, Cl-, and K+ during digestion: implications for homeostatic balance in freshwater rainbow trout, Am. J. Physiol., 2006, 291, R1764–1772 CAS.
- H. W. Smith, Observations on the flora of the alimentary tract of animals and factors affecting its composition, J. Pathol. Bacteriol., 1965, 89, 95–122 CrossRef CAS PubMed.
- J. S. Fordtran and T. W. Locklear, Ionic constituents and osmolarity of gastric and small-intestinal fluids after eating, Am. J. Dig. Dis., 1966, 11, 503–521 CrossRef CAS PubMed.
- Y. Tanaka, T. Hara, R. Waki and S. Nagata, Regional differences in the components of luminal water from rat gastrointestinal tract and comparison with other species, J. Pharm. Pharm. Sci., 2012, 15, 510–518 CAS.
- M. Mitchell and M. Moretó, Absorptive function of the small intestine: adaptations meeting demand, in Avian Gut Function Health and Disease, ed. G. C. Perry, CAB International, Wallingford, UK, 2006, pp. 43–63 Search PubMed.
- S. Hurwitz and A. Bar, Activity, concentration, and lumen-blood electrochemical potential difference of calcium in the intestine of the laying hen, J. Nutr., 1968, 95, 647–654 CrossRef CAS PubMed.
- A. G. Oomen, C. J. Rompelberg, M. A. Bruil, C. J. Dobbe, D. P. Pereboom and A. J. Sips, Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants, Arch. Environ. Contam. Toxicol., 2003, 44, 281–287 CrossRef CAS PubMed.
- D. M. Mudie, G. L. Amidon and G. E. Amidon, Physiological parameters for oral delivery and in vitro testing, Mol. Pharmaceutics, 2010, 7, 1388–1405 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food - an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- W. Peters and V. Walldorf, Endodermal secretion of chitin in the cuticle of the earthworm gizzard, Tissue Cell, 1986, 18, 361–374 CrossRef CAS PubMed.
- W. Peters, Peritrophic Membranes, Zoophysiology series, Springer-Verlag, Berlin, 2012, vol. 30 Search PubMed.
- C. A. Edwards and P. J. Bohlen, Biology and Ecology of Earthworms, Chapman and Hall, London, 3rd edn, 1996 Search PubMed.
- S. P. Hopkin, Ecophysiology of Metals in Terrestrial Invertebrates, Elsevier Applied Science Publishers, 1989 Search PubMed.
- G. Bryan and P. Gibbs, Occurrence, composition and function of intracellular calcium phosphate granules in the musculature of nephtyid polychaetes (Annelida), J. Mar. Biol. Assoc. U. K., 1986, 66, 343–365 CrossRef CAS.
- I. Dimov and M. F. Maduro, The C. elegans intestine: organogenesis, digestion, and physiology, Cell Tissue Res., 2019, 1–14 Search PubMed.
- D. G. Albertson and J. Thompson, The pharynx of Caenorhabditis elegans, Philos. Trans. R. Soc., B, 1976, 275, 299–325 CAS.
- R. S. Edgar, G. N. Cox, M. Kusch and J. C. Politz, The cuticle of Caenorhabditis elegans, J. Nematol., 1982, 14, 248 CAS.
- H. Fares and B. Grant, Deciphering endocytosis in Caenorhabditis elegans, Traffic, 2002, 3, 11–19 CrossRef PubMed.
- B. Jackson, P. Williams, A. Lanzirotti and P. Bertsch, Evidence for biogenic pyromorphite formation by the nematode Caenorhabditis elegans, Environ. Sci. Technol., 2005, 39, 5620–5625 CrossRef CAS PubMed.
- A. Lobo-da-Cunha and C. Batista-Pinto, Stomach of Aplysia depilans (Mollusca, Opisthobranchia): a histochemical, ultrastructural, and cytochemical study, J. Morphol., 2003, 256, 360–370 CrossRef PubMed.
- H. H. Boer and K. S. Kits, Histochemical and ultrastructural study of the alimentary tract of the freshwater snail Lymnaea stagnalis, J. Morphol., 1990, 205, 97–111 CrossRef CAS PubMed.
- M. M. Desouky, Tissue distribution and subcellular localization of trace metals in the pond snail Lymnaea stagnalis with special reference to the role of lysosomal granules in metal sequestration, Aquat. Toxicol., 2006, 77, 143–152 CrossRef CAS PubMed.
- P. Mrak, U. Bogataj, J. Strus and N. Znidarsic, Formation of the hindgut cuticular lining during embryonic development of Porcellio scaber (Crustacea, Isopoda), Zookeys, 2015, 93–109, DOI:10.3897/zookeys.515.9468.
- J.-W. Wägele, U. Welsch and W. Müller, Fine structure and function of the digestive tract of Cyathura carinata (Krøyer)(Crustacea, Isopoda), Zoomorphology, 1981, 98, 69–88 CrossRef.
- G. Vernon, L. Herold and E. Witkus, Fine structure of the digestive tract epithelium in the terrestrial isopod, Armadillidium vulgare, J. Morphol., 1974, 144, 337–359 CrossRef CAS PubMed.
- N. N. Smirnov, Physiology of the Cladocera, Academic Press, London, 2nd edn, 2017 Search PubMed.
- U. Hansen and W. Peters, Structure and permeability of the peritrophic membranes of some small crustaceans, Zool. Anz., 1998, 236, 103–108 Search PubMed.
- T. W. Schultz and J. R. Kennedy, The fine structure of the digestive system of Daphnia pulex (Crustacea: Cladocera), Tissue Cell, 1976, 8, 479–490 CrossRef CAS PubMed.
- A. Quaglia, B. Sabelli and L. Villani, Studies on the intestine of Daphnidae (Crustacea, Cladocera) ultrastructure of the midgut of Daphnia magna and Daphnia obtusa, J. Morphol., 1976, 150, 711–725 CrossRef CAS PubMed.
- P. Griffiths, Morphological and ultrastructural effects of sublethal cadmium poisoning on Daphnia, Environ. Res., 1980, 22, 277–284 CrossRef CAS PubMed.
- W. Humbert, The midgut of Tomocerus minor Lubbock (insecta, collembola): ultrastructure, cytochemistry, ageing and renewal during a moulting cycle, Cell Tissue Res., 1979, 196, 39–57 CrossRef CAS PubMed.
- M. Pawert, R. Triebskorn, S. Graff, M. Berkus, J. Schulz and H. R. Kohler, Cellular alterations in collembolan midgut cells as a marker of heavy metal exposure: Ultrastructure and intracellular metal distribution, Sci. Total Environ., 1996, 181, 187–200 CrossRef CAS PubMed.
- D. E. Walter and H. C. Proctor, Mites. Ecology, Evolution and Behaviour, CAB International, Wallingford, 1999 Search PubMed.
- J. Bucking, in Acarid Phylogeny and Evolution. Adaptations in Mites and Ticks, ed. F. Bernini, R. Nannelli, G. Nuzzaci and E. de Lillo, Kluwer Academic Publishers, Dordrecht, 2002, pp. 217–225 Search PubMed.
- M. Ludwig, M. Kratzmann and G. Alberti, Observations on the proventricular glands (Organes Racemiformes) of the oribatid mite chamobates-borealis (Acari, Oribatida) - an organ of interest for studies on adaptation of animals to acid soils, Exp. Appl. Acarol., 1992, 15, 49–57 CrossRef.
- F. B. Dade, Anatomy and dissection of the honeybee, International Bee Research Association, United Kingdom, 2009 Search PubMed.
- R. E. Snodgrass, Anatomy of the Honey Bee, Cornell University Press, USA, 1984 Search PubMed.
- S. H. P. Maddrell and B. O. C. Gardiner, The permeability of the cuticular lining of the insect alimentary canal, J. Exp. Biol., 1980, 85, 227–237 CAS.
- M. Holtof, C. Lenaerts, D. Cullen and J. V. Broeck, Extracellular nutrient digestion and absorption in the insect gut, Cell Tissue Res., 2019, 377, 397–414 CrossRef PubMed.
- H. Raes, W. Bohyn, P. De Rycke and F. Jacobs, Membrane-bound iron-rich granules in fat cells and midgut cells of the adult honeybee (Apis mellifera L.), Apidologie, 1989, 20, 327–337 CrossRef.
- D. E. Sadava, D. M. Hillis, H. C. Heller and M. Berenbaum, Life: The Science of Biology, Sinauer Associates, Sunderland, MA, 10th edn, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00174k |
| This journal is © The Royal Society of Chemistry 2020 |
