Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges†
Jitao
Lv
a,
Peter
Christie
 a and
Shuzhen
Zhang
a and
Shuzhen
Zhang
 *ab
*ab
aState Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China. E-mail: szzhang@rcees.ac.cn
bUniversity of the Chinese Academy of Sciences, Beijing 100049, China
First published on 31st October 2018
Abstract
Interactions between engineered nanoparticles (ENPs) and plants represent one of the fundamental problems we must face in the rapid development of nanotechnology. Hundreds of studies have addressed this issue in the past decade. This review summarizes recent research progress on the uptake, translocation and transformation of metal-based ENPs in higher plants. The integrated uptake and transport pathways of ENPs in plants are summarized and the key physiological barriers to plant uptake of ENPs are proposed. Transformation of ENPs in the soil–plant system is discussed, paying particular attention to the effects of phyllosphere and rhizosphere processes on the transformation and plant uptake of ENPs. The advances, limitations and challenges of analytical techniques for the qualitative and quantitative analysis for ENPs in plants are addressed. Furthermore, the key challenges in each field are thoroughly assessed and future perspectives are proposed. This review is intended to provide an unambiguous assessment of the present knowledge on the uptake, translocation and transformation of NPs in higher plants, and also to provide guidance for future research.
Environmental significanceInteractions between engineered nanoparticles (ENPs) and plants is one of the fundamental issues we must face in the development of nanotechnology, because ENPs released into the environment will inevitably interact with plants, a basic component of ecosystems. Although hundreds of studies have addressed plant uptake, translocation, accumulation, transformation, and phytotoxicity of ENPs and even their transmission in the food chain, there remain some critical issues present in the field of NP–plant interactions. In this critical review, mechanisms regarding the uptake and translocation pathways and transformations of ENPs in plants are systematically reviewed. In particular, the analytical technique developments, methodological challenges and future perspectives for related fields are proposed. |
1. Introduction
The rapid development of nanotechnology and extensive commercial applications of engineered nanomaterials raise the risk of discharge of engineered nanoparticles (ENPs) into the environment and especially the soil–plant system.1–4 Briefly, there are two main routes by which ENPs can enter the soil–plant system. One is through the agricultural application of sewage sludges which often contain SiO2, TiO2, ZnO, and Ag NPs.5–8 The other route is through application of nano-agrochemicals such as nano-pesticides, nano-fertilizers and nano-amendments, resulting in the direct entry of SiO2, TiO2, Zn/ZnO, Fe/FeOx, Cu/CuO/Cu(OH)2, CeO2 and Ag NPs into the soil–plant system.9–11After entering the soil–plant system, NPs will inevitably interact with plants and thereby potentially influence plant physiology and possibly food security. Nano-phytotoxicity is the most widely studied aspect of studies related to the interactions between plants and ENPs (over 430 papers have been published in the past decade), and both harmful and beneficial effects on plants at the physiological, biochemical and genetic levels have been reported.12–21 The uptake, translocation and accumulation of ENPs in plants play critical roles in the determination of ENP phytotoxicity and may also further influence human food security. Plant uptake of NPs is usually studied in combination with their phytotoxicity, and papers focusing on phytotoxicity, uptake, translocation and accumulation of ENPs in plants have been reviewed from different perspectives.17,22–25 Frankly speaking, present knowledge on the uptake and translocation mechanisms of ENPs in plants remains very limited and is not systematic. Schwab et al. have comprehensively reviewed the various physiological barriers to the uptake and transport of ENPs in plants.23 However, they are concerned only with the influence of plant physiology on plant uptake and transport of ENPs. Another important aspect is that ENPs are highly unstable and abiotic or biotic transformations of ENPs such as redox reactions, aggregation and dissolution of ENPs may occur in the rhizosphere or inside plants, and this will greatly alter the bioavailability, toxicity and fate of the ENPs.26–31 A timely and integrated review of current knowledge on the uptake, translocation, accumulation, and transformation of ENPs in the soil–plant system is therefore needed.
Furthermore, understanding of the uptake, translocation and transformation of NPs in plants or other organisms is highly dependent on the development of specialized analytical techniques. In the past decade various advanced analytical techniques have been used to detect the speciation and location of NPs in organisms at tissue, cellular and sub-cellular levels, but great challenges remain in this field especially for quantitative analysis and in situ detection of NPs in complex matrices such as soils, plants and other organisms.32 Here, state-of-the-art developments in the available and potential techniques for the analysis of interactions between NPs and plants are reviewed, and critical challenges and future needs are proposed.
2. Uptake and translocation pathways of NPs in plants
2.1 Foliar exposure to and uptake of NPs
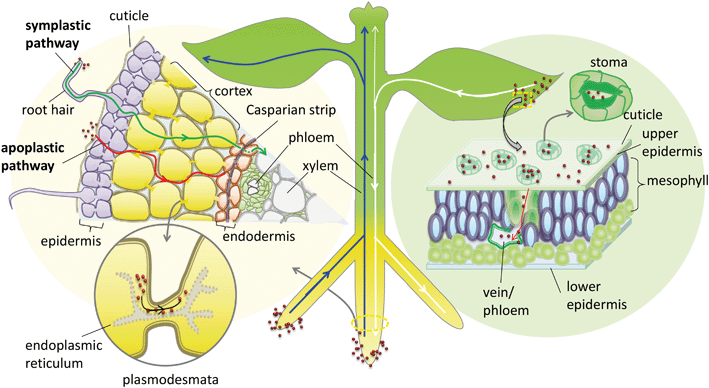
In addition to the cuticular pathway, studies have demonstrated the uptake of hydrophilic substances through stomatal openings (stomatal pathway) (Fig. 1). The morphological size of stomatal apertures is approximately 25 μm in length and 3–10 μm in width.36 However, due to the unique geometric construction and physiological function of stomata, the actual size exclusion limit (SEL) of stomatal aperture for NP penetration is still far from known. Eichert and Goldbach, using an indirect calculation method, estimated the equivalent pore radius of this pathway to be larger than 20 nm.34 This stomatal pathway is the only confirmed pathway of foliar uptake of NPs from the leaf surface to the internal tissues. Numerous studies support this pathway of NP uptake, including the observation of various NPs or their aggregates in leaf stomata and the deeper tissues of different plant species including Citrullus lanatus, Cucurbita pepo, Allium porrum, Lactuca sativa, and Arabidopsis thaliana using TEM, CLSM or μ-XRF.36–40 Eichert et al. investigated the SEL and lateral heterogeneity of the stomatal foliar uptake pathway for water-suspended fluorescent polystyrene NPs.36 The uptake of 43 nm NPs through the stomatal pathway was detected by CLSM, whereas no uptake of 1.1 μm particles was observed. They also observed the distribution of fluorescent NPs in the leaf apoplast once they entered the substomatal cavern. The experimental results indicate that the pore radius of the stomatal pathway estimated by Eichert and Goldbach was an underestimate. Kim et al. found that nano-zerovalent iron (nZVI) induced the activation of plasma membrane H+-ATPase activity and promoted stomatal aperture opening.41 In addition, plant species with different leaf morphology, stomatal size and density are expected to have different capacities for foliar uptake of NPs.37 In a recent study, foliar exposure of the vegetables lettuce, collard greens and kale to CuO NPs (20–100 nm) was studied using single-particle inductively coupled plasma-mass spectrometry (SP-ICP-MS).42 The results indicated that most of the NPs and their aggregates could be rinsed with water, and the retained capacity of the NPs for leaf tissues was dependent on their hydrophobicity and surface roughness.42 Further studies are encouraged to systematically investigate the stomatal pathway of NP uptake by different plant species.
NPs may undergo long-distance transport via the vascular system after entering the leaf apoplast through the stomatal pathway. Traditionally photosynthate, sugars and macromolecules in the leaf, including small RNA and proteins, are able to transport downward via the phloem system to shoots and roots.43 In general, the long-distance transport of liquids in higher plants occurs via the vascular system, which is composed of the xylem and phloem conductive tissues. In the xylem system the direction of flow is from bottom to top (from root to shoot), while in the phloem system the flow direction is from top to bottom (from shoot to root) (Fig. 1). This plant vascular system is noncirculatory in nature, which means that materials moving downward in the phloem do not circulate back to their original sites through the xylem.43 Therefore, the phloem system is the only possible foliar uptake pathway for NPs to translocate from leaf to root. Although many studies have identified the foliar uptake of NPs, no study has provided direct evidence to support the subsequent phloem translocation pathway of NPs in plants. Wang et al. studied the foliar uptake of four metal oxide NPs of size range 24–47 nm by watermelon. They found that the small NPs could penetrate watermelon leaves following the stomatal pathway using TEM, and the metal elements were detected in the shoots and roots, therefore they concluded that NPs passed through the shoots and finally reached the roots through the phloem sieve tubes.44 Hong et al. investigated foliar uptake of CeO2 NPs of primary size 8 ± 1 nm by cucumber (Cucumis sativus). Ce was detected by ICP-OES in all the tissues of the CeO2 NP treated plants and Ce containing particles in roots were also observed using TEM.45 Quantified by ICP-MS, Zhao et al. found that 97–99% of Cu was sequestered in the leaves and only 1–3% of Cu accumulated in root tissues after the exposure of lettuce plants to Cu(OH)2 NPs (∼50 to 1000 nm) through foliar spray for one month. They suggested that leaf exudates could form weak acids in the presence of water and thus accelerated the dissolution of Cu(OH)2 NPs, resulting in a pathway for Cu ions to penetrate the epidermal cells and translocate to other tissues.46 One important concern is that no evidence was provided to confirm whether the metal contents detected by elemental analysis or particles detected by TEM in shoots and roots were from NPs or from dissolved metallic ions. This is a common disadvantage of using only elemental analysis to track NP delivery to plants. Wang et al., using split-root experiments and high-resolution TEM observation, found that 20–40 nm CuO NPs were translocated from maize roots to shoots via the xylem and then from the shoots back to the roots via the phloem.47 During this translocation, CuO NPs may be reduced from Cu(II) to Cu(I). Ma et al. also observed the xylem- and phloem-based transport of 25 nm CeO2 NPs in cucumber using split-root experiments. They further found, using μ-XRF and μ-XANES, that CeO2 NPs were transported from roots to shoots through the xylem and 15% of the NPs were reduced to Ce(III), while only CeO2 NPs were transported back from shoots to roots through the phloem.48 These results support the notion that NPs can transport upward through the xylem and downward through the phloem, although how the NPs transport between the xylem and phloem remains unknown. The NPs and their transformed products translocated in roots may subsequently be exuded into the rhizosphere, and the ones translocated in leaves may be exuded into the phyllosphere, which can further influence the microbial community in the rhizosphere or the phyllosphere.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 are used as nanopesticides to be sprayed on diseased leaf surfaces, thus increasing the risks of NP foliar uptake and then transport to other tissues.
54 are used as nanopesticides to be sprayed on diseased leaf surfaces, thus increasing the risks of NP foliar uptake and then transport to other tissues.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 017
017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260
260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 km2, approximately twice as large as the land surface.49 Experiences of PM2.5 indicate that the filtration effectiveness of atmospheric NPs may be highly dependent on plant species.55,56 In order to evaluate the effects of plants on the remediation of air pollution and especially as atmospheric NP captors, experiments involving systematic simulation and on-line field monitoring of atmospheric NPs are required.
200 km2, approximately twice as large as the land surface.49 Experiences of PM2.5 indicate that the filtration effectiveness of atmospheric NPs may be highly dependent on plant species.55,56 In order to evaluate the effects of plants on the remediation of air pollution and especially as atmospheric NP captors, experiments involving systematic simulation and on-line field monitoring of atmospheric NPs are required.
2.2 Root uptake and translocation of NPs in plants
One of the most important properties impacting plant root uptake of NPs is particle size. It is certain that size selection exists in the uptake of NPs by plants, but inconsistent results have been reported in the literature. Sabo-Attwood et al. using μ-XRF and TEM observed root uptake of 3.5 nm Au NPs by tobacco but 18 nm AuNPs remained agglomerated on the root outer surfaces.59 However, Taylor et al. using TEM found that Au NPs (from 7 to 108 nm) were not directly taken up by Arabidopsis thaliana roots.60 Moreover, Slomberg and Schoenfisch using TEM observed that SiO2 NPs up to 200 nm were able to be taken up into the roots of Arabidopsis thaliana, although fewer particles were observed as the particle size increased (14, 50, 200 nm).61 Larue performed an elegant experiment to reveal the size-dependent uptake and transport of TiO2 NPs in wheat (Triticum aestivum) using TEM and μ-XRF, which provides evidence that NPs with primary diameters less than 36 nm accumulated in roots and were distributed throughout the whole plant tissues without dissolution or transformation, while NPs with primary diameters in the range 36–140 nm accumulated in wheat root parenchyma but did not reach the stele and consequently did not translocate to the shoots, and NPs larger than 140 nm did not accumulate in wheat roots.62 It is still impossible from current studies to estimate the SEL for NP uptake by roots. One factor is that the SEL is different for different plant species and growth stages. Another important factor is that the sizes of NPs in the rhizosphere are entirely different from their original ones and highly dynamic and broadly distributed. However, the particle sizes used in most studies have been based on the average size of the original NPs. In fact, the uptake of NPs by plants is dependent on the minimum, but not the average, size of NPs.
Surface charge is another factor impacting the root uptake and translocation of NPs in plants. Generally, the plant root cap is protected by a border cell mucilage layer consisting of negatively charged root secretions. Avellan et al. using X-ray computed nanotomography (nano-CT) and hyperspectral imaging microscopy (HSI) found that Arabidopsis thaliana roots secreted mucilages that were adsorbed to positively charged Au NPs (∼12 nm) and prevented the translocation of Au NPs into the root tissues. However, negatively charged Au NPs (∼12 nm) did not adsorb the mucilages and were able to translocate into the apoplast of roots.63 Koelmel et al. using laser ablation-inductively coupled-mass spectrometry (LA-ICP-MS) further found that surface functionalization greatly affected the root uptake and translocation of AuNPs (core diameter 2 nm) in rice (Oryza sativa L.). The accumulated Au concentrations in roots followed the order: Au NPs(+) > Au NPs(0) > Au NPs(−), while the reverse order was obtained in shoots, indicating preferential translocation of negatively charged NPs through the vascular system.64 Similar effects were observed in wheat (Triticum aestivum) exposed to positively charged, neutral and negatively charged CeO2 NPs (∼4 nm).65 In some cases the surface charges of NPs can be reversed due to the formation of nano-coronas by coatings of negatively charged root exudates on the surfaces of the positively charged NPs. These nano-coronas may transport through the vascular system like negatively charged NPs. More complicated scenarios include root exudates possibly inducing aggregation, partial dissolution or transformation of NPs and further impacting on their root uptake and bioavailability, and which mechanism is the dominant one in the rhizosphere is dependent on the chemical properties of the NPs and the exudates as well as the rhizosphere microenvironment. In addition, different plant species and plants at different growth stages secrete different root exudates, further affecting the size, surface charge and speciation of NPs. Therefore, the influence of plant species and growth stage on plant root uptake of NPs is complicated.
Few studies have focused on the influence of physiological factors such as plant species, disease and rhizosphere microorganisms on the uptake, accumulation and transformation of NPs in the soil–plant system.66 Different plant species display different uptake capacities for NPs, likely due to differences in plant physiology and metabolic function. For example, a higher Ce translocation rate was observed in dicotyledons than in monocotyledons.67 Glenn et al. using TEM and ICP-MS analysis found that root uptake of Au NPs was also plant species dependent; both 4 nm and 18 nm Au NPs were taken up by Azolla caroliniana Willd, whereas only 4 nm Au NPs were taken up by Myriophyllum simulans Orch, and Egeria densa Planch did not take up Au NPs of either size.68 Judy et al. using μ-XRF and LA-ICP-MS found that 10–50 nm Au NPs were taken up by tobacco roots but not by wheat.69 Understanding species-dependent NP uptake by plants is helpful to agricultural risk management. Mycorrhizal fungi can form symbiotic partnerships with higher plants and increase effective plant root surfaces by up to 10 times.23 This promotes plant uptake of water and nutrients and may influence the uptake and bioavailability of NPs.23 Studies indicate that tomato plants colonized by mycorrhizal fungi accumulate lower concentration of Ag in their tissues when exposed to 2 nm Ag NPs than do non-mycorrhizal plants, but this effect was not evident when the plants were exposed to 15 nm Ag NPs.70 Another study found that symbiotic mycorrhizal colonization lowered Zn accumulation in tissues of maize when exposed to ZnO NPs (90 ± 10 nm).71 Root diseases such as root rot, root knot and rhizopus disease also influence the uptake of NPs due to the damage to the physiological barriers of roots against NP entry.72 More work is needed to investigate the influence of rhizosphere biotic and abiotic processes on the bioavailability and fate of NPs in the plant–soil system.
When approaching the root epidermis there are two basic pathways for root uptake and transport of NPs in higher plants. Most studies have proposed the apoplastic pathway (Fig. 1) in which NPs firstly penetrate the pores of the cell wall and then diffuse into the space between the cell wall and the plasma membrane or pass through the intercellular space without crossing the cell membrane. A large number of studies have observed NPs or their aggregates in the root apoplastic space using TEM or CLSM, and therefore suggest the existence of an apoplastic pathway for NPs in plant roots. For example, 20 nm ZnO NPs in ryegrass roots,74 12 nm Au NPs in Arabidopsis thaliana roots,63 20–80 nm Ag NPs in Arabidopsis thaliana roots,75 43 nm CuO NPs in Elsholtzia splendens roots,76 and 22 nm La2O3 NPs in cucumber roots.29 An intractable problem is that the pore diameters of the plant cell walls are estimated to be in the range 5 to 20 nm.12 Logically, only NPs smaller than 20 nm can pass through the cell walls. However, many NPs larger than 20 nm have been observed in intercellular spaces. One possible mechanism is that NPs may induce the destruction of the cell wall and enlarge the pore size.77 Another plausible hypothesis is that NPs enter the intercellular spaces or even the xylem through diseased roots or physical wounding as a consequence of belowground herbivores and mechanical injuries, such as accidents during seedling transplantation.17 In all, by the apoplastic pathway, NPs can pass through the epidermis, cortex and reach the endodermis, but are prevented by the Casparian strip, a belt of specialized cell wall material sealed by lipophilic hydrocarbons located in adjacent cells of the endodermis around the vascular system, to prevent the entry of macromolecules and NPs into the vascular cylinder.78 However, under some special conditions NPs may enter the vascular system and avoid the Casparian strip through the root apoplast, for example through the root tip region where the Casparian strip has not yet formed,31,79 or the lateral root junction where the Casparian strip is disconnected.17,31,80 Experimental evidence of this pathway was provided by Lv et al. who found using TEM, μ-XRF and fluorescence tracking that 30 nm ZnO NPs gathered at the lateral root junction of maize and inside the connected xylem cylinder (Fig. 2).31 Therefore, the lateral root junction may be an important apoplastic pathway by which NPs enter the vascular system, and future studies are needed to confirm whether this pathway occurs in other plant species, and to specify the size selectivity of this pathway.
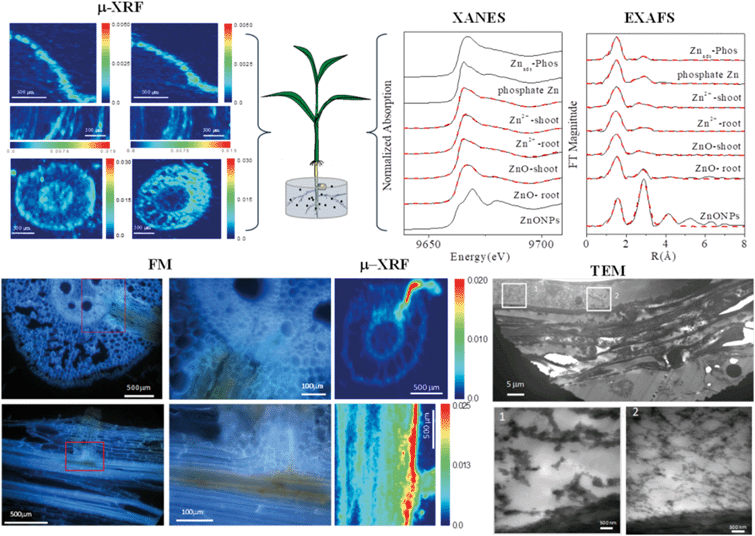 | ||
| Fig. 2 Combining micro-X-ray fluorescence microscopy (μ-XRF), X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS), fluorescence microscopy (FM) and transmission electron microscopy (TEM) to study the uptake, translocation and transformation of ZnO NPs in maize plants.31 | ||
The other hypothesized pathway is the symplastic route, which is a cell-to-cell transport pathway (Fig. 1). In addition to the traverse across the cell wall there are two barriers by which NPs may be transported through the symplastic pathway. One is penetration by NPs of the cell membrane and entry into the cytoplasm. The other is transport of NPs to the adjacent cells after entering the cell through plasmodesmata. Several hypotheses have been proposed for the transmembrane transport of NPs by plant cells, e.g. through aquaporins, interconnected ion channels, endocytosis and broken membrane intubation. However, ion channels work for specific ions, and although aquaporins are non-selective in nature, the protein channels of aquaporins are generally below 1 nm in diameter.81 The hypothesis of a water or ion channel pathway for NP cellular internalization must face challenges from the basic principles of plant physiology.82 Broken membranes have been observed in cellular internalization of carbon nanotubes but this is a destructive invasion that may induce membranolysis and cell death.83,84 According to the literature, the highest feasibility transmembrane pathway for NPs is through endocytosis. Plant cell endocytosis is poorly understood compared with that in animal systems. However, recent studies on NP internalization using isolated plant cells suggest that endocytosis pathways are involved. For example, Onelli et al. using CLSM observed both clathrin independent and clathrin dependent pathways for the endocytosis of Au NPs into tobacco protoplasts.85 Etxeberria et al. using CLMS observed the fluid phase endocytic uptake of 40 nm polystyrene nano-spheres and 20 nm CdSe/ZnS quantum dots (DQ) by cultured sycamore cells,86 and polystyrene nano-spheres were delivered to the central vacuole while CdSe/ZnS nano-dots were sequestered into cytoplasmic vesicular structures.86 Torney et al. further confirmed that the surface properties of NPs play a crucial role in plant cell endocytosis, and endocytic uptake of triethylene glycol (TEG) functionalized mesoporous silica nanoparticles (MSN) by tobacco mesophyll protoplasts took place, but un-functionalized MSN were not taken up. The internalized NPs remained in endocytotic vesicles in the cytoplasm, with sizes ranging from 0.2 to 3 μm.87 However, at present, direct evidence for the endocytic uptake of NPs in situ in plant roots is still lacking, except for some indirect TEM evidence supported by the observations of vesicles containing nano-dots in the cytoplasm of plant roots.47,88,89
Cell walls separate individual plant cells. Symplastic transport takes place intercellularly through plasmodesmata (PD). Plasmodesmata are channels that span the plant cell wall and enable intercellular communication by linking the cytoplasm between adjacent cells (Fig. 1).90 Botanists have provided abundant evidence that PD facilitate the cell-to-cell trafficking of biological macromolecules such as proteins, nucleic acids, and RNA, and the roles of PD as intercellular channels for macromolecular transport in plants have been well reviewed.90–93 Specifically, non-targeted traffic of proteins by diffusion in the range of approximately 3–20 kDa with maximum size of about 3 nm was demonstrated,93,94 providing a model for intercellular NP traffic. Geisler-Lee, using TEM, for the first time directly observed Ag0 containing NPs and aggregated clumps inside root cell PD and middle lamella in the first 1–2 mm of Arabidopsis thaliana root tips (plant root exposure to 20, 40 and 80 nm Ag NPs).75 Zhai et al. using TEM observed the presence of Au NPs or their aggregates in root cell cytoplasm, cell walls, plastids, mitochondria, and especially the PD of poplar plants (Populus deltoides × nigra) exposed to 15, 25 and 50 nm Au NPs.95 Notably, both studies also observed Ag0 or Au0-containing black dots in Ag+-treated or Au+-treated root cells.75,95 Thus, whether NPs observed by TEM in root cells were taken up intact or reduced or assimilated Ag+ or Au+ released from NPs in vivo is unclear. It should be noted that numerous studies have demonstrated a natural source for the synthesis of Ag or Au NPs inside plants96–98 which requires particular attention when using Ag or Au NPs as models to study the NP uptake pathway in plants. Until now, there has been no direct observation of other NPs inside PD except for Ag or Au NPs. It is still difficult to clarify whether this is due to the special physico-chemical properties of Ag or Au NPs providing them with the capacity to shuttle through PD or merely due to the reduction of Ag+in situ. Therefore, the hypothesis of intercellular NP transport through the PD channel still lacks sufficient evidence. Future studies are needed to provide more solid evidence to support this hypothesis using inactive NPs, and microinjection strategies that have been used to establish the transport of proteins via PD are worth pursuing to reveal the SEL of NPs for PD channels.
Some physiological and technological challenges still need to be resolved to clarify the integrated mechanisms of root uptake and translocation of NPs by plants, such as identifying the apoplastic and symplastic pathways, the energy source of phloem-based transport and the precise particle size analysis of NPs in the rhizosphere and plant tissues. Future studies of plant NP uptake and translocation must be concerned more about how this occurs rather than just that it occurs. Effective cooperation between plant physiologists, material scientists, environmental scientists and analysts is necessary to solve these problems.
3. Transformation of NPs in the rhizosphere and in plants
3.1 Transformation of NPs in rhizosphere
NPs are highly dynamic and unstable in the environment compared with their bulk counterparts due to their small size, high surface-to-volume ratio and reactivity.99 Many biotic and abiotic processes may occur when NPs are released into the environment, resulting in changes in their agglomeration state, surface chemistry or speciation.26 Therefore, NPs exposed to plants in the real environment may not maintain the properties of pristine NPs. Numerous studies have focused on the transformation of NPs in soils, sediments, wastewater and activated sludge in the past few years.5,100–106 For example, Ag NPs were found to convert to Ag2S in sludge;100 ZnO NPs were transformed to Zn3(PO4)2, ZnS and Zn associated Fe oxy/hydroxides (Zn–FeOOH) in sludge and biosolids.102 More information on the transformation and fate of various NPs in the environment can be found in previous reviews.26,107–109Here, we focus on the transformation of NPs in the rhizosphere environment. The rhizosphere is a chemically and biologically active region enriched with root exudates and microorganisms110 which can induce the transformation of NPs prior to their approach to root surfaces. For example, Huang et al. found that the strong binding capacity of Cu NPs (∼40 nm) and a synthetic root exudate influenced the oxidation and reduction transformations of Cu NPs to Cu(I) and Cu(II), and also significantly decreased Cu uptake and bioaccumulation in cucumber (Cucumis sativus).111 Gao et al. investigated the dissolution of CuO NPs (∼40 nm) in wheat (Triticum aestivum) rhizosphere soil, and found that Cu in the readily available fraction (extracted by CaCl2) increased and the labile fraction of Cu (extracted by DTPA) decreased in the rhizosphere soil compared to the bulk soil.112 Rico et al. investigated the transformation of CeO2 NPs (67 nm × 8 nm) using synchrotron-based μ-XRF and μ-XANES to analyze their spatial localization and speciation in thin sections of intact roots of barley (Hordeum vulgare L.) at the soil–root interface. Their results showed that 84–90% of Ce was localized as CeO2 in the soil and at root surfaces, while a few Ce accumulation “hot spots” on root surfaces revealed highly significant reduction (56–98%) of CeO2 NPs to Ce(III) species.113 The mechanism inducing NP dissolution and transformation at the soil–root surface is complicated. The rhizosphere process is not only affected by, but also induces, the changes in soil pH, organic matter, mineral constituents, and microbial community.114,115 The changes in these soil factors can further result in either opposite or negative effects on NP transformations.116
In addition, most of the reactions between NPs and soil matrices are thermodynamically feasible but kinetically slow, therefore kinetics is one of most important factors determining the transformation of NPs in the rhizosphere. For example, after 14 d of cultivation of wheat (Triticum aestivum), the extractable Cu in the rhizosphere soil spiked with Cu NPs pre-aged for 28 d was found to be higher than that of those without aging,112 which was opposite to the trend observed in soil spiked with CuSO4.112 The aging process is also affected by surface coatings and the particle size of NPs. For example, Coutris et al. have observed that 20 nm uncoated Ag NPs in soil are more resistant to aging than Ag ions or 5 nm citrate-coated Ag NPs, but they can act as continuous sources of bioaccessible Ag ions during a 70 d aging process.117 Although related studies are still in their infancy they remind us that aged NPs in soils may be quite different from their ionic form, and this needs to be considered in the risk assessment of NPs in soils. More field experiments, long-term studies and systematic projects are urgently needed in order to explore the dissolution and transformation of NPs in soils and especially those representing high exposure risks to plants such as Cu-based NPs and ZnO, TiO2, CeO2 and Ag NPs.
3.2 Plant uptake of transformation products of NPs
Transformation of NPs at the rhizosphere soil–root interface will influence the phytotoxicity and bioavailability of NPs.112,113 However, to date the majority of studies have focused only on plant uptake and phytotoxicity of pristine NPs, and very few studies have attempted to address the behaviors of their transformation products. Wang et al. investigated the uptake, accumulation and toxicity of Ag2S NPs, a widespread transformation product of Ag NPs in the environment, in cucumber and wheat and found that Ag2S NPs with sizes up to 120 nm were taken up by plant roots and subsequently delivered as Ag2S NPs into leaf tissues without transformation.118 However, the interesting problem as to whether sulfuration increases or decreases the bioavailability and accumulation of Ag NPs in plants was not addressed. Stegemeier et al. further compared the bioavailability of AgNO3, Ag NPs (6.3 nm) and Ag2S NPs (7.8 nm). They suggested that despite accumulating a similar total amount of Ag in roots (>99%) and shoots (<1%), different forms of Ag interacted with the roots in different manners. For example, Ag+ accumulated uniformly throughout roots, Ag NPs accumulated mainly in the (columella) border cells and elongation zones, and Ag2S NPs remained largely adhering to the root exteriors.119 Spielman-Sun et al. investigated the impacts of speciation and solubility of Cu-based NPs on Cu uptake and translocation in roots of wheat (Triticum aestivum). Their results showed that Cu(OH)2 NPs with a high solubility were quickly taken up by roots and further reduced and/or sulfidized, while CuO or CuS NPs with low solubility were more persistent over the 48 h post-exposure period with as much as 80% of the NPs nontransformed.120 In order to better understand and predict the fate, bioavailability and risk of NPs in environmental and agricultural ecosystems, future work needs to focus on the uptake, accumulation and toxicity of not only the ENPs but also the environmentally abundant NP transformation products.3.3 Biotransformation of ENPs in plants
In addition to transformation in rhizosphere environments, transformation of NPs has also been found to occur inside or on the surface of plant tissues, resulting in different accumulated elemental speciation in plants. Yin et al. found for the first time that Ag was partially oxidized in the root tissues of Lolium multiflorum. Two hypotheses were proposed to explain this observation: (i) direct uptake of the Ag NPs by roots followed by oxidative transformation in root tissues and (ii) dissolution of Ag NPs outside the root surface followed by the uptake of ionic species by roots.121 Wang et al. identified the cellular internalization and intracellular biotransformation of NPs to Ag2S and Ag-thiolates in Chlamydomonas reinhardtii;122 but similar transformation of Ag NPs inside higher plant tissues has not been observed. Au NPs are less active than Ag NPs and no study has reported the oxidative transformation of Au NPs inside plants. In contrast, both Ag+ and Au+ were able to be reduced by plants to form NPs in plant roots and shoots.96–98 Distinguishing the pristine Ag or Au NPs from phytosynthesized NPs is a major challenge, and measuring the values of Ag or Au isotope ratios may offer some prospect of distinguishing them more clearly.123Metal oxide NPs such as TiO2 and SiO2 NPs are the most stable of the commonly studied NPs and are present in their pristine speciation in plants.61,124,125 By contrast, NPs such as ZnO, CuO, NiO, CeO2, Yb2O3 and La2O3 were found to be able to transform, resulting in changes in the accumulated speciation in plants. The transformations of ZnO NPs exposed to various plants have been determined using synchrotron X-ray absorption spectroscopy (XAS). Lv et al. found that the majority of Zn accumulated in maize roots and shoots was in forms such as Zn phosphate under hydroponic exposure of ZnO NPs, mainly due to the enhanced dissolution of ZnO NPs in the rhizosphere and the plant uptake and translocation of Zn in the ionic form (Fig. 2).31 Similar Zn accumulated speciation was also found in wheat cultivated in soil.126,127 Other types of accumulated speciation such as Zn-citrate and Zn-nitrate in soybean,128,129 Zn-citrate, Zn-histidine, and Zn-phytate in cowpea,30 and Zn-nitrate in velvet mesquite130 have also been reported. Although different Zn speciation has been found in plants, a consistent conclusion obtained is that no ZnO has been observed in shoots when roots have been exposed to ZnO NPs, thus Zn uptake, transport and accumulation in plants are mainly in the form of Zn2+ released from ZnO NPs. Exposure to CuO NPs and Cu2+ resulted in similar Cu accumulated speciation in wheat, except that for CuO part of the Cu(II) was reduced to Cu(I) inside the plants and formed Cu(I)–sulfur complexes.126,127 Similar reduction of Cu(II) to Cu(I) in plants was also observed in soil-cultivated rice and maize.47,131 Peng et al. further found that 40 nm CuO NPs were transported from rice roots to shoots and dissolved Cu(II) was mainly combined with cysteine, citrate, and phosphate ligands and some of the Cu(II) was reduced to Cu2O.132
In early studies, rare earth oxide (REO) NPs such as CeO2 NPs were considered to be highly stable and not to undergo transformation in the surrounding environment and plants.133–135 However, Zhang and his colleagues have conducted systematic studies on plant uptake and accumulation of REO NPs and they found that REO NPs such as CeO2, Yb2O3 and La2O3 NPs were able to be biologically transformed in plants and the rhizosphere. For example, Zhang et al. found that after hydroponic exposure of cucumber plants to 7 nm CeO2 NPs for 21 days, part of the CeO2 was reduced to Ce(III) and formed needle-like clusters of CePO4 in intercellular regions and the epidermis of cucumber roots, and formed Ce(III) complexes with carboxylates during translocation to the shoots (Fig. 3).28 They speculated that organic acids in root exudates promoted the dissolution of CeO2 NPs, and the reducing root exudates did induce the reduction of Ce(IV) to Ce(III) in the rhizosphere (Fig. 3).28 Similar reduction and transformation of CeO2 NPs were also observed in agar-cultivated asparagus lettuce136 and soil-cultivated soybean.128 Ma et al. further investigated the transformation sites of CeO2 NPs in cucumber plants. They found that in the root exposure mode, Ce was present as a mixture of Ce(IV) and Ce(III) in all the plant tissues, while Ce(III) was absent from plant tissues in petiole exposure mode, supporting the role of the rhizosphere in the reduction of CeO2 NPs.137 By using a split-root exposure mode of 25 nm CeO2 NPs to cucumber, Ma et al. further found that Ce was transported from roots to shoots through the xylem as a mixture of Ce(IV) and Ce(III), but Ce transported from the shoots back to the roots through the phloem was almost completely in the form of CeO2.48 Similar to the dissolution and transformation processes of ZnO NPs, 22 nm La2O3 NPs or 15 nm Yb2O3 NPs exposed to hydroponic cultivated cucumber were observed to be partially dissolved due to organic acids exuded from the roots, and transformed to needle-like LaPO4 or YbPO4 nanoclusters in the intercellular regions of the cucumber roots.29,138
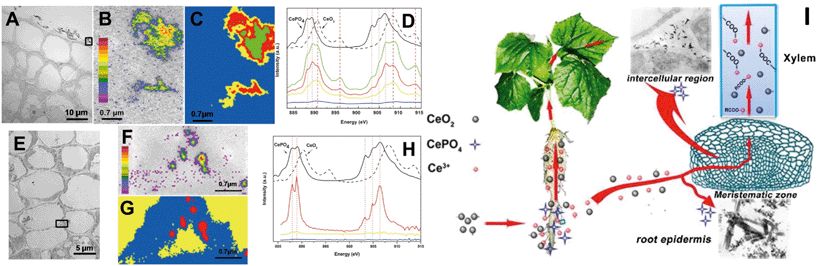 | ||
| Fig. 3 Uptake and biological transformation of CeO2 NPs in cucumber plants detected by TEM (A and E) and STXM (B–D and F–H); (B and F), Ce maps of rectangular area in panels A and E. Panels D and H are, respectively, the XAFS spectra extracted from the image sequences of panels C and G.28 I is the proposed schematic diagram of CeO2 NP uptake and transformation in cucumber plants. | ||
4. Advanced analytical techniques and future needs to investigate NPs in plants or other organisms
In order to obtain objective knowledge on the uptake, translocation and transformation of NPs in plants, comprehensive information is needed including the size distribution, concentration, speciation, and locations of NPs in plants and this presents an unprecedented challenge to analytical techniques.139 In recent years many advanced techniques have been proposed to obtain the above information. Here, we provide a comprehensive summary of the techniques available for quantitative and qualitative analysis of NPs in plants (Table 1). The main features of the techniques are introduced, and some potential techniques which have been used in organisms or complex environmental matrices are also proposed.| Technique | Content required | Limit of quantitation | Speciation | Lateral resolution | Particle size | Sample treatment | Element information | Special information | Testing environment |
|---|---|---|---|---|---|---|---|---|---|
| ICP-MS | 10−9 | 10−9 | Unable | Unable | Unable | Acid digestion | Yes | No | Solution |
| SP-ICP-MS | 10−9 | 10−9 | Unable | Unable | Yes | Enzyme digestion | Yes | No | Solution |
| MC-ICP-MS | 10−9 | 10−9 | Unable | Unable | Unable | Acid digestion | Yes | Isotope | Solution |
| LA-ICP-MS | 10−9 | 10−9 | Unable | 1–10 μm | Unable | Tissue section or living body | Yes | No | Unconstrained |
| (HR)TEM | — | Unable | Unable | nm | Yes | Tissue section | No | Crystalline | Anhydrous/vacuum |
| STEM | 10−3 | Rel. | Unable | nm | Yes | Tissue section | Yes | No | Anhydrous/vacuum |
| μ-XRF | 10−5 | Rel. | Unable | μm | Unable | Tissue section | Yes | No | Unconstrained |
| μ-XANES | 10−5 | Unable | Yes | Yes | Unable | Tissue section or living body | Yes | No | Unconstrained |
| STXM | 10−5 | Rel. | Yes | 10 nm | Unable | Tissue section | Yes | No | Unconstrained |
| NanoSIMS | 10−5 | Rel. | Unable | 50 nm | Unable | Tissue section | Yes | Isotope | Anhydrous/vacuum |
| CLSM | 10−6 | Unable | Unable | μm | Unable | Living body | No | Fluorescence | Unconstrained |
| SR-FTIR | — | Unable | Unable | μm | Unable | Tissue section | No | Infrared | Anhydrous |
| CRM | — | Unable | Unable | μm | Unable | Tissue section or living body | No | Raman | Unconstrained |
| HSI | — | Unable | Unable | 2.5 nm | Unable | Tissue section or living body | No | VNIR | Unconstrained |
4.1 Quantitative analysis
To date, precise quantitative information on metal or rare-earth based NPs is still dependent on the analysis of their metallic element concentration by methods such as those based on inductively coupled plasma mass spectrometry (ICP-MS). Although the lateral resolution of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) is lower than that of electron or X-ray microanalysis, it allows an accurate determination of metal distribution in biological materials in situ.140 LA-ICP-MS has been used to investigate the tissue level distribution of elemental Au in rice and tobacco under exposure to Au NPs,64,69 but whether the observed Au element exists as ions or NPs needs to be confirmed using other techniques. With the help of enzyme digestion, single-particle ICP-MS (SP-ICP-MS) can provide concentrations of NPs with different sizes in plant tissues.141,142 It should be noted that the enzyme digestion procedure may induce the dissolution of, and changes in, the speciation of NPs. Until now the vast majority of applications of SP-ICP-MS have been limited to inactive NPs such as Au, Ag, CeO2, CuO and TiO2 NPs,42,141,143–145 and further studies are needed to establish the digestion procedures applicable to more types of NPs as well as NPs in non-biodegradable plant components. Combined with special separation techniques such as capillary electrophoresis (CE),146,147 size exclusion chromatography (SEC),148,149 hydrodynamic chromatography (HDC),150 and field-flow fractionation (FFF),151,152 NPs with different particle sizes and ions can be distinguished and quantified. However, most of these techniques have been used only in simple solutions or environmental waters at the most. Only FFF-ICP-MS has been used to analyze and characterize natural colloids and NPs in complex environmental media such as wastewaters and soil pore water.153 Although not reported, we expect that these techniques can be used to detect NPs in the rhizosphere and root exudates. Similar to SP-ICP-MS, detecting NPs in biological samples by these hyphenated analytical techniques needs digestion or enzymolysis pretreatment of biological tissues or extraction of NPs from biological tissues, and this is a key issue to overcome for their application. Future studies are encouraged to extend these techniques to detect NPs in more complex matrices including biological media.4.2 Speciation and location analysis
Transmission electron microscopy (TEM) is the most widely used technique for direct detection of distributions of NPs or aggregates inside plants at the subcellular level. The crystalline structure of NPs can be determined using high resolution TEM (HRTEM) combined with selected area electron diffraction (SAED).47,122 Furthermore, the nanoscale distribution of elements in plants at the subcellular level can be obtained using scanning transmission electron microscopy (STEM),68 but with low sensitivity. Confocal laser scanning microscopy (CLSM) or fluorescence microscopy (FM) has been used to track the distribution of fluorescence-labeled NPs and quantum dots (QD) in plant tissues.31,134,154 One advantage of CLSM is that living cells or plant tissues can be observed directly, providing the possibility to detect NP internalization by plant cells in situ, but it has the disadvantage of low lateral resolution and the necessity of labeling using fluorescent dyes for most NPs.High resolution secondary ion mass spectrometry (NanoSIMS) is a nanoscale ion microprobe that is able to map most of the elements in the periodic table with high lateral resolution (down to 50 nm) and high elemental isotope sensitivity.155 We have used NanoSIMS to detect Ag NP-exposed algae and observed the overlap between the mappings of 32S− and 107Ag− in the cell walls and cytoplasm, suggesting combined accumulation of Ag and S in algal cells, which indirectly confirms the presence of Ag–sulfur complexes inside algal cells.122 Using NanoSIMS, Aubert found that nanosized molybdenum octahedral clusters were abundantly present in the apoplast and symplast of the root epidermis, endodermis (cortex) and stele, with a concentration gradient decreasing from the epidermis to the stele.156
All the above techniques except for CLSM require complicated sample preparation and a rigorous testing environment (anhydrous and high vacuum) and this is not conducive to obtaining information on undisturbed samples. In particular, the chemical speciation of NPs cannot be addressed using electronic and secondary ion beam-based techniques. Synchrotron radiation (SR) based techniques display a unique advantage in their minimal sample preparation, non-destructive testing, and optimum balance between sensitivity, chemical specificity and spatial resolution.32 In addition, a convenient combination with other techniques can provide comprehensive chemical information for the majority of the elements in the periodic table. The combination of microbeam X-ray fluorescence mapping (μ-XRF) and X-ray absorption near edge structure spectroscopy (μ-XANES) is a powerful technique. μ-XRF provides multi-elemental (heavier elements) mapping with μm lateral resolution, and μ-XANES provides the elemental speciation of hotspots. This technique has been widely used to study plant uptake and transformation of NPs such as Ag, Au, ZnO, TiO2, and CeO2.31,48,59,125,128,157 The newly developed full spectral XANES (FS-XANES) imaging technique using the Maia detector array allows one to obtain XANES spectroscopic information at each pixel over the entire mapping area at the microscale (down to 0.6 μm).158 The greatest disadvantage of these techniques used in NP–plant interactions is that the lateral resolution of most μ-XRF/μ-XAS beamlines in the world is above 1 μm or a few hundred nm. Fortunately, some advanced nano-XRF/nano-XAS beamlines with spatial resolution down to a few tens of nm have been set up.159 Castillo-Michel et al. recently wrote a comprehensive review on the application of synchrotron-based μ-XRF and μ-XAS in plant–NP interaction research and optimized sample preparation methods were also proposed.32 Zhu et al. reviewed the recent advances in XRF-based sub-100 nm resolution cell imaging and projected the future development of this technique,160 which provides the opportunity of using nano-XRF to reveal the subcellular distribution and speciation of NPs in plant tissues.
In addition to synchrotron-based hard X-ray (energy above 4 keV) μ-XRF and μ-XAS, soft X-ray (energy below ∼2 keV) scanning transmission X-ray microscopy (STXM) has the potential to provide high spatial resolution down to a few nm and is able to characterize samples at the sub-cellular level.161 Many of the heavy elements' L or M edge energies are in the soft X-ray range, e.g. Ag (M), Ce (M), Fe (L), Zn (L), and Cu (L), and the speciation and nanoscale distribution of NPs containing these elements in plant cells or tissues can be detected using STXM. Zhang and his colleagues have used STXM to study the uptake, translocation and transformation of rare earth oxide NPs including CeO2, La2O3 and Yb2O3 NPs in plants.28,29,137,138 Peng et al., using multiple synchrotron radiation based techniques, demonstrated the translocation and transformation of CuO NPs in rice; specifically, CuO NPs and Cu-citrate were observed in root cells using STXM.131 Gilbert et al., using STXM, studied the fate of ZnO NPs administered to human bronchial epithelial cells,162 and this technique may also be feasible for studies of plant cells.
Other techniques such as synchrotron Fourier transform infrared (SR-FTIR) microspectroscopy,163–165 confocal Raman microspectroscopy (CRM)166,167 and hyperspectral imaging (HSI) microscopy168 can be used as important supplementary techniques for the study of NP–plant interactions in addition to the above mentioned techniques which have been used in studies of NP–plant interactions. SR-FTIR microspectroscopy can acquire high-quality spectral images quickly and this helps to identify and locate functional groups on NPs with infrared absorption. The most recently developed method can straightforwardly determine the infrared absorption spectra of samples with a spatial resolution of 20 nm (namely nano-FTIR).139,164 More importantly, this technique allows one to obtain FTIR spectral imaging of plant tissue simultaneously and this is beneficial in diagnosing plant chemical responses to NP exposure.169 However, application of synchrotron radiation-based methods for such studies is largely limited by the availability of synchrotron radiation-based facilities.
HSI microscopy is an advanced visualization technique to enable the rapid identification of materials at the micro- and nanoscales.168 Mortimer et al. determined the potential of HSI for the analysis of cellular internalization of different metal-based NPs including Ag, Au, CdSe/ZnS, CuO and TiO2 NPs by the ciliated protozoan Tetrahymena thermophile. They obtained the hyperspectral images of all the NPs internalized in the protozoan at a spectral resolution of 2.5 nm, except for TiO2 NPs because they showed spectral similarities to the unexposed control cells.170 CRM allows high-speed acquisition of nondestructive chemical and structural imaging of heterogeneous samples with a microscale spatial resolution (∼1 μm).166 One of the greatest advantages of CRM is its capacity to analyze living hydrated samples which is of benefit for in situ detection. Kang et al. have used high-speed CRM to observe the real-time uptake of individual single-walled carbon nanotubes (SWCNT) by living macrophages via transient spatial Raman mapping.167 Eder et al. used CRM to visualize chains of magnetite crystals in magnetotactic bacteria at ∼100 nm lateral resolution.171 We still expect techniques such as HSI and CRM to have great potential for application in studies of NP–plant interactions, although they have not yet been used to detect NPs in plant tissues.
4.3 Stable isotope-based analytical techniques/methods
Isotope fractionation has been found to occur in many physical and chemical transformations of metal elements. NPs from different sources may therefore have special isotope ratios which can be used as intrinsic tracers to probe the source and fate of NPs.172,173 The rapid development of the multicollector ICP-MS (MC-ICP-MS) technique has confirmed the excellent accuracy and precision of non-traditional stable isotopic analysis which can provide the opportunity to track and distinguish between natural and manufactured NPs in environmental media or organisms.123 How to identify the contribution of ions and particles to the bioavailability of NPs is always a great difficulty faced by environmental and analytical scientists. A recently developed method using isotope labeling especially multi-isotope labeling combined with high-precision mass spectrometry mainly as MC-ICP-MS provides a new opportunity to resolve this problem. For example, Khan et al. studied the waterborne uptake and efflux kinetics of aqueous 68Zn, 68ZnO NPs, and 68ZnO bulk particles by an estuarine snail (Peringia ulvae), and indicated that the solubility of ZnO NPs in the exposure media was a key parameter that determined the bioavailability of the Zn constituent.174 Laycock et al. further employed double stable isotope labeled 68ZnO NPs and soluble 64ZnCl2 to test the bioavailability of ZnO NPs to earthworms by soil exposure. From the 68Zn/64Zn ratios determined in earthworms, soils, and pore waters, they provided direct evidence that rapid dissolution of the ZnO NPs was the most likely explanation for the indistinguishable environmental distribution and uptake of Zn ions and particles.175 Unfortunately, there has been no study to date using similar methods to distinguish between the uptake of particles and ions by plants.As introduced above, NanoSIMS has excellent isotopic resolution and can provide isotopic mapping of samples. However, this advantage has not been used adequately to investigate NPs in organisms. The combination of NanoSIMS and isotope labeling techniques will unprecedentedly contribute to distinguishing the tissue distribution and translocation of NPs and ions in organisms, including plants. In addition, double stable isotope labeled NPs can be used to study root and foliar exposure of NPs to plants simultaneously, and the uptake route of NPs under different exposure modes can be investigated. We therefore consider that the power of NanoSIMS for tracking NPs in organisms should be explored as quickly as possible.
It should be noted that it is impossible to use a single technique to obtain comprehensive information on the uptake, translocation and transformation of NPs in plants, therefore the use of a combination of multiple techniques is highly recommended, and the development of novel methods and techniques is to be encouraged in this field.
5. Conclusions and perspectives
Interactions between ENPs and plants represent one of the fundamental problems we must face at a time of rapidly developing nanotechnology because ENPs released into the environment will inevitably interact with plants, not to mention the application of nano-agriculture. This issue relates not only to ecological risk but also concerns human food safety. Here, we systematically review recent advances in studies on the uptake, translocation and transformation of metal based ENPs in the soil–plant system and summarize the integrated chain of these processes. Key conclusions can be drawn from previous studies as follows. (1) There are various pathways for the uptake of ENPs by plants, and which pathway is the dominant one depends on the materials, morphology and particle size and plant species, growth stage and physiological and growing conditions although the detailed mechanisms are still far from clear. (2) Phyllosphere and rhizosphere processes including secretion of root exudates and phyllospheric or rhizospheric microorganisms play important roles in the surface chemistry, size distribution, dissolution and transformation of ENPs, which further influence the uptake of ENPs by plants. (3) In addition to the uptake and accumulation of ENPs in plant, biotransformation of ENPs such as ZnO, Cu, Cu(OH)2, Ag, Ce2O3, Y2O3 are evidenced in plants and this greatly influences the fate of ENPs in the plant–soil system. However, there remain challenges in fully addressing the uptake, translocation and transformation mechanisms of NPs in the soil–plant system, particularly arising from the following issues. (1) Thousands of ENPs have been produced in the past few decades and the number will continue to increase greatly in the future. Thus, a priority list of ENPs for research according to their physicochemical characteristics, production, applications, discharge, exposure scenarios and ultimate effects must be established. (2) Characteristics such as chemical composition, size, morphology and surface coating of NPs determine their plant bioavailability and phytotoxicity, therefore comprehensive analysis of these characteristics of ENPs is highly recommended. Furthermore, certified nanoparticle reference materials are very limited and more are needed in order to ensure comparability among studies. (3) Some basic plant physiological problems concerning the mechanisms of plant uptake, translocation and transformation of ENPs are still unresolved. For example, how ENPs penetrate the cuticle layer of roots or leaves, except by exploiting disease and physical damage. What are the size exclusion limits of ENPs to pass through cell walls, cytomembranes, PD channels, and stomatal pores of plants? Where are the locations of NPs that accumulate and are transformed inside plants? Are there organ functions or special enzymes to help ENPs translocate or transform? More direct evidence is needed to support the apoplastic and symplastic pathways of NP transport in plants. (4) The effects of environmental matrices and phyllosphere or rhizosphere processes on the transformation and bioavailability of NPs in the soil–plant system require detailed investigation. The uptake, accumulation and toxicity of the environmentally abundant transformation products of ENPs require study in addition to the original ENPs. (5) Long-term exposure of plants, especially crops and vegetables, to ENPs has been neglected and more experimental evidence is needed to evaluate whether and to what extent NPs and their ionic form can accumulate in the edible parts of plants, whether ENPs can transfer along the human food chain and even undergo biomagnification, and whether ENPs can be a threat to food safety. (6) Developments in analytical methods/technologies, for example high spatial resolution, high sensitivity and multi-informative techniques or combined methods, and stable isotope based techniques, are urgently required to support the fundamental studies on the uptake and transport pathways of NPs in plants, and to obtain qualitative and quantitative information on the accumulation, location and speciation of ENPs in plants. In addition, interactions between NPs and plants commonly occur but until now almost all studies have focused on well-designed ENPs and have neglected unintentional nanoparticles (UNPs) and natural nanoparticles (NNPs).176–179 In fact, the environmental concentrations of UNPs and NNPs are several orders of magnitude greater than those of ENPs.178 The uptake of UNPs and NNPs by plants and their influences on the uptake of contaminants, nutrients and water by plants therefore require urgent investigation. The interactions between NPs and plants is a highly cross disciplinary field requiring effective teamwork and cooperation between plant physiologists and environmental, agricultural and material scientists and analysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Key Research and Development Program of China (2016YFA0203102), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDB14020202), and the National Natural Science Foundation of China (Projects 21621064 and 21537005).References
- K. A. D. Guzman, M. R. Taylor and J. F. Banfield, Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000-2004, Environ. Sci. Technol., 2006, 40, 1401–1407 CrossRef PubMed.
- M. Scheringer, Nanoecotoxicology-Environmental risks of nanomaterials, Nat. Nanotechnol., 2008, 3, 322–323 CrossRef CAS PubMed.
- M. R. Wiesner, G. V. Lowry, K. L. Jones, M. F. Hochella, R. T. Di Giulio, E. Casman and E. S. Bernhardt, Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials, Environ. Sci. Technol., 2009, 43, 6458–6462 CrossRef CAS PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Fate and risks of nanomaterials in aquatic and terrestrial environments, Acc. Chem. Res., 2013, 46, 854–862 CrossRef CAS PubMed.
- A. E. Pradas del Real, H. Castillo-Michel, R. Kaegi, B. Sinnet, V. Magnin, N. Findling, J. Villanova, M. Carriere, C. Santaella, A. Fernandez-Martinez, C. Levard and G. Sarret, Fate of Ag-NPs in sewage sludge after application on agricultural soils, Environ. Sci. Technol., 2016, 50, 1759–1768 CrossRef CAS PubMed.
- B. Pan and B. S. Xing, Applications and implications of manufactured nanoparticles in soils: A review, Eur. J. Soil Sci., 2012, 63, 437–456 CrossRef CAS.
- F. Gottschalk and B. Nowack, The release of engineered nanomaterials to the environment, J. Environ. Monit., 2011, 13, 1145–1155 RSC.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- M. Kah, R. S. Kookana, A. Gogos and T. D. Bucheli, A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues, Nat. Nanotechnol., 2018, 13, 677–684 CrossRef CAS PubMed.
- H. Chhipa, Nanofertilizers and nanopesticides for agriculture, Environ. Chem. Lett., 2017, 15, 15–22 CrossRef CAS.
- M. Kah, Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation?, Front. Chem., 2015, 3, 64 Search PubMed.
- X. M. Ma, J. Geiser-Lee, Y. Deng and A. Kolmakov, Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation, Sci. Total Environ., 2010, 408, 3053–3061 CrossRef CAS PubMed.
- M. Ghosh, M. Bandyopadhyay and A. Mukherjee, Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels plant and human lymphocytes, Chemosphere, 2010, 81, 1253–1262 CrossRef CAS PubMed.
- S. Pakrashi, N. Jain, S. Dalai, J. Jayakumar, P. T. Chandrasekaran, A. M. Raichur, N. Chandrasekaran and A. Mukherjee, In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations, PLoS One, 2014, 9, e87789 CrossRef PubMed.
- S. Wang, J. Kurepa and J. A. Smalle, Ultra-small TiO2 nanoparticles disrupt microtubular networks in Arabidopsis thaliana, Plant, Cell Environ., 2011, 34, 811–820 CrossRef CAS PubMed.
- Y. S. El-Temsah and E. J. Joner, Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil, Environ. Toxicol., 2012, 27, 42–49 CrossRef CAS PubMed.
- K. J. Dietz and S. Herth, Plant nanotoxicology, Trends Plant Sci., 2011, 16, 582–589 CrossRef CAS PubMed.
- L. J. Zhao, B. Peng, J. A. Hernandez-Viezcas, C. Rico, Y. P. Sun, J. R. Peralta-Videa, X. L. Tang, G. H. Niu, L. X. Jin, A. Varela-Ramirez, J. Y. Zhang and J. L. Gardea-Torresdey, Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid Peroxidation, ACS Nano, 2012, 6, 9615–9622 CrossRef CAS PubMed.
- L. J. Zhao, Y. X. Huang, H. J. Zhou, A. S. Adeleye, H. T. Wang, C. Ortiz, S. J. Mazer and A. A. Keller, GC-TOF-MS based metabolomics and ICP-MS based metallomics of cucumber (Cucumis sativus) fruits reveal alteration of metabolites profile and biological pathway disruption induced by nano copper, Environ. Sci.: Nano, 2016, 3, 1114–1123 RSC.
- L. J. Zhao, Q. R. Hu, Y. X. Huang, A. N. Fulton, C. Hannah-Bick, A. S. Adeleye and A. A. Keller, Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a Cu(OH)2 nanopesticide, Environ. Sci.: Nano, 2017, 4, 1750–1760 RSC.
- L. J. Zhao, Y. X. Huang, J. Hu, H. J. Zhou, A. S. Adeleye and A. A. Keller, H-1 NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress, Environ. Sci. Technol., 2016, 50, 2000–2010 CrossRef CAS PubMed.
- D. K. Tripathi, A. Tripathi, S. Singh Shweta, Y. Singh, K. Vishwakarma, G. Yadav, S. Sharma, V. K. Singh, R. K. Mishra, R. G. Upadhyay, N. K. Dubey, Y. Lee and D. K. Chauhan, Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review, Front. Microbiol., 2017, 8, 07 Search PubMed.
- F. Schwab, G. S. Zhai, M. Kern, A. Turner, J. L. Schnoor and M. R. Wiesner, Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants-Critical review, Nanotoxicology, 2016, 10, 257–278 CAS.
- P. Miralles, T. L. Church and A. T. Harris, Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants, Environ. Sci. Technol., 2012, 46, 9224–9239 CrossRef CAS PubMed.
- J. L. Gardea-Torresdey, C. M. Rico and J. C. White, Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments, Environ. Sci. Technol., 2014, 48, 2526–2540 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- D. H. Lin, X. L. Tian, F. C. Wu and B. S. Xing, Fate and transport of engineered nanomaterials in the environment, J. Environ. Qual., 2010, 39, 1896–1908 CrossRef PubMed.
- P. Zhang, Y. H. Ma, Z. Y. Zhang, X. He, J. Zhang, Z. Guo, R. Z. Tai, Y. L. Zhao and Z. F. Chai, Biotransformation of ceria nanoparticles in cucumber plants, ACS Nano, 2012, 6, 9943–9950 CrossRef CAS PubMed.
- Y. H. Ma, X. He, P. Zhang, Z. Y. Zhang, Z. Guo, R. Z. Tai, Z. J. Xu, L. J. Zhang, Y. Y. Ding, Y. L. Zhao and Z. F. Chai, Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus), Nanotoxicology, 2011, 5, 743–753 CrossRef CAS PubMed.
- P. Wang, N. W. Menzies, E. Lombi, B. A. McKenna, B. Johannessen, C. J. Glover, P. Kappen and P. M. Kopittke, Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata), Environ. Sci. Technol., 2013, 47, 13822–13830 CrossRef CAS PubMed.
- J. T. Lv, S. Z. Zhang, L. Luo, J. Zhang, K. Yang and P. Christie, Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize, Environ. Sci.: Nano, 2015, 2, 68–77 RSC.
- H. A. Castillo-Michel, C. Larue, A. E. Pradas del Real, M. Cotte and G. Sarret, Practical review on the use of synchrotron based micro- and nano- X-ray fluorescence mapping and X-ray absorption spectroscopy to investigate the interactions between plants and engineered nanomaterials, Plant Physiol. Biochem., 2017, 110, 13–32 CrossRef CAS PubMed.
- M. Pollard, F. Beisson, Y. H. Li and J. B. Ohlrogge, Building lipid barriers: Biosynthesis of cutin and suberin, Trends Plant Sci., 2008, 13, 236–246 CrossRef CAS PubMed.
- T. Eichert and H. E. Goldbach, Equivalent pore radii of hydrophilic foliar uptake routes instomatous and astomatous leaf surfaces-further evidencefor a stomatal pathway, Physiol. Plant., 2008, 132, 491–502 CrossRef CAS PubMed.
- C. Popp, M. Burghardt, A. Friedmann and M. Riederer, Characterization of hydrophilic and lipophilic pathways of Hedera helix L. cuticular membranes: Permeation of water and uncharged organic compounds, J. Exp. Bot., 2005, 56, 2797–2806 CrossRef CAS PubMed.
- T. Eichert, A. Kurtz, U. Steiner and H. E. Goldbach, Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles, Physiol. Plant., 2008, 134, 151–160 CrossRef CAS PubMed.
- G. Uzu, S. Sobanska, G. Sarret, M. Munoz and C. Dumat, Foliar lead uptake by lettuce exposed to atmospheric fallouts, Environ. Sci. Technol., 2010, 44, 1036–1042 CrossRef CAS PubMed.
- E. Corredor, P. S. Testillano, M. J. Coronado, P. Gonzalez-Melendi, R. Fernandez-Pacheco, C. Marquina, M. R. Ibarra, J. M. de la Fuente, D. Rubiales, A. Perez-De-Luque and M. C. Risueno, Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification, BMC Plant Biol., 2009, 9, 45–53 CrossRef PubMed.
- C. Larue, H. Castillo-Michel, S. Sobanska, L. Cecillon, S. Bureau, V. Barthes, L. Ouerdane, M. Carriere and G. Sarret, Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation, J. Hazard. Mater., 2014, 264, 98–106 CrossRef CAS PubMed.
- J. Kurepa, T. Paunesku, S. Vogt, H. Arora, B. M. Rabatic, J. J. Lu, M. B. Wanzer, G. E. Woloschak and J. A. Smalle, Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana, Nano Lett., 2010, 10, 2296–2302 CrossRef CAS PubMed.
- J. H. Kim, Y. Oh, H. Yoon, I. Hwang and Y. S. Chang, Iron nanoparticle-induced activation of plasma membrane H(+)-ATPase promotes stomatal opening in Arabidopsis thaliana, Environ. Sci. Technol., 2015, 49, 1113–1119 CrossRef CAS PubMed.
- A. A. Keller, Y. X. Huang and J. Nelson, Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS, J. Nanopart. Res., 2018, 20, 101 CrossRef.
- T. J. Lough and W. J. Lucas, Integrative plant biology: Role of phloem long-distance macromolecular trafficking, Annu. Rev. Plant Biol., 2006, 57, 203–232 CrossRef CAS PubMed.
- W. N. Wang, J. C. Tarafdar and P. Biswas, Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake, J. Nanopart. Res., 2013, 15, 1417 CrossRef.
- J. Hong, J. R. Peralta-Videa, C. Rico, S. Sahi, M. N. Viveros, J. Bartonjo, L. Zhao and J. L. Gardea-Torresdey, Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants, Environ. Sci. Technol., 2014, 48, 4376–4385 CrossRef CAS PubMed.
- L. Zhao, C. Ortiz, A. S. Adeleye, Q. Hu, H. Zhou, Y. Huang and A. A. Keller, Metabolomics to detect response of lettuce (Lactuca sativa) to Cu(OH)2 nanopesticides: Oxidative stress response and detoxification mechanisms, Environ. Sci. Technol., 2016, 50, 9697–9707 CrossRef CAS PubMed.
- Z. Wang, X. Xie, J. Zhao, X. Liu, W. Feng, J. C. White and B. Xing, Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.), Environ. Sci. Technol., 2012, 46, 4434–4441 CrossRef CAS PubMed.
- Y. Ma, X. He, P. Zhang, Z. Zhang, Y. Ding, J. Zhang, G. Wang, C. Xie, W. Luo, J. Zhang, L. Zheng, Z. Chai and K. Yang, Xylem and phloem based transport of CeO2 nanoparticles in hydroponic cucumber plants, Environ. Sci. Technol., 2017, 51, 5215–5221 CrossRef CAS PubMed.
- J. A. Vorholt, Microbial life in the phyllosphere, Nat. Rev. Microbiol., 2012, 10, 828–840 CrossRef CAS PubMed.
- S. E. Lindow and M. T. Brandl, Microbiology of the phyllosphere, Appl. Environ. Microbiol., 2003, 69, 1875–1883 CrossRef CAS PubMed.
- S. L. Honour, J. N. Bell, T. W. Ashenden, J. N. Cape and S. A. Power, Responses of herbaceous plants to urban air pollution: Effects on growth, phenology and leaf surface characteristics, Environ. Pollut., 2009, 157, 1279–1286 CrossRef CAS PubMed.
- S. Sankaran, A. Mishra, R. Ehsani and C. Davis, A review of advanced techniques for detecting plant diseases, Comput. Electron. Agric., 2010, 72, 1–13 CrossRef.
- M. Kah and T. Hofmann, Nanopesticide research: Current trends and future priorities, Environ. Int., 2014, 63, 224–235 CrossRef CAS PubMed.
- H. J. Park, S. H. Kim, H. J. Kim and S. H. Choi, A new composition of nanosized silica-silver for control of various plant diseases, Plant Pathol. J., 2006, 22, 295–302 CrossRef.
- T. Nguyen, X. X. Yu, Z. M. Zhang, M. M. Liu and X. H. Liu, Relationship between types of urban forest and PM2.5 capture at three growth stages of leaves, J. Environ. Sci., 2015, 27, 33–41 CrossRef PubMed.
- K. Dzierzanowski, R. Popek, H. Gawronska, A. Saebo and S. W. Gawronski, Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species, Int. J. Phytorem., 2011, 13, 1037–1046 CrossRef CAS PubMed.
- A. D. Maynard and E. D. Kuempel, Airborne nanostructured particles and occupational health, J. Nanopart. Res., 2005, 7, 587–614 CrossRef CAS.
- A. Seaton, L. Tran, R. Aitken and K. Donaldson, Nanoparticles, human health hazard and regulation, J. R. Soc., Interface, 2010, 7(Suppl 1), S119–S129 CrossRef CAS PubMed.
- T. Sabo-Attwood, J. M. Unrine, J. W. Stone, C. J. Murphy, S. Ghoshroy, D. Blom, P. M. Bertsch and L. A. Newman, Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings, Nanotoxicology, 2012, 6, 353–360 CrossRef CAS PubMed.
- A. F. Taylor, E. L. Rylott, C. W. Anderson and N. C. Bruce, Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold, PLoS One, 2014, 9, e93793 CrossRef PubMed.
- D. L. Slomberg and M. H. Schoenfisch, Silica nanoparticle phytotoxicity to Arabidopsis thaliana, Environ. Sci. Technol., 2012, 46, 10247–10254 CAS.
- C. Larue, J. Laurette, N. Herlin-Boime, H. Khodja, B. Fayard, A. M. Flank, F. Brisset and M. Carriere, Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase, Sci. Total Environ., 2012, 431, 197–208 CrossRef CAS PubMed.
- A. Avellan, F. Schwab, A. Masion, P. Chaurand, D. Borschneck, V. Vidal, J. Rose, C. Santaella and C. Levard, Nanoparticle uptake in plants: Gold nanomaterial localized in roots of Arabidopsis thaliana by X-ray computed nanotomography and hyperspectral imaging, Environ. Sci. Technol., 2017, 51, 8682–8691 CrossRef CAS PubMed.
- J. Koelmel, T. Leland, H. H. Wang, D. Amarasiriwardena and B. S. Xing, Investigation of gold nanoparticles uptake and their tissue level distribution in rice plants by laser ablation-inductively coupled-mass spectrometry, Environ. Pollut., 2013, 174, 222–228 CrossRef CAS PubMed.
- E. Spielman-Sun, E. Lombi, E. Donner, D. Howard, J. M. Unrine and G. V. Lowry, Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum), Environ. Sci. Technol., 2017, 51, 7361–7368 CrossRef CAS PubMed.
- A. A. Keller, A. S. Adeleye, J. R. Conway, K. L. Garner, L. J. Zhao, G. N. Cherr, J. Hong, J. L. Gardea-Torresdey, H. A. Godwin and S. S. Hanna, et al., Comparative environmental fate and toxicity of copper nanomaterials, Nanoimpact, 2017, 7, 28–40 CrossRef.
- C. Layet, M. Auffan, C. Santaella, C. Chevassus-Rosset, M. Montes, P. Ortet, M. Barakat, B. Collin, S. Legros, M. N. Bravin, B. Angeletti, I. Kieffer, O. Proux, J. L. Hazemann and E. Doelsch, Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles, Environ. Sci. Technol., 2017, 51, 9756–9764 CrossRef CAS PubMed.
- J. B. Glenn, S. A. White and S. J. Klaine, Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent, Environ. Toxicol. Chem., 2012, 31, 194–201 CrossRef CAS PubMed.
- J. D. Judy, J. M. Unrine, W. Rao, S. Wirick and P. M. Bertsch, Bioavailability of gold nanomaterials to plants: Importance of particle size and surface coating, Environ. Sci. Technol., 2012, 46, 8467–8474 CrossRef CAS PubMed.
- A. Noori, J. C. White and L. A. Newman, Mycorrhizal fungi influence on silver uptake and membrane protein gene expression following silver nanoparticle exposure, J. Nanopart. Res., 2017, 19, 66 CrossRef.
- F. Wang, X. Liu, Z. Shi, R. Tong, C. A. Adams and X. Shi, Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants--A soil microcosm experiment, Chemosphere, 2016, 147, 88–97 CrossRef CAS PubMed.
- A. Servin, W. Elmer, A. Mukherjee, R. De la Torre-Roche, H. Hamdi, J. C. White, P. Bindraban and C. Dimkpa, A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield, J. Nanopart. Res., 2015, 17, 92 CrossRef.
- D. M. Zhou, S. Y. Jin, L. Z. Li, Y. Wang and N. Y. Weng, Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions, J. Environ. Sci., 2011, 23, 1852–1857 CrossRef CAS.
- D. H. Lin and B. S. Xing, Root uptake and phytotoxicity of ZnO nanoparticles, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS PubMed.
- J. Geisler-Lee, Q. Wang, Y. Yao, W. Zhang, M. Geisler, K. Li, Y. Huang, Y. Chen, A. Kolmakov and X. Ma, Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana, Nanotoxicology, 2013, 7, 323–337 CrossRef CAS PubMed.
- J. Shi, C. Peng, Y. Yang, J. Yang, H. Zhang, X. Yuan, Y. Chen and T. Hu, Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens, Nanotoxicology, 2014, 8, 179–188 CrossRef CAS PubMed.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Nanoparticulate material delivery to plants, Plant Sci., 2010, 179, 154–163 CrossRef CAS.
- N. Geldner, D. Roppolo, B. De Rybel, V. D. Tendon, A. Pfister, J. Alassimone, J. E. M. Vermeer, M. Yamazaki, Y. D. Stierhof and T. Beeckman, A novel protein family mediates Casparian strip formation in the endodermis, Nature, 2011, 473, 380–384 CrossRef PubMed.
- S. Schymura, T. Fricke, H. Hildebrand and K. Franke, Elucidating the role of dissolution in CeO2 nanoparticle plant uptakeby smart radiolabeling, Angew. Chem., Int. Ed., 2017, 56, 7411–7414 CrossRef CAS PubMed.
- M. McCully, How do real roots work-some new views of root structure, Plant Physiol., 1995, 109, 1–6 CrossRef CAS PubMed.
- D. T. Luu and C. Maurel, Aquaporins in a challenging environment: molecular gears for adjusting plant water status, Plant, Cell Environ., 2005, 28, 85–96 CrossRef CAS.
- E. Steudle and C. A. Peterson, How does water get through roots?, J. Exp. Bot., 1998, 49, 775–788 CAS.
- E. Wild and K. C. Jones, Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants, Environ. Sci. Technol., 2009, 43, 5290–5294 CrossRef CAS PubMed.
- M. S. Serag, M. F, N. Kaji, C. Gaillard, Y. Okamoto, K. Terasaka, M. Jabasini, M. Tokeshi, H. Mizukami, A. Bianco and Y. Baba, Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells, ACS Nano, 2011, 5, 493–499 CrossRef CAS PubMed.
- E. Onelli, C. Prescianotto-Baschong, M. Caccianiga and A. Moscatelli, Clathrin-dependent and independent endocytic pathways in tobacco protoplasts revealed by labelling with charged nanogold, J. Exp. Bot., 2008, 59, 3051–3068 CrossRef CAS PubMed.
- E. Etxeberria, P. Gonzalez, E. Baroja-Fernandez and J. P. Romero, Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells, Plant Signaling Behav., 2006, 1, 196–200 CrossRef.
- F. Torney, B. G. Trewyn, V. S. Y. Lin and K. Wang, Mesoporous silica nanoparticles deliver DNA and chemicals into plants, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS PubMed.
- P. Zhang, Y. Ma, Z. Zhang, X. He, Z. Guo, R. Tai, Y. Ding, Y. Zhao and Z. Chai, Comparative toxicity of nanoparticulate/bulk Yb2O3 and YbCl3 to cucumber (Cucumis sativus), Environ. Sci. Technol., 2012, 46, 1834–1841 CrossRef CAS PubMed.
- H. Li, X. Ye, X. Guo, Z. Geng and G. Wang, Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato, J. Hazard. Mater., 2016, 314, 188–196 CrossRef CAS PubMed.
- P. Zambryski and K. Crawford, Plasmodesmata: Gatekeepers for cell-to-cell transport of developmental signals in plants, Annu. Rev. Cell Dev. Biol., 2000, 16, 393–421 CrossRef CAS PubMed.
- W. J. Lucas, Plasmodesmata: Intercellular channels for macromolecular transport in plants, Curr. Opin. Cell Biol., 1995, 7, 673–680 CrossRef CAS PubMed.
- W. J. Lucas, L. K. Ham and J. Y. Kim, Plasmodesmata-bridging the gap between neighboring plant cells, Trends Cell Biol., 2009, 19, 495–503 CrossRef CAS PubMed.
- W. J. Lucas and J. Y. Lee, Plant cell biology-Plasmodesmata as a supracellular control network in plants, Nat. Rev. Mol. Cell Biol., 2004, 5, 712–726 CrossRef CAS PubMed.
- K. J. Oparka and R. Turgeon, Sieve elements and companion cells-traffic control centers of the phloem, Plant Cell, 1999, 11, 739–750 CAS.
- G. S. Zhai, K. S. Walters, D. W. Peate, P. J. J. Alvarez and J. L. Schnoor, Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar, Environ. Sci. Technol. Lett., 2014, 1, 146–151 CrossRef CAS PubMed.
- J. L. Gardea-Torresdey, E. Gomez, J. R. Peralta-Videa, J. G. Parsons, H. Troiani and M. Jose-Yacaman, Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles, Langmuir, 2003, 19, 1357–1361 CrossRef CAS.
- J. L. Gardea-Torresdey, J. G. Parsons, E. Gomez, J. Peralta-Videa, H. E. Troiani, P. Santiago and M. J. Yacaman, Formation and growth of Au nanoparticles inside live alfalfa plants, Nano Lett., 2002, 2, 397–401 CrossRef CAS.
- I. R. Beattie and R. G. Haverkamp, Silver and gold nanoparticles in plants: Sites for the reduction to metal, Metallomics, 2011, 3, 628–632 RSC.
- M. Auffan, J. Rose, J. Y. Bottero, G. V. Lowry, J. P. Jolivet and M. R. Wiesner, Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Fate and transformation of silver nanoparticles in urban wastewater systems, Water Res., 2013, 47, 3866–3877 CrossRef CAS PubMed.
- F. Gomez-Rivera, J. A. Field, D. Brown and R. Sierra-Alvarez, Fate of cerium dioxide (CeO2) nanoparticles in municipal wastewater during activated sludge treatment, Bioresour. Technol., 2012, 108, 300–304 CrossRef CAS PubMed.
- E. Lombi, E. Donner, E. Tavakkoli, T. W. Turney, R. Naidu, B. W. Miller and K. G. Scheckel, Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge, Environ. Sci. Technol., 2012, 46, 9089–9096 CrossRef CAS PubMed.
- J. T. Lv, S. Z. Zhang, S. S. Wang, L. Luo, H. L. Huang and J. Zhang, Chemical transformation of zinc oxide nanoparticles as a result of interaction with hydroxyapatite, Colloids Surf., A, 2014, 461, 126–132 CrossRef CAS.
- J. T. Lv, S. Z. Zhang, L. Luo, W. Han, J. Zhang, K. Yang and P. Christie, Dissolution and microstructural transformation of zno nanoparticles under the influence of phosphate, Environ. Sci. Technol., 2012, 46, 7215–7221 CrossRef CAS PubMed.
- B. C. Reinsch, B. Forsberg, R. L. Penn, C. S. Kim and G. V. Lowry, Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents, Environ. Sci. Technol., 2010, 44, 3455–3461 CrossRef CAS PubMed.
- G. V. Lowry, B. P. Espinasse, A. R. Badireddy, C. J. Richardson, B. C. Reinsch, L. D. Bryant, A. J. Bone, A. Deonarine, S. Chae, M. Therezien, B. P. Colman, H. Hsu-Kim, E. S. Bernhardt, C. W. Matson and M. R. Wiesner, Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland, Environ. Sci. Technol., 2012, 46, 7027–7036 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Jr., Environmental transformations of silver nanoparticles: impact on stability and toxicity, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- K. L. Garner and A. A. Keller, Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies, J. Nanopart. Res., 2014, 16, 2503 CrossRef.
- D. M. Mitrano, S. Motellier, S. Clavaguera and B. Nowack, Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products, Environ. Int., 2015, 77, 132–147 CrossRef CAS PubMed.
- B. Seshadri, N. S. Bolan and R. Naidu, Rhizosphere-induced heavy metal(loid) transformation in relation to bioavailability and remediation, J. Soil Sci. Plant Nutr., 2015, 15, 524–548 CAS.
- Y. Huang, L. Zhao and A. A. Keller, Interactions, transformations, and bioavailability of nano-copper exposed to root exudates, Environ. Sci. Technol., 2017, 51, 9774–9783 CrossRef CAS PubMed.
- X. Gao, A. Avellan, S. Laughton, R. Vaidya, S. M. Rodrigues, E. A. Casman and G. V. Lowry, CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil, Environ. Sci. Technol., 2018, 52, 2888–2897 CrossRef CAS PubMed.
- C. M. Rico, M. G. Johnson and M. A. Marcus, Cerium oxide nanoparticles transformation at the root-soil interface of barley (Hordeum vulgare L.), Environ. Sci.: Nano, 2018, 5, 1807–1812 RSC.
- K. Zhalnina, K. B. Louie, Z. Hao, N. Mansoori, U. N. da Rocha, S. Shi, H. Cho, U. Karaoz, D. Loque, B. P. Bowen, M. K. Firestone, T. R. Northen and E. L. Brodie, Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly, Nat. Microbiol., 2018, 3, 470–480 CrossRef CAS PubMed.
- A. Jilling, M. Keiluweit, A. R. Contosta, S. Frey, J. Schimel, J. Schnecker, R. G. Smith, L. Tiemann and A. S. Grandy, Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes, Biogeochemistry, 2018, 139, 103–122 CrossRef CAS.
- B. Nowack and T. D. Bucheli, Occurrence, behavior and effects of nanoparticles in the environment, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS PubMed.
- C. Coutris, E. J. Joner and D. H. Oughton, Aging and soil organic matter content affect the fate of silver nanoparticles in soil, Sci. Total Environ., 2012, 420, 327–333 CrossRef CAS PubMed.
- P. Wang, E. Lombi, S. K. Sun, K. G. Scheckel, A. Malysheva, B. A. McKenna, N. W. Menzies, F. J. Zhao and P. M. Kopittke, Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants, Environ. Sci.: Nano, 2017, 4, 448–460 RSC.
- J. P. Stegemeier, F. Schwab, B. P. Colman, S. M. Webb, M. Newville, A. Lanzirotti, C. Winkler, M. R. Wiesner and G. V. Lowry, Speciation matters: Bioavailability of silver and silver sulfide nanoparticles to alfalfa (Medicago sativa), Environ. Sci. Technol., 2015, 49, 8451–8460 CrossRef CAS PubMed.
- E. Spielman-Sun, E. Lombi, E. Donner, A. Avellan, B. Etschmann, D. Howard and G. V. Lowry, Temporal evolution of copper distribution and speciation in roots of Triticum aestivum exposed to CuO, Cu(OH)2, and CuS nanoparticles, Environ. Sci. Technol., 2018, 52, 9777–9784 CrossRef CAS PubMed.
- L. Y. Yin, Y. W. Cheng, B. Espinasse, B. P. Colman, M. Auffan, M. Wiesner, J. Rose, J. Liu and E. S. Bernhardt, More than the ions: The effects of silver nanoparticles on Lolium multiflorum, Environ. Sci. Technol., 2011, 45, 2360–2367 CrossRef CAS PubMed.
- S. Wang, J. Lv, J. Ma and S. Zhang, Cellular internalization and intracellular biotransformation of silver nanoparticles in Chlamydomonas reinhardtii, Nanotoxicology, 2016, 10, 1129–1135 CrossRef CAS PubMed.
- D. Lu, Q. Liu, T. Zhang, Y. Cai, Y. Yin and G. Jiang, Stable silver isotope fractionation in the natural transformation process of silver nanoparticles, Nat. Nanotechnol., 2016, 11, 682–686 CrossRef CAS PubMed.
- A. D. Servin, H. Castillo-Michel, J. A. Hernandez-Viezcas, B. C. Diaz, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Synchrotron micro-XRE and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants, Environ. Sci. Technol., 2012, 46, 7637–7643 CrossRef CAS PubMed.
- C. Larue, H. Khodja, N. Herlin-Boime, F. Brisset, A. M. Flank, B. Fayard, S. Chaillou and M. Carrière, Investigation of titanium dioxide nanoparticles toxicity and uptake by plants, J. Phys.: Conf. Ser., 2011, 304, 012057 CrossRef.
- C. O. Dimkpa, D. E. Latta, J. E. McLean, D. W. Britt, M. I. Boyanov and A. J. Anderson, Fate of CuO and ZnO nano and micro particles in the plant environment, Environ. Sci. Technol., 2013, 47, 4734–4742 CrossRef CAS PubMed.
- C. O. Dimkpa, J. E. McLean, D. E. Latta, E. Manangon, D. W. Britt, W. P. Johnson, M. I. Boyanov and A. J. Anderson, CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat, J. Nanopart. Res., 2012, 14, 1125 CrossRef.
- J. A. Hernandez-Viezcas, H. Castillo-Michel, J. C. Andrews, M. Cotte, C. Rico, J. R. Peralta-Videa, Y. Ge, J. H. Priester, P. A. Holden and J. L. Gardea-Torresdey, In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max), ACS Nano, 2013, 7, 1415–1423 CrossRef CAS PubMed.
- M. L. Lopez-Moreno, G. de la Rosa, J. A. Hernandez-Viezcas, H. Castillo-Michel, C. E. Botez, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants, Environ. Sci. Technol., 2010, 44, 7315–7320 CrossRef CAS PubMed.
- J. A. Hernandez-Viezcas, H. Castillo-Michel, A. D. Servin, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina (velvet mesquite) treated with ZnO nanoparticles, Chem. Eng. J., 2011, 170, 346–352 CrossRef CAS PubMed.
- C. Peng, C. Xu, Q. Liu, L. Sun, Y. Luo and J. Shi, Fate and transformation of CuO nanoparticles in the soil-rice system during the life cycle of rice plants, Environ. Sci. Technol., 2017, 51, 4907–4917 CrossRef CAS PubMed.
- C. Peng, D. Duan, C. Xu, Y. Chen, L. Sun, H. Zhang, X. Yuan, L. Zheng, Y. Yang, J. Yang, X. Zhen, Y. Chen and J. Shi, Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants, Environ. Pollut., 2015, 197, 99–107 CrossRef CAS PubMed.
- M. L. Lopez-Moreno, G. de la Rosa, J. A. Hernandez-Viezcas, J. R. Peralta-Videa and J. L. Gardea-Torresdey, X-ray Absorption Spectroscopy (XAS) Corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species, J. Agric. Food Chem., 2010, 58, 3689–3693 CrossRef CAS PubMed.
- L. J. Zhao, J. R. Peralta-Videa, A. Varela-Ramirez, H. Castillo-Michel, C. Q. Li, J. Y. Zhang, R. J. Aguilera, A. A. Keller and J. L. Gardea-Torresdey, Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: Insight into the uptake mechanism, J. Hazard. Mater., 2012, 225, 131–138 CrossRef PubMed.
- K. Birbaum, R. Brogioli, M. Schellenberg, E. Martinoia, W. J. Stark, D. Gunther and L. K. Limbach, No evidence for cerium dioxide nanoparticle translocation in maize plants, Environ. Sci. Technol., 2010, 44, 8718–8723 CrossRef CAS PubMed.
- D. Cui, P. Zhang, Y. H. Ma, X. He, Y. Y. Li, J. Zhang, Y. C. Zhao and Z. Y. Zhang, Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium, Environ. Sci.: Nano, 2014, 1, 459–465 RSC.
- Y. H. Ma, P. Zhang, Z. Y. Zhang, X. He, J. Z. Zhang, Y. Y. Ding, J. Zhang, L. R. Zheng, Z. Guo, L. J. Zhang, Z. F. Chai and Y. L. Zhao, Where does the transformation of precipitated ceria nanoparticles in hydroponic plants take place?, Environ. Sci. Technol., 2015, 49, 10667–10674 CrossRef CAS PubMed.
- P. Zhang, Y. H. Ma, Z. Y. Zhang, X. He, Z. Guo, R. Z. Tai, Y. Y. Ding, Y. L. Zhao and Z. F. Chai, Comparative toxicity of nanoparticulate/bulk Yb2O3 and YbCl3 to cucumber (Cucumis sativus), Environ. Sci. Technol., 2012, 46, 1834–1841 CrossRef CAS PubMed.
- P. Wang, E. Lombi, F. J. Zhao and P. M. Kopittke, Nanotechnology: A new opportunity in plant sciences, Trends Plant Sci., 2016, 21, 699–712 CrossRef CAS PubMed.
- D. Pozebon, G. L. Scheffler and V. L. Dressler, Recent applications of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for biological sample analysis: A follow-up review, J. Anal. At. Spectrom., 2017, 32, 890–919 RSC.
- Y. Deng, E. J. Petersen, K. E. Challis, S. A. Rabb, R. D. Holbrook, J. F. Ranville, B. C. Nelson and B. Xing, Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants, Environ. Sci. Technol., 2017, 51, 10615–10623 CrossRef CAS PubMed.
- J. Jimenez-Lamana, J. Wojcieszek, M. Jakubiak, M. Asztemborska and J. Szpunar, Single particle ICP-MS characterization of platinum nanoparticles uptake and bioaccumulation by Lepidium sativum and Sinapis alba plants, J. Anal. At. Spectrom., 2016, 31, 2321–2329 RSC.
- Y. B. Dan, W. L. Zhang, R. M. Xue, X. M. Ma, C. Stephan and H. L. Shi, Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma-mass spectrometry analysis, Environ. Sci. Technol., 2015, 49, 3007–3014 CrossRef CAS PubMed.
- Y. Dan, X. Ma, W. Zhang, K. Liu, C. Stephan and H. Shi, Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles, Anal. Bioanal. Chem., 2016, 408, 5157–5167 CrossRef CAS PubMed.
- D. P. Bao, Z. G. Oh and Z. Chen, Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis, Front. Plant Sci., 2016, 7, 32 Search PubMed.
- L. Liu, B. He, Q. Liu, Z. Yun, X. Yan, Y. Long and G. Jiang, Identification and accurate size characterization of nanoparticles in complex media, Angew. Chem., Int. Ed., 2014, 53, 14476–14479 CrossRef CAS PubMed.
- M. Matczuk, K. Anecka, F. Scaletti, L. Messori, B. K. Keppler, A. R. Timerbaev and M. Jarosz, Speciation of metal-based nanomaterials in human serum characterized by capillary electrophoresis coupled to ICP-MS: A case study of gold nanoparticles, Metallomics, 2015, 7, 1364–1370 RSC.
- G. T. Wei, F. K. Liu and C. R. Wang, Shape separation of nanometer gold particles by size-exclusion chromatography, Anal. Chem., 1999, 71, 2085–2091 CrossRef CAS PubMed.
- X. X. Zhou, J. F. Liu and F. L. Geng, Determination of metal oxide nanoparticles and their ionic counterparts in environmental waters by size exclusion chromatography coupled to ICP-MS, NanoImpact, 2016, 1, 13–20 CrossRef.
- K. Tiede, A. B. A. Boxall, D. Tiede, S. P. Tear, H. David and J. Lewis, A robust size-characterisation methodology for studying nanoparticle behaviour in 'real' environmental samples, using hydrodynamic chromatography coupled to ICP-MS, J. Anal. At. Spectrom., 2009, 24, 964–972 RSC.
- L. J. Gimbert, R. E. Hamon, P. S. Casey and P. J. Worsfold, Partitioning and stability of engineered ZnO nanoparticles in soil suspensions using flow field-flow fractionation, Environ. Chem., 2007, 4, 8–10 CrossRef CAS.
- Z. Q. Tan, J. F. Liu, X. R. Guo, Y. G. Yin, S. K. Byeon, M. H. Moon and G. B. Jiang, Toward full spectrum speciation of silver nanoparticles and ionic silver by on-line coupling of hollow fiber flow field-flow fractionation and minicolumn concentration with multiple detectors, Anal. Chem., 2015, 87, 8441–8447 CrossRef CAS PubMed.
- M. Baalousha, B. Stolpe and J. R. Lead, Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: a critical review, J. Chromatogr. A, 2011, 1218, 4078–4103 CrossRef CAS PubMed.
- D. A. Navarro, M. A. Bisson and D. S. Aga, Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants, J. Hazard. Mater., 2012, 211, 427–435 CrossRef PubMed.
- K. L. Moore, E. Lombi, F. J. Zhao and C. R. M. Grovenor, Elemental imaging at the nanoscale: NanoSIMS and complementary techniques for element localisation in plants, Anal. Bioanal. Chem., 2012, 402, 3263–3273 CrossRef CAS PubMed.
- T. Aubert, A. Burel, M. A. Esnault, S. Cordier, F. Grasset and F. Cabello-Hurtado, Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters, J. Hazard. Mater., 2012, 219, 111–118 CrossRef PubMed.
- A. J. Bone, B. P. Colman, A. P. Gondikas, K. M. Newton, K. H. Harrold, R. M. Cory, J. M. Unrine, S. J. Klaine, C. W. Matson and R. T. Di Giulio, Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles: part 2-toxicity and Ag speciation, Environ. Sci. Technol., 2012, 46, 6925–6933 CrossRef CAS PubMed.
- L. Monico, K. Janssens, M. Alfeld, M. Cotte, F. Vanmeert, C. G. Ryan, G. Falkenberg, D. L. Howard, B. G. Brunetti and C. Miliani, Full spectral XANES imaging using the Maia detector array as a new tool for the study of the alteration process of chrome yellow pigments in paintings by Vincent van Gogh, J. Anal. At. Spectrom., 2015, 30, 613–626 RSC.
- G. Martinez-Criado, J. Villanova, R. Tucoulou, D. Salomon, J. P. Suuronen, S. Laboure, C. Guilloud, V. Valls, R. Barrett, E. Gagliardini, Y. Dabin, R. Baker, S. Bohic, C. Cohen and J. Morse, ID16B: a hard X-ray nanoprobe beamline at the ESRF for nano-analysis, J. Synchrotron Radiat., 2016, 23, 344–352 CrossRef CAS PubMed.
- Y. Zhu, J. C. Zhang, A. G. Li, Y. Q. Zhang and C. H. Fan, Synchrotron-based X-ray microscopy for sub-100 nm resolution cell imaging, Curr. Opin. Chem. Biol., 2017, 39, 11–16 CrossRef CAS PubMed.
- D. A. Shapiro, Y. S. Yu, T. Tyliszczak, J. Cabana, R. Celestre, W. L. Chao, K. Kaznatcheev, A. L. D. Kilcoyne, F. Maia, S. Marchesini, Y. S. Meng, T. Warwick, L. L. Yang and H. A. Padmore, Chemical composition mapping with nanometre resolution by soft X-ray microscopy, Nat. Photonics, 2014, 8, 765–769 CrossRef CAS.
- B. Gilbert, S. C. Fakra, T. Xia, S. Pokhrel, L. Madler and A. E. Nel, The fate of ZnO nanoparticles administered to human bronchial epithelial cells, ACS Nano, 2012, 6, 4921–4930 CrossRef CAS PubMed.
- L. G. Thygesen, M. M. Lokke, E. Micklander and S. B. Engelsen, Vibrational microspectroscopy of food. Raman vs. FT-IR, Trends Food Sci. Technol., 2003, 14, 50–57 CrossRef CAS.
- S. Amarie, P. Zaslansky, Y. Kajihara, E. Griesshaber, W. W. Schmahl and F. Keilmann, Nano-FTIR chemical mapping of minerals in biological materials, Beilstein J. Nanotechnol., 2012, 3, 312–323 CrossRef CAS PubMed.
- F. Huth, A. Govyadinov, S. Amarie, W. Nuansing, F. Keilmann and R. Hilenbrand, Nano-FTIR absorption spectroscopy of molecular fingerprints at 20 nm spatial resolution, Nano Lett., 2012, 12, 3973–3978 CrossRef CAS PubMed.
- S. Bernard, O. Beyssac and K. Benzerara, Raman mapping using advanced line-scanning systems: Geological applications, Appl. Spectrosc., 2008, 62, 1180–1188 CrossRef CAS PubMed.
- J. W. Kang, F. T. Nguyen, N. Lue, R. R. Dasari and D. A. Heller, Measuring Uptake dynamics of multiple identifiable carbon nanotube species via high-speed confocal raman imaging of live cells, Nano Lett., 2012, 12, 6170–6174 CrossRef CAS PubMed.
- G. A. Roth, S. Tahiliani, N. M. Neu-Baker and S. A. Brenner, Hyperspectral microscopy as an analytical tool for nanomaterials, Wires Nanomed. Nanobi., 2015, 7, 565–579 CrossRef CAS PubMed.
- M. C. Martin, U. Schade, P. Lerch and P. Dumas, Recent applications and current trends in analytical chemistry using synchrotron-based Fourier-transform infrared microspectroscopy, TrAC, Trends Anal. Chem., 2010, 29, 453–463 CrossRef CAS.
- M. Mortimer, A. Gogos, N. Bartolome, A. Kahru, T. D. Bucheli and V. I. Slaveykova, Potential of hyperspectral imaging microscopy for semi-quantitative analysis of nanoparticle uptake by protozoa, Environ. Sci. Technol., 2014, 48, 8760–8767 CrossRef CAS PubMed.
- S. H. K. Eder, A. M. Gigler, M. Hanzlik and M. Winklhofer, Sub-micrometer-scale mapping of magnetite crystals and sulfur globules in magnetotactic bacteria using confocal raman micro-spectrometry, PLoS One, 2014, 9, e107356 CrossRef PubMed.
- Y. Yin, Z. Tan, L. Hu, S. Yu, J. Liu and G. Jiang, Isotope tracers to study the environmental fate and bioaccumulation of metal-containing engineered nanoparticles: Techniques and applications, Chem. Rev., 2017, 117, 4462–4487 CrossRef CAS PubMed.
- S. J. Yu, Y. G. Yin, X. X. Zhou, L. J. Dong and J. F. Liu, Transformation kinetics of silver nanoparticles and silver ions in aquatic environments revealed by double stable isotope labeling, Environ. Sci.: Nano, 2016, 3, 883–893 RSC.
- F. R. Khan, A. Laycock, A. Dybowska, F. Larner, B. D. Smith, P. S. Rainbow, S. N. Luoma, M. Rehkamper and E. Valsami-Jones, Stable isotope tracer to determine uptake and efflux dynamics of ZnO nano- and bulk particles and dissolved Zn to an estuarine snail, Environ. Sci. Technol., 2013, 47, 8532–8539 CrossRef CAS PubMed.
- A. Laycock, A. Romero-Freire, J. Najorka, C. Svendsen, C. A. M. van Gestel and M. Rehkamper, Novel multi-isotope tracer approach to test ZnO nanoparticle and soluble Zn bioavailability in joint soil exposures, Environ. Sci. Technol., 2017, 51, 12756–12763 CrossRef CAS PubMed.
- V. K. Sharma, J. Filip, R. Zboril and R. S. Varma, Natural inorganic nanoparticles-formation, fate, and toxicity in the environment, Chem. Soc. Rev., 2015, 44, 8410–8423 RSC.
- S. Wagner, A. Gondikas, E. Neubauer, T. Hofmann and F. von der Kammer, Spot the difference: Engineered and natural nanoparticles in the environment-release, behavior, and fate, Angew. Chem., Int. Ed., 2014, 53, 12398–12419 CAS.
- M. F. Hochella, M. G. Spencer and K. L. Jones, Nanotechnology: Nature's gift or scientists' brainchild?, Environ. Sci.: Nano, 2015, 2, 114–119 RSC.
- L. Becker, Fullerenes in the 1.85-billion-year-old sudbury impact structure, Science, 1994, 265, 1644–1644 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00645h |
| This journal is © The Royal Society of Chemistry 2019 |