Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Near-infrared photochemistry at interfaces based on upconverting nanoparticles
Si
Wu
 * and
Hans-Jürgen
Butt
* and
Hans-Jürgen
Butt
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wusi@mpip-mainz.mpg.de
First published on 3rd May 2017
Abstract
Near-infrared (NIR) light is better suited than ultraviolet (UV) light for biomedical applications because it penetrates deeper into tissue and causes less photodamage to biological systems. The use of NIR light to control biointerfaces has attracted increasing interest. Here, we review NIR photoreactions at interfaces based on upconverting nanoparticles (UCNPs). UCNPs can convert NIR light to UV or visible light, which can then induce photoreactions of photosensitive compounds. This process is referred to as UCNP-assisted photochemistry. Recently, we and others demonstrated UCNP-assisted photochemistry at interfaces to control interfacial properties of nano-carriers, implants, emulsions, and cells. We introduce the fundamentals of UCNP-assisted photochemistry at interfaces, highlight its potential applications, and discuss remaining challenges.
1. Introduction
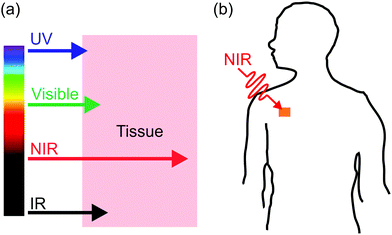
Interfacial properties of materials are important for diverse applications in our daily lives, fundamental research, and industry. Light provides a non-contact way to control interfacial properties with high spatiotemporal resolution. The development of photosensitive interfaces has attracted a lot of attention (Fig. 1).1–8 Grafting photosensitive compounds on a substrate can prepare photosensitive surfaces (Fig. 1a). Photoreactions such as photoisomerization, photopolymerization, photolysis, and photo-coupling on a substrate can control surface properties and functions.1,2,4–10 These surface photoreactions have been used for controlling wettability,2,4,6,11,12 preparing coatings and patterns,7,13–15 controlling motions of objectives on a surface,2,4 regulating adhesion,16,17 and switching electromagnetic devices.18,19 A topic under intense investigation is photosensitive surfaces of nanoparticles (NPs) (Fig. 1b). NPs have large surface areas; their stability, properties and functions are strongly influenced by their surface properties. Inducing photoreactions at NP surfaces is an elegant way to manipulate NPs.20–23 A topic related to photoreactions at surfaces is photoreactions at other interfaces (Fig. 1c). Photosensitive surfactants, amphiphiles, lipids, and colloids are usually used for interfacial photoreactions.24–26 Interfacial photoreactions can modulate the interfacial tension,25 drive the movement of objectives at an interface,25,27 control transportation across membranes,28,29 change morphologies of membranes,30,31 and control Marangoni and coffee-ring effects.32,33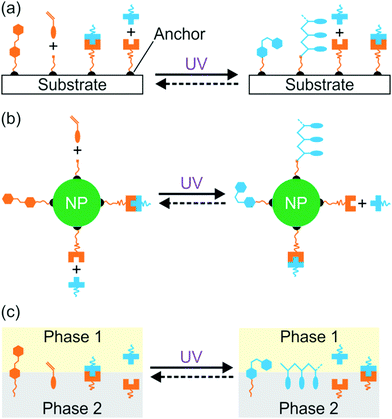 | ||
| Fig. 1 Photoreactions induced by UV light at the surface of a substrate (a), the surface of a nanoparticle (b), and an interface (c). | ||
Photoreactions at interfaces have been used for biomedical applications. For example, UV-light-controlled protein adsorption and cell adhesion have been demonstrated.34–38 Fan et al. improved targeting specificity of nano-carriers by uncaging targeting groups using UV light.21 In the above-mentioned examples, UV light was used to induce photoreactions at interfaces. However, UV light is problematic for some biomedical applications. In contrast, near-infrared (NIR) light in the “therapeutic window” is better suited than UV light for these applications because NIR light can penetrate deeper into tissue and causes less photodamage to cells (Fig. 2a).39–42 Therefore, NIR light is superior to UV light for controlling interfacial properties of implants in the body or regulate interfacial functions of nano-carriers for improved therapeutic efficiency (Fig. 2b).
Conventional NIR photoreactions are induced by simultaneous two-photon absorption.43 Two NIR photons (e.g., λ = 800 nm) need to be simultaneously absorbed by a UV-sensitive compound (e.g., absorption at 400 nm). NIR photoreactions at interfaces based on two-photon absorption have been extensively studied, e.g. for potential applications in lithography and controlled drug release.44–46 However, two-photon absorption is not efficient even when high-intensity (typical pulse intensity: >106 W cm−2)44,45 femtosecond lasers are employed. Additionally, two-photon absorption only occurs at the laser focus. As the femtosecond laser will defocus while passing through tissue, the two-photon absorption approach is impractical to trigger deep-tissue photoreactions.
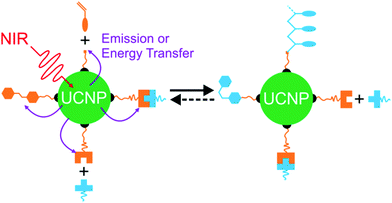
Recently, NIR photoreactions assisted by lanthanide-doped upconverting nanoparticles (UCNPs) have been developed (Fig. 3a).39,47 UCNPs are inorganic NPs which can convert NIR light to UV or visible light.48,49 The upconverted short-wavelength light can induce photoreactions of photosensitive compounds. This process is referred to as UCNP-assisted photochemistry. Compared with NIR photochemistry based on two-photon absorption, UCNP-assisted photoreactions are more efficient. The excitation intensity for UCNP-assisted photochemistry is several orders of magnitude lower than that for two-photon absorption.39 UCNP-assisted photoreactions are induced by inexpensive NIR laser diodes and do not require high-intensity, expensive femtosecond lasers.39 Additionally, it has been demonstrated that NIR light can induce UCNP-assisted photoreactions after passing through tissue with a thickness of a few millimeters.39 Moreover, UCNPs are highly stable under laser excitation.
 | ||
| Fig. 3 (a) Schematic illustration of UCNP-assisted photochemistry. Four types of UCNP-assisted photoreactions including photoisomerization, photopolymerization, photolysis, and photo-coupling have been investigated. TEM images of (b) NaYF4:Yb/Tm@NaYF4 (core = NaYF4:30 mol% Yb3+/0.5 mol% Tm3+; shell = NaYF4) and (c) NaYF4:Yb/Er@NaYF4 (core = NaYF4:30 mol% Yb3+/2 mol% Er3+; shell = NaYF4) UCNPs. Insets in (b and c) are photographs of UCNPs upon a 980 nm laser exposure. Panels (b and c) adapted with permission.82 Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
We will not introduce the mechanism of photon upconversion and the synthesis of UCNPs in this perspective because there are comprehensive and very good reviews.49–53 Photodynamic therapy and NIR light-sensitive materials based on UCNPs have also been reviewed.39,54,55 In the current perspective article, we concentrate on UCNP-assisted photochemistry at interfaces, highlight some potential applications, and discuss future challenges.
2. General concept of UCNP-assisted photochemistry
In UCNP-assisted photochemistry, UCNPs convert NIR light to UV or visible light (Fig. 3). Two typical UCNPs, NaYF4:Yb/Tm UCNPs (β-phase NaYF4 doped with Yb3+ and Tm3+) and NaYF4:Yb/Er UCNPs (β-phase NaYF4 doped with Yb3+ and Er3+) are shown in Fig. 3b and c. In these UCNPs, Yb3+ is the sensitizer which can harvest NIR light and transfer energy to Tm3+ or Er3+. The energy transfer process from Yb3+ to Tm3+ or Er3+ may occur for several times so that Tm3+ or Er3+ can be excited to a higher excited state. Tm3+ is a UV/blue emitter; Er3+ is a green/red emitter.The second important component for UCNP-assisted photochemistry is the photosensitive substance. Conventional photosensitive compounds are usually sensitive to UV or visible light but are not sensitive to NIR light because of their short absorption wavelengths. To make them NIR photosensitive, they are combined with UCNPs. The upconverted UV or visible light from UCNPs can induce photoreactions of conventional photosensitive compounds (Fig. 3a). A requirement for UCNP-assisted photochemistry is that the emission wavelengths of UCNPs should overlap with the absorption wavelengths of photosensitive compounds. If photosensitive compounds are very close to UCNPs, energy transfer from excited UCNPs to photosensitive compounds may occur. Usually, photosensitive compounds re-absorb upconverted light which induces photoreactions.
UCNPs have assisted different types of photoreactions such as photoisomerization,47,56–65 photopolymerization,66–70 photolysis,71–91 and photo-coupling92 (Fig. 3a). (1) UCNP-assisted photoisomerization can be triggered reversibly using NIR light in the presence of UCNPs and photoswitchable compounds such as azobenzene derivatives,59,63 spiropyran derivatives,64,93 and dithienylethene derivatives.47,56 (2) UCNP-assisted photopolymerization is useful to cure thick samples (up to 13.7 cm).70 Different monomers have been polymerized under NIR light irradiation in the presence of photoinitiators and UCNPs.67 (3) UCNP-assisted photolysis is conducted by combining UCNPs with photolytic compounds including o-nitrobenzyl derivatives,72,73 coumarin derivatives,80,82 Ru complexes,81–84,91 and other photocaged compounds.85–88 UCNP-assisted photolysis has been extensively studied because of its wide applications such as drug delivery,72 activation of sensors,74 and controlling protein adsorption and cell adhesion.81,90 (4) UCNP-assisted photo-coupling is a new type of UCNP-assisted photoreactions, which was demonstrated by us and our collaborators very recently.92 These UCNP-assisted photoreactions at different interfaces will be discussed in the next sections.
3. UCNP-assisted photochemistry at nanoparticle surfaces
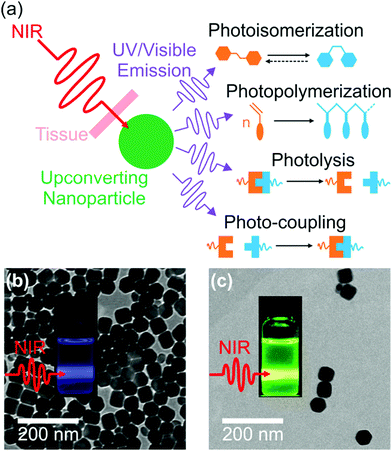
NIR light can trigger reactions of photosensitive compounds grafted on UCNPs (Fig. 4). Upon NIR irradiation, the grafted photosensitive compounds may absorb the upconverted light that induces photoreactions. The short distance between the grafted photosensitive compounds and UCNPs may allow non-radiative energy transfer from the excited UCNPs to the photosensitive compounds. Therefore, UCNP-assisted photoreactions at UCNP surfaces are usually more efficient than UCNP-assisted photoreactions in solution, where photosensitive compounds are far away from UCNPs.The first UCNP-assisted photoreaction at UCNP surfaces was reported by Branda and co-workers.71 UCNPs were grafted with 3′,5′-di(carboxymethoxy)benzoin cage 1a (Fig. 5). UCNPs converted 980 nm NIR light into 290 nm UV light, which induced the release of the caged species on the UCNP surface. This work opened up the avenue of using UCNPs for NIR-controlled drug delivery, surface modification, and activation of sensors, prodrugs and targeting groups.
 | ||
| Fig. 5 UCNP-assisted photolysis at surfaces of UCNPs. Decoration of UCNPs with 3′,5′-di(carboxymethoxy)benzoin cage 1a produces the remote-control release system (1a[NaYF4:TmYb]), which can be triggered by indirect irradiation with NIR light to generate 2a[NaYF4:TmYb] and release a carboxylic acid. Reproduced with permission.71 Copyright 2010, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
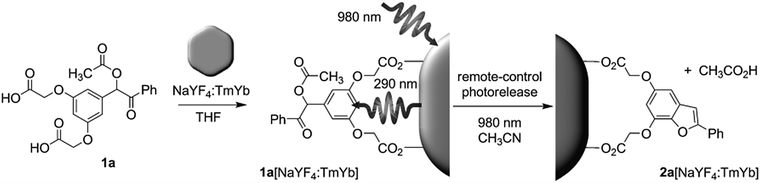
NIR light-induced release of caged species was used to activate cell targeting groups on UCNP surfaces (Fig. 6).77 To achieve phototargeting, the tumor-homing agent folate was caged with a photolytic o-nitrobenzyl group and grafted onto UCNPs. Upon NIR irradiation, the upconverted UV light uncaged the o-nitrobenzyl group and allowed folate-conjugated UCNPs to target cancer cells. After the UCNPs were taken up by cancer cells, the antitumor drug doxorubicin grafted on the surface of UCNPs via a disulfide bond was cleaved by lysosomal enzymes within the cancer cells. The released doxorubicin inhibited the growth of cancer cells. The photocaged UNCPs can serve as a platform for the improvement of selective targeting and reduction of side effects in cancer therapy.
 | ||
| Fig. 6 Illustration of NIR-light-controlled targeting of cancer cells via UCNP-assisted photochemistry. Targeting of cancer cells can be achieved by the activation of the photocaged folic acids on the surfaces of the UCNPs using NIR light. Reproduced with permission.77 Copyright 2013, American Chemical Society. | ||
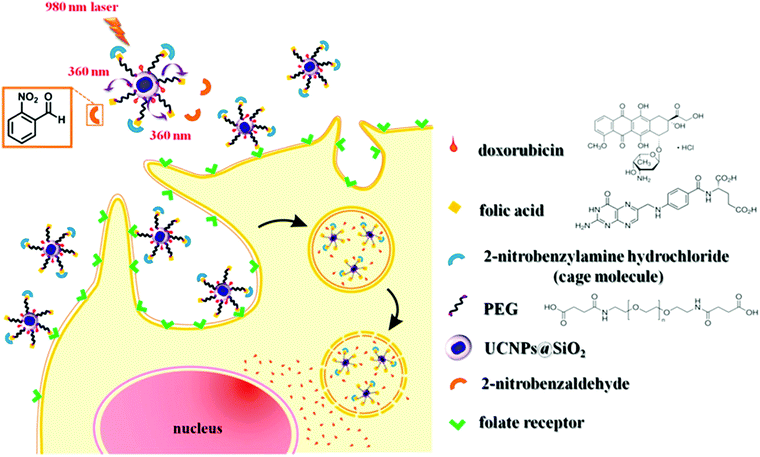
Another application for UCNP-assisted photochemistry at NP surfaces is activation of fluorescent dyes for bioimaging (Fig. 7).74 Yang et al. demonstrated in vitro and in vivo uncaging and bioluminescence imaging based on photocaged UCNPs. A photocaged D-luciferin was conjugated to UCNPs. UCNPs upconverted NIR light into UV light, which uncaged the o-nitrobenzyl group from D-luciferin. As a result, the released D-luciferin effectively conferred enhanced fluorescence and bioluminescence signals. Shen et al. also used NIR light to activate a fluorescent dye grafted on UCNPs.76 Caged fluorescein, which can recover fluorescence upon removing the photolytic o-nitrobenzyl group, was grafted on UCNPs. UCNPs converted NIR light into UV light to uncage fluorescein in live cells. NIR photoactivation for enhanced bioimaging is an important technique that may offer new possibilities for analyzing cell lineages, probing cellular protein dynamics, and monitoring the dynamic functions of cells.
 | ||
| Fig. 7 UCNP-assisted photochemistry at surfaces of core/shell UCNPs for bioimaging. NIR light induced uncaging of D-luciferin and subsequent bioluminescence. Adapted with permission.74 Copyright 2012, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Several groups have constructed NIR-controlled drug delivery systems based on UCNP-assisted photochemistry at NP surfaces.60,62,79,83,85,86 For example, we coated UCNPs with a mesoporous silica shell, loaded the anticancer drug doxorubicin in the pores, and grafted photocleavable Ru complexes onto the NP surfaces (Fig. 8a).83 The Ru complexes acted as molecular valves, which prevented drug leakage. Upon NIR irradiation, the upconverted blue light induces cleavage of Ru complexes that releases the drug which inhibited cancer cells growth. The advantage of this system is that drug release was triggered by 974 nm light with an intensity as low as 0.35 W cm−2. Such a low light intensity minimized overheating problems and prevented photodamage to the biological system.
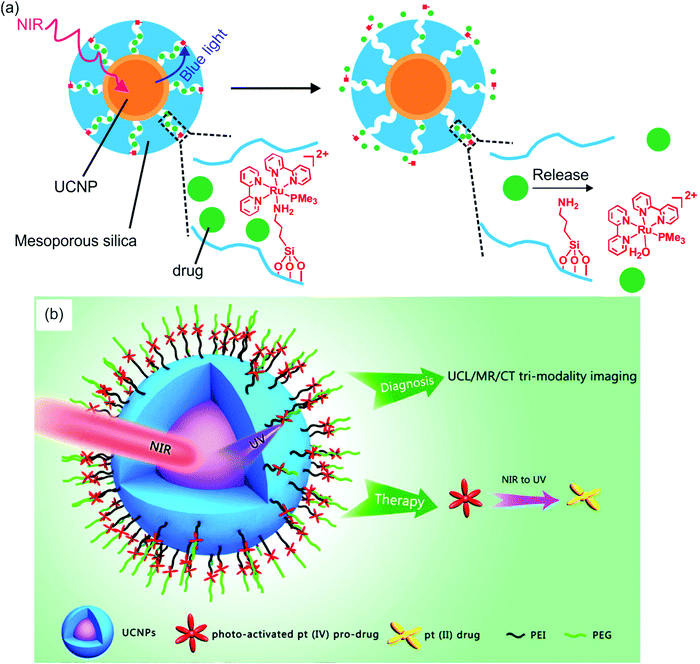 | ||
| Fig. 8 UCNP-assisted photochemistry at NP surfaces for drug delivery. (a) NIR light-induced drug release using UCNP/mesoporous silica core/shell NPs functionalized with photocleavable Ru complexes. Under NIR excitation, the upconverted blue light triggers cleavage of the Ru complexes and release of the drug doxorubicin (green balls). (b) Schematic illustration of UCNPs functionalized with a photoactivated Pt(IV) prodrug. Panel (a) adapted with permission.83 Copyright 2015, Royal Society of Chemistry. Panel (b) reproduced with permission.85 Copyright 2013, American Chemical Society. | ||
In another drug delivery system, photoactivatable prodrugs have been grafted onto UCNPs for phototherapy. Dai et al. used NIR light to activate a Pt(IV) prodrug grafted on NaYF4:Yb3+/Tm3+@NaGdF4:Yb3+ core–shell UCNPs (Fig. 8b).85 The UCNPs can effectively carry the grafted Pt(IV) prodrugs into cancer cells by endocytosis. The mice treated with the NPs under NIR irradiation demonstrated better inhibition of tumor growth than those under direct UV irradiation. This system is multifunctional, which could be used for upconversion luminescence/magnetic resonance/computer tomography trimodality imaging. Almost at the same time, Min et al. reported the conjugation of a similar photoactivatable Pt(IV) prodrug to UCNPs.86 NIR light can activate the Pt(IV) prodrug and simultaneously be used for real-time imaging of apoptosis induced by activated cytotoxicity.
UCNP-assisted photochemistry has also been used for surface coating. Haupt and co-workers demonstrated that UV or visible light emitted from UCNPs can be used to photopolymerize a thin polymer coating around the NPs for their protection, functionalization, and conjugation (Fig. 9).67 This approach enables to polymerize a large variety of monomers with different functionalities on UCNP surfaces. A second layer, exemplified by a molecularly imprinted polymer shell specific for trypsin, can also be synthesized. UCNP-assisted photopolymerization is easy to apply, rapid, and it allows different surface chemistries for further functionalization. Thus, this method provides a platform for the preparation of polymer-coated UCNPs for applications in bioassays, sensing, imaging, and drug delivery.
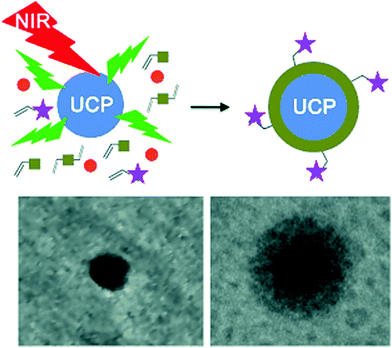 | ||
| Fig. 9 UCNP-assisted photopolymerization at NP surfaces. Reproduced with permission.67 Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
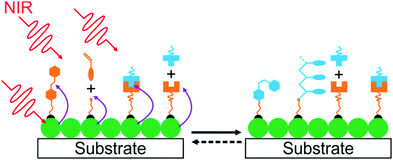
4. UCNP-assisted photochemistry at surfaces of substrates
UCNP-assisted photochemistry is also conducted at substrate surfaces (Fig. 10). To prepare an NIR photosensitive surface, UCNPs are deposited on a substrate. Photosensitive compounds are then grafted on the surface. Upon NIR excitation, the upconverted UV or visible light can trigger photoreactions at the surface.Our group studied UCNP-assisted photocleavage of Ru complexes at a substrate surface (Fig. 11).81 In our design, proteins and an UCNP-decorated substrate were linked via blue-light-cleavable Ru complexes. The substrate was irradiated using NIR light with a photomask. In the exposed areas, UCNPs converted the NIR light into blue light, which induced cleavage of the Ru complexes and release of the proteins. Thus, protein adsorption on the surface can be controlled with NIR light. In addition, photon upconversion lithography is a general method for the patterning of biomaterials using NIR light. Patterned areas, as large as whole wafers, can be prepared using photon upconversion lithography. The smallest line width obtained was ∼15 μm. Thus, we envision using photon upconversion lithography to control cell migration, guide neuron development, and regulate inflammation and vascularization of biomaterials. In this work, quartz and silicon wafers were used as model substrates. Our ongoing work in this direction is to fabricate implants with similar surfaces for NIR light-controlled surface properties for in vivo applications.
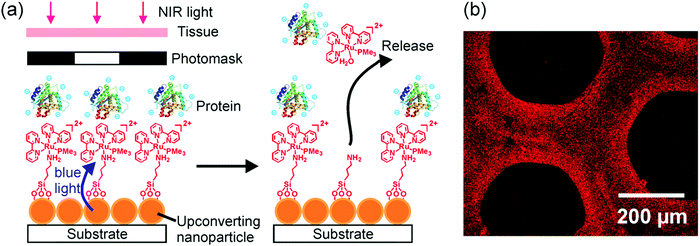 | ||
| Fig. 11 (a) Schematic of photon upconversion lithography for the patterning of proteins. Polyethylene glycol, which is co-grafted with Ru complexes on the UCNPs, is not shown for clarity. (b) Confocal laser scanning microscopy image of a protein pattern fabricated using photon upconversion lithography. Adapted with permission.81 Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
UCNP-assisted photochemistry also showed applications in NIR-controlled cell adhesion. One successful example is the work of Qu and co-workers (Fig. 12a).90 They fabricated an UCNP-decorated substrate. A UV-photocleavable 4-(hydroxymethyl)-3-nitrobenzoic acid group and a cell adhesive RGD ligand were grafted on the surface. Cells can adhere on such a surface. To detach cells from this surface, UCNPs harvested NIR light and convert it into local UV light, which resulted in cleavage of the photocleavable linkers and on-demand release of the cells. The authors also demonstrated that NIR light passed through 4 mm-thick tissue and controlled cell adhesion on the substrate. By liking UCNPs and gene nano-carriers with the same photolytic linker, Zheng et al. demonstrated NIR light-controlled gene selective expression.94 Both studies have demonstrated that this approach facilitates the design of UCNP-based multifunctional cell scaffold for dynamic study of biological process, regeneration medicine, and disease-related cell isolation and analysis.
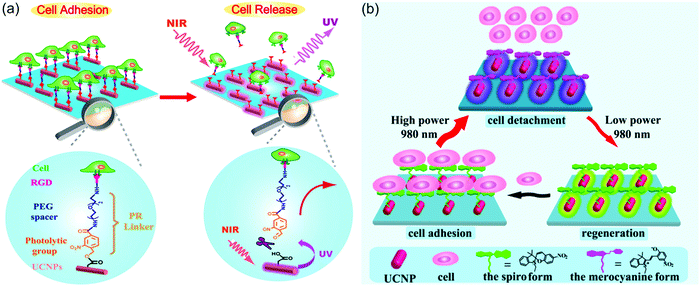 | ||
| Fig. 12 (a) Irreversible control of cell adhesion using NIR light based on UCNPs. Upon 980 nm light excitation, the UCNPs emit UV photons, which cleave the photolytic linker. As a result, the cells dissociate from the substrate. (b) Reversible control of cell adhesion/detachment using NIR light with different intensities based on UCNPs. At a high intensity (8 W cm−2), the UCNPs emit UV photons and activate the isomerization from the spiropyran form to the merocyanine form, resulting in the cell detachment. Conversely, when exposed to a low intensity (0.5 W cm−2), the same UCNPs can emit visible light to drive the merocyanine form back to the spiropyran form, leading to cell adhesion again. Panel (a) reproduced with permission.90 Copyright 2014, American Chemical Society. Panel (b) reproduced with permission.65 Copyright 2015, American Chemical Society. | ||
The above-mentioned work demonstrated irreversible control of cell adhesion on a substrate using NIR light. To achieve reversible control, photoswitchable spiropyran was grafted on UCNP-modified substrates (Fig. 12b).65 It is well known that the spiropyran-to-merocyanine isomerization is induced using UV light and merocyanine-to-spiropyran back isomerization is induced using green light. Qu and co-workers synthesized NaYF4:Tm/Yb@NaYF4@NaYF4:Er/Yb@NaYF4 core–shell–shell–shell UCNPs, which emit more UV light under the excitation of high-intensity 980 nm light (e.g., 8 W cm−2) and emit more green light under the excitation of low-intensity 980 nm light (e.g., 0.5 W cm−2).65 Thus, the two-way isomerization of spiropyran on the UCNPs merely depends on the excitation intensity. At a high intensity, the spiropyran-to-merocyanine isomerization was prominent, whereas its reverse isomerization occurred upon irradiation by the same laser but with a lower intensity. Such NIR-controlled reversible isomerization made the interactions between surface-grafted spiropyran and cell surface protein fibronectin switchable, thus leading to reversible cell adhesion and detachment. Moreover, efficient adhesion-and-detachment of cells was realized even after 10 cycles. NIR light showed little damage toward cells and could control cell adhesion after passing through 4 mm-thick tissue. This approach is promising for in vivo dynamically manipulating cell-molecule interactions and biological processes.
5. UCNP-assisted photochemistry at liquid/liquid interfaces
NIR light can also trigger UCNP-assisted photoreactions at liquid/liquid interfaces. Recently, Chen et al. demonstrated UCNP-assisted photoisomerization at water/oil interfaces (Fig. 13).95 UCNPs conjugated with photoswitchable spiropyran was hydrophobic. Light can reversibly control hydrophobicity/hydrophilicity of the NPs (Fig. 13d). UCNPs converted NIR light to UV light, which induced spiropyran-to-merocyanine isomerization and hydrophobic-to-hydrophilic transition. Subsequently, visible light induced the reverse merocyanine-to-spiropyran isomerization and hydrophilic-to-hydrophobic transition. Similar to other colloids,96,97 the spiropyran-conjugated UCNPs with interfacial activity are suitable to prepare Pickering emulsions. The Pickering emulsions stabilized by spiropyran-conjugated UCNPs can be inversed under light irradiation (Fig. 13b and c). Spiropyran-conjugated UCNPs can stabilize water-in-oil (w/o) emulsions at the bottom of the sample and prevent sedimentation (Fig. 13b, left). Upon NIR irradiation and homogenization, spiropyran-conjugated UCNPs transferred to the upper layer and oil-in-water (o/w) emulsions were formed (Fig. 13b, middle). Spiropyran-conjugated UCNPs could transfer back to the bottom layer after exposing to visible light and shaking (Fig. 13b, right). A model enantioselective biocatalytic active bacterium was loaded in the aqueous phase (Fig. 13a). The Pickering emulsion enhanced the catalytic performance. In addition, product recovery, and biocatalysts and colloid emulsifiers recycling could be easily realized based on the light-controlled inversion of the Pickering emulsion. Importantly, the utilization of NIR/visible light to perform the reversible inversion without any chemical auxiliaries showed little damage toward the biocatalysts, which was highlighted by the high catalytic efficiency and high enantioselectivity even after 10 cycles. NIR/visible light controlled Pickering emulsions showed promising potential for biocatalysis in biphasic systems.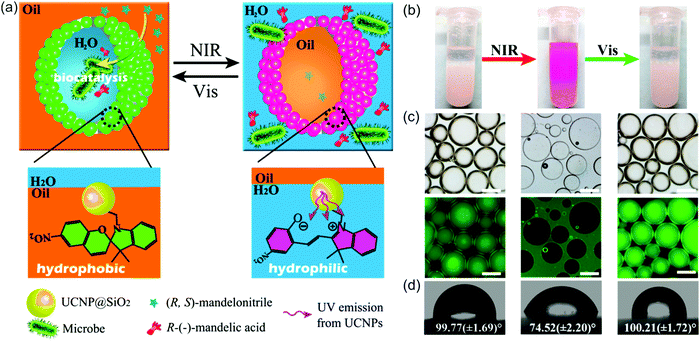 | ||
| Fig. 13 (a) Schematic illustration of the NIR light-responsive Pickering emulsions with reversible inversion ability for biphasic enantioselective biocatalysis. (b) Photograph of Pickering emulsions stabilized by spiropyran-conjugated UCNPs: w/o (left), o/w (middle), and w/o (right) before and after the irradiation of light with different wavelengths. (c) Fluorescence microscopy images of the corresponding Pickering emulsions shown in (b) (scale bar: 200 μm). (d) Water contact angles on a filter paper deposited with spiropyran-conjugated UCNPs irradiated using light with wavelengths. Reproduced with permission.95 Copyright 2014, American Chemical Society. | ||
Another example of UCNP-assisted photochemistry at interfaces is NIR light-induced photoisomerization in the bilayer membranes of vesicles (Fig. 14).98 Phospholipid modified UCNPs and the anticancer drug DOX were encapsulated in azobenzene-doped liposomes. NIR light can be converted into UV and visible light to trigger photoisomerization of azobenzene amphiphiles, which resulted in drug release. The release amount and rate of the anticancer drug can be well controlled by tuning the intensity and duration of the NIR laser. The NIR light-triggered drug release ability makes this novel drug delivery system an effective chemotherapy to overcome multidrug resistance by spatiotemporal control. This drug delivery system provides great values in the advancement of clinical NIR light-regulated precise drug release and drug-resistant tumor treatment.
 | ||
| Fig. 14 Schematic illustration of the NIR-triggered azobenzene-liposome/UCNP hybrid vesicles for controlled drug delivery. The phosphatidylcholine coated UCNPs and the anticancer drug DOX are loaded together within the hydrophilic compartment of the liposome. The amphiphilic azobenzene derivatives are embedded in the liposome bilayers consisting of phospholipids (DSPC/DOTAP). Reproduced with permission.98 Copyright 2016, Wiley-VCH Verlag GmbH & Co. KGaA. | ||
6. Outlook
From the chemistry point of view, it is interesting to develop new NIR photoreactions at interfaces based on UCNPs. UCNP-assisted photoisomerization, photolysis, and photopolymerization at interfaces have been extensively studied and some potential applications have been demonstrated. In contrast, UCNP-assisted photo-coupling reactions have been reported by us and our collaborators only recently;92 this type of reactions has not been widely investigated (Fig. 15). We envision the use of UCNP-assisted photo-coupling reactions to modify biointerfaces. A possible application is NIR light-induced NP coupling, which can result in NP aggregates with enhanced therapeutic effects due to the size effect.99 Another possible application of UCNP-assisted photo-coupling reactions at interfaces is to glue biological tissues with NIR light.100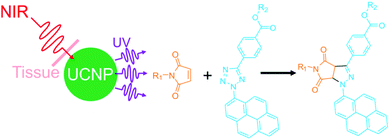 | ||
| Fig. 15 NIR photo-coupling reaction based on nitrile imine-mediated tetrazole-ene cycloadditions in the presence of UCNPs. This type of photoreaction at interfaces still awaits exploration. | ||
Improving spatial resolution of photon upconversion lithography for the patterning of biomaterials is still a challenge. Two-photon lithography is a standard technique in some labs and in industry to fabricate patterns with a feature size of a few hundred nanometers. However, the smallest feature size fabricated by photon upconversion lithography is only ∼15 μm.81 It is too large for some studies on interactions between patterned biomolecules and cells.101 It is highly desirable to improve the spatial resolution of photon upconversion lithography. We and our collaborators have shown that the spatial resolution of upconversion can be improved using pulsed excitation because pulsed excitation can avoid saturation in the upconversion process.102 This technique has been adapted to high-resolution photopatterning by us, which will be reported in due course.
NIR-controlled cell adhesion based on UCNPs has been demonstrated and is promising for in vivo applications. The next step in this direction would be guide cell migration and stem cell fate. Currently, light-guided cell migration and stem cell fate are based on UV photopatterning or two-photon lithography.38,103 Photon upconversion lithography with the spatial solution of tens of μm should be suitable for these applications.
UCNP-assisted photochemistry at interfaces still faces some problems that may hinder its applications. A big problem for UCNP-assisted photochemistry is photothermal overheating. The quantum yield of UCNPs is usually less than 1%.104 To obtain sufficient UV or visible light for photoreactions, UCNPs are excited by high-intensity NIR light; typical excitation intensities at 980 nm are 0.35–560 W cm−2.39 Because the maximum permissible exposure for skin at 980 nm is ∼0.726 W cm−2,105,106 high-intensity 980 nm light can overheat and damage biological systems.82 We and others partially addressed the overheating problem using Ru complexes as photosensitive compounds,82,83 high-efficient UCNPs,76 and UCNPs that can be excited by 808 nm light.107–112 Although NIR light at 980 nm is better suited than UV light for biomedical applications, NIR light at 808 nm is even better because the penetration depth of 808 nm light in bovine tissue is 54% deeper than that of 980 nm light.41 The development of highly efficient UCNPs, which can be excited with 808 nm light, is currently a topic of intense investigation. At the moment, different strategies are under investigation to enhance the efficiency of upconversion.113 In particular, enhancing upconversion using surface plasmons and photonic structures is a topic under intense investigation because of the potential to achieve large enhancement.114–119 Different strategies might be combined in a system to optimize upconversion efficiency and minimize the overheating problem for deep-tissue applications.
Another interesting application of UCNP-assisted photochemistry at interfaces is photovoltaics such as solar cells.120–123 The incorporation of UCNPs into dye-sensitized solar cells enables the use of the NIR region of the solar spectrum.120–123 This design requires the development of highly efficient UCNPs that can be excited by broadband NIR light.
Acknowledgements
S. W. acknowledges the Deutsche Forschungsgemeinschaft (DFG, WU 787/2-1) and the Fonds der Chemischen Industrie (FCI, No. 661548) for their financial support. H.-J. B. acknowledges financial support from the ERC advanced grant 340391-SuPro. Open Access funding provided by the Max Planck Society.References
- J. X. Cui, V. San Miguel and A. del Campo, Macromol. Rapid Commun., 2013, 34, 310–329 CrossRef CAS PubMed.
- S. T. Wang, Y. L. Song and L. Jiang, J. Photochem. Photobiol., C, 2007, 8, 18–29 CrossRef CAS.
- M. Paven, H. Mayama, T. Sekido, H. J. Butt, Y. Nakamura and S. Fujii, Adv. Funct. Mater., 2016, 26, 3199–3206 CrossRef CAS.
- K. Ichimura, S. K. Oh and M. Nakagawa, Science, 2000, 288, 1624–1626 CrossRef CAS PubMed.
- E. Blasco, M. Piñol, L. Oriol, B. V. K. J. Schmidt, A. Welle, V. Trouillet, M. Bruns and C. Barner-Kowollik, Adv. Funct. Mater., 2013, 23, 4011–4019 CrossRef CAS.
- C. Li, Y. Y. Zhang, J. Ju, F. T. Cheng, M. J. Liu, L. Jiang and Y. L. Yu, Adv. Funct. Mater., 2012, 22, 760–763 CrossRef CAS.
- J. X. Cui, T. H. Nguyen, M. Ceolin, R. Berger, O. Azzaroni and A. del Campo, Macromolecules, 2012, 45, 3213–3220 CrossRef CAS.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schuwer, C. Sugnaux, S. Tugulu and H. A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS PubMed.
- J. T. Huang, S. Wu, S. Beckemper, A. Gillner, Q. J. Zhang and K. Y. Wang, Opt. Lett., 2010, 35, 2711–2713 CrossRef CAS PubMed.
- J. T. Huang, S. Beckemper, S. Wu, J. Shen, Q. J. Zhang, K. Y. Wang and A. Gillner, Phys. Chem. Chem. Phys., 2011, 13, 16150–16158 RSC.
- R. Rosario, D. Gust, M. Hayes, F. Jahnke, J. Springer and A. A. Garcia, Langmuir, 2002, 18, 8062–8069 CrossRef CAS.
- J. Groten, C. Bunte and J. Rühe, Langmuir, 2012, 28, 15038–15046 CrossRef CAS PubMed.
- S. Wu and J. T. Huang, RSC Adv., 2012, 2, 12084–12087 RSC.
- P. Weis, D. S. Wang and S. Wu, Macromolecules, 2016, 49, 6368–6373 CrossRef CAS.
- T. Pauloehrl, G. Delaittre, M. Bruns, M. Meissler, H. G. Borner, M. Bastmeyer and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2012, 51, 9181–9184 CrossRef CAS PubMed.
- H. Zhou, C. Xue, P. Weis, Y. Suzuki, S. Huang, K. Koynov, G. K. Auernhammer, R. Berger, H. J. Butt and S. Wu, Nat. Chem., 2017, 9, 145–151 CrossRef CAS PubMed.
- J. Nishida, M. Kobayashi and A. Takahara, ACS Macro Lett., 2013, 2, 112–115 CrossRef CAS.
- M. Suda, N. Kameyama, A. Ikegami and Y. Einaga, J. Am. Chem. Soc., 2009, 131, 865–870 CrossRef CAS PubMed.
- A. Ikegami, M. Suda, T. Watanabe and Y. Einaga, Angew. Chem., Int. Ed., 2010, 49, 372–374 CrossRef CAS PubMed.
- D. S. Wang and S. Wu, Langmuir, 2016, 32, 632–636 CrossRef CAS PubMed.
- N. C. Fan, F. Y. Cheng, J. A. Ho and C. S. Yeh, Angew. Chem., Int. Ed., 2012, 51, 8806–8810 CrossRef PubMed.
- R. Klajn, P. J. Wesson, K. J. M. Bishop and B. A. Grzybowski, Angew. Chem., Int. Ed., 2009, 48, 7035–7039 CrossRef CAS PubMed.
- H. Zhao, S. Sen, T. Udayabhaskararao, M. Sawczyk, K. Kucanda, D. Manna, P. K. Kundu, J. W. Lee, P. Kral and R. Klajn, Nat. Nanotechnol., 2016, 11, 82–88 CrossRef CAS PubMed.
- Y. Zhou, D. S. Wang, S. L. Huang, G. Auernhammer, Y. J. He, H. J. Butt and S. Wu, Chem. Commun., 2015, 51, 2725–2727 RSC.
- A. Diguet, R. M. Guillermic, N. Magome, A. Saint-Jalmes, Y. Chen, K. Yoshikawa and D. Baigl, Angew. Chem., Int. Ed., 2009, 48, 9281–9284 CrossRef CAS PubMed.
- L. D. Zarzar, V. Sresht, E. M. Sletten, J. A. Kalow, D. Blankschtein and T. M. Swager, Nature, 2015, 518, 520–524 CrossRef CAS PubMed.
- N. Kavokine, M. Anyfantakis, M. Morel, S. Rudiuk, T. Bickel and D. Baigl, Angew. Chem., Int. Ed., 2016, 55, 11183–11187 CrossRef CAS PubMed.
- X. Wang, G. Liu, J. Hu, G. Zhang and S. Liu, Angew. Chem., Int. Ed., 2014, 53, 3138–3142 CrossRef CAS PubMed.
- Y. R. Choi, G. C. Kim, H.-G. Jeon, J. Park, W. Namkung and K.-S. Jeong, Chem. Commun., 2014, 50, 15305–15308 RSC.
- A. Diguet, M. Yanagisawa, Y. J. Liu, E. Brun, S. Abadie, S. Rudiuk and D. Baigl, J. Am. Chem. Soc., 2012, 134, 4898–4904 CrossRef CAS PubMed.
- W. Su, Y. H. Luo, Q. Yan, S. Wu, K. Han, Q. J. Zhang, Y. Q. Gu and Y. M. Li, Macromol. Rapid Commun., 2007, 28, 1251–1256 CrossRef CAS.
- M. Anyfantakis and D. Baigl, Angew. Chem., Int. Ed., 2014, 53, 14077–14081 CrossRef CAS PubMed.
- S. N. Varanakkottu, M. Anyfantakis, M. Morel, S. Rudiuk and D. Baigl, Nano Lett., 2016, 16, 644–650 CrossRef CAS PubMed.
- M. Schutt, S. S. Krupka, A. G. Milbradt, S. Deindl, E. K. Sinner, D. Oesterhelt, C. Renner and L. Moroder, Chem. Biol., 2003, 10, 487–490 CrossRef CAS PubMed.
- J. Auernheimer, C. Dahmen, U. Hersel, A. Bausch and H. Kessler, J. Am. Chem. Soc., 2005, 127, 16107–16110 CrossRef CAS PubMed.
- J. Edahiro, K. Sumaru, Y. Tada, K. Ohi, T. Takagi, M. Kameda, T. Shinbo, T. Kanamori and Y. Yoshimi, Biomacromolecules, 2005, 6, 970–974 CrossRef CAS PubMed.
- S. Petersen, J. M. Alonso, A. Specht, P. Duodu, M. Goeldner and A. del Campo, Angew. Chem., Int. Ed., 2008, 47, 3192–3195 CrossRef CAS PubMed.
- T. T. Lee, J. R. Garcia, J. I. Paez, A. Singh, E. A. Phelps, S. Weis, Z. Shafiq, A. Shekaran, A. del Campo and A. J. Garcia, Nat. Mater., 2015, 14, 352–360 CrossRef CAS PubMed.
- S. Wu and H. J. Butt, Adv. Mater., 2016, 28, 1208–1226 CrossRef CAS PubMed.
- P. Juzenas, A. Juzeniene, O. Kaalhus, V. Iani and J. Moan, Photochem. Photobiol. Sci., 2002, 1, 745–748 CAS.
- D. E. Hudson, D. O. Hudson, J. M. Wininger and B. D. Richardson, Photomed. Laser Surg., 2013, 31, 163–168 CrossRef PubMed.
- R. Steiner, in Laser and IPL Technology in Dermatology and Aesthetic Medicine, ed. C. Raulin and S. Karsai, Springer Berlin Heidelberg, 2011, ch. 2, pp. 23–36 DOI:10.1007/978-3-642-03438-1_2.
- T. Furuta, S. S. H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Callaway, W. Denk and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1193–1200 CrossRef CAS.
- M. Alvarez, A. Best, S. Pradhan-Kadam, K. Koynov, U. Jonas and M. Kreiter, Adv. Mater., 2008, 20, 4563–4567 CrossRef CAS.
- M. Alvarez, A. Best, A. Unger, J. M. Alonso, A. del Campo, M. Schmelzeisen, K. Koynov and M. Kreiter, Adv. Funct. Mater., 2010, 20, 4265–4272 CrossRef CAS.
- T. M. Guardado-Alvarez, L. Sudha Devi, M. M. Russell, B. J. Schwartz and J. I. Zink, J. Am. Chem. Soc., 2013, 135, 14000–14003 CrossRef CAS PubMed.
- C. J. Carling, J. C. Boyer and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 10838–10839 CrossRef CAS PubMed.
- J. A. Capobianco, F. Vetrone, T. D'Alesio, G. Tessari, A. Speghini and M. Bettinelli, Phys. Chem. Chem. Phys., 2000, 2, 3203–3207 RSC.
- M. Haase and H. Schafer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed.
- Z. Y. Cheng and J. Lin, Macromol. Rapid Commun., 2015, 36, 790–827 CrossRef CAS PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Y. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- S. Wu, J. Blinco and C. Barner-Kowollik, Chem. – Eur. J., 2017 DOI:10.1002/chem.201700658.
- J. C. Boyer, C. J. Carling, B. D. Gates and N. R. Branda, J. Am. Chem. Soc., 2010, 132, 15766–15772 CrossRef CAS PubMed.
- T. Q. Wu, M. Barker, K. M. Arafeh, J. C. Boyer, C. J. Carling and N. R. Branda, Angew. Chem., Int. Ed., 2013, 52, 11106–11109 CrossRef CAS PubMed.
- L. Wang, H. Dong, Y. N. Li, R. Liu, Y. F. Wang, H. K. Bisoyi, L. D. Sun, C. H. Yan and Q. Li, Adv. Mater., 2015, 27, 2065–2069 CrossRef CAS PubMed.
- W. Wu, L. M. Yao, T. S. Yang, R. Y. Yin, F. Y. Li and Y. L. Yu, J. Am. Chem. Soc., 2011, 133, 15810–15813 CrossRef CAS PubMed.
- J. A. Liu, W. B. Bu, L. M. Pan and J. L. Shi, Angew. Chem., Int. Ed., 2013, 52, 4375–4379 CrossRef CAS PubMed.
- J. N. Liu, J. W. Bu, W. B. Bu, S. J. Zhang, L. M. Pan, W. P. Fan, F. Chen, L. P. Zhou, W. J. Peng, K. L. Zhao, J. L. Du and J. L. Shi, Angew. Chem., Int. Ed., 2014, 53, 4551–4555 CrossRef CAS PubMed.
- X. M. Li, L. Zhou, Y. Wei, A. M. El-Toni, F. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2014, 136, 15086–15092 CrossRef CAS PubMed.
- L. Wang, H. Dong, Y. N. Li, C. M. Xue, L. D. Sun, C. H. Yan and Q. Li, J. Am. Chem. Soc., 2014, 136, 4480–4483 CrossRef CAS PubMed.
- B. F. Zhang, M. Frigoli, F. Angiuli, F. Vetrone and J. A. Capobianco, Chem. Commun., 2012, 48, 7244–7246 RSC.
- W. Li, Z. Chen, L. Zhou, Z. Li, J. Ren and X. Qu, J. Am. Chem. Soc., 2015, 137, 8199–8205 CrossRef CAS PubMed.
- A. Stepuk, D. Mohn, R. N. Grass, M. Zehnder, K. W. Kramer, F. Pelle, A. Ferrier and W. J. Stark, Dent. Mater., 2012, 28, 304–311 CrossRef CAS PubMed.
- S. Beyazit, S. Ambrosini, N. Marchyk, E. Palo, V. Kale, T. Soukka, B. T. S. Bui and K. Haupt, Angew. Chem., Int. Ed., 2014, 53, 8919–8923 CrossRef CAS PubMed.
- Q. B. Xiao, Y. T. Ji, Z. H. Xiao, Y. Zhang, H. Z. Lin and Q. B. Wang, Chem. Commun., 2013, 49, 1527–1529 RSC.
- J. Mendez-Ramos, J. C. Ruiz-Morales, P. Acosta-Mora and N. M. Khaidukov, J. Mater. Chem. C, 2016, 4, 801–806 RSC.
- R. Liu, H. Chen, Z. Q. Li, F. Shi and X. Y. Liu, Polym. Chem., 2016, 7, 2457–2463 RSC.
- C. J. Carling, F. Nourmohammadian, J. C. Boyer and N. R. Branda, Angew. Chem., Int. Ed., 2010, 49, 3782–3785 CrossRef CAS PubMed.
- B. Yan, J. C. Boyer, N. R. Branda and Y. Zhao, J. Am. Chem. Soc., 2011, 133, 19714–19717 CrossRef CAS PubMed.
- B. Yan, J. C. Boyer, D. Habault, N. R. Branda and Y. Zhao, J. Am. Chem. Soc., 2012, 134, 16558–16561 CrossRef CAS PubMed.
- Y. M. Yang, Q. Shao, R. R. Deng, C. Wang, X. Teng, K. Cheng, Z. Cheng, L. Huang, Z. Liu, X. G. Liu and B. G. Xing, Angew. Chem., Int. Ed., 2012, 51, 3125–3129 CrossRef CAS PubMed.
- M. K. G. Jayakumar, N. M. Idris and Y. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8483–8488 CrossRef CAS PubMed.
- J. Shen, G. Y. Chen, T. Y. Ohulchanskyy, S. J. Kesseli, S. Buchholz, Z. P. Li, P. N. Prasad and G. Han, Small, 2013, 9, 3213–3217 CrossRef CAS PubMed.
- Y. H. Chien, Y. L. Chou, S. W. Wang, S. T. Hung, M. C. Liau, Y. J. Chao, C. H. Su and C. S. Yeh, ACS Nano, 2013, 7, 8516–8528 CrossRef CAS PubMed.
- M. L. Viger, M. Grossman, N. Fomina and A. Almutairi, Adv. Mater., 2013, 25, 3733–3738 CrossRef CAS PubMed.
- Y. M. Yang, B. Velmurugan, X. G. Liu and B. G. Xing, Small, 2013, 9, 2937–2944 CrossRef CAS PubMed.
- L. Z. Zhao, J. J. Peng, Q. Huang, C. Y. Li, M. Chen, Y. Sun, Q. N. Lin, L. Y. Zhu and F. Y. Li, Adv. Funct. Mater., 2014, 24, 363–371 CrossRef CAS.
- Z. J. Chen, S. Q. He, H. J. Butt and S. Wu, Adv. Mater., 2015, 27, 2203–2206 CrossRef CAS PubMed.
- Z. Chen, W. Sun, H.-J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165–9170 CrossRef CAS PubMed.
- S. Q. He, K. Krippes, S. Ritz, Z. J. Chen, A. Best, H. J. Butt, V. Mailander and S. Wu, Chem. Commun., 2015, 51, 431–434 RSC.
- E. Ruggiero, A. Habtemariam, L. Yate, J. C. Mareque-Rivas and L. Salassa, Chem. Commun., 2014, 50, 1715–1718 RSC.
- Y. L. Dai, H. H. Xiao, J. H. Liu, Q. H. Yuan, P. A. Ma, D. M. Yang, C. X. Li, Z. Y. Cheng, Z. Y. Hou, P. P. Yang and J. Lin, J. Am. Chem. Soc., 2013, 135, 18920–18929 CrossRef CAS PubMed.
- Y. Z. Min, J. M. Li, F. Liu, E. K. L. Yeow and B. G. Xing, Angew. Chem., Int. Ed., 2014, 53, 1012–1016 CrossRef CAS PubMed.
- P. T. Burks, J. V. Garcia, R. GonzalezIrias, J. T. Tillman, M. T. Niu, A. A. Mikhailovsky, J. P. Zhang, F. Zhang and P. C. Ford, J. Am. Chem. Soc., 2013, 135, 18145–18152 CrossRef CAS PubMed.
- A. E. Pierri, P. J. Huang, J. V. Garcia, J. G. Stanfill, M. Chui, G. Wu, N. Zheng and P. C. Ford, Chem. Commun., 2015, 51, 2072–2075 RSC.
- L. L. Fedoryshin, A. J. Tavares, E. Petryayeva, S. Doughan and U. J. Krull, ACS Appl. Mater. Interfaces, 2014, 6, 13600–13606 CAS.
- W. Li, J. S. Wang, J. S. Ren and X. G. Qu, J. Am. Chem. Soc., 2014, 136, 2248–2251 CrossRef CAS PubMed.
- Z. J. Chen, Y. B. Xiong, R. Etchenique and S. Wu, Chem. Commun., 2016, 52, 13959–13962 RSC.
- P. Lederhose, Z. J. Chen, R. Muller, J. P. Blinco, S. Wu and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2016, 55, 12195–12199 CrossRef CAS PubMed.
- J. P. Lai, Y. X. Zhang, N. Pasquale and K. B. Lee, Angew. Chem., Int. Ed., 2014, 53, 14419–14423 CrossRef CAS PubMed.
- B. Zheng, L. Su, H. Pan, B. Hou, Y. Zhang, F. Zhou, X. Wu, X. Gong, H. Wang and J. Chang, Adv. Mater., 2016, 28, 707–714 CrossRef CAS PubMed.
- Z. W. Chen, L. Zhou, W. Bing, Z. J. Zhang, Z. H. Li, J. S. Ren and X. G. Qu, J. Am. Chem. Soc., 2014, 136, 7498–7504 CrossRef CAS PubMed.
- H. Maeda, M. Okada, S. Fujii, Y. Nakamura and T. Furuzono, Langmuir, 2010, 26, 13727–13731 CrossRef CAS PubMed.
- S. Fujii, M. Okada, T. Nishimura, H. Maeda, T. Sugimoto, H. Hamasaki, T. Furuzono and Y. Nakamura, J. Colloid Interface Sci., 2012, 374, 1–8 CrossRef CAS PubMed.
- C. Yao, P. Wang, X. Li, X. Hu, J. Hou, L. Wang and F. Zhang, Adv. Mater., 2016, 28, 9341–9348 CrossRef CAS PubMed.
- T. Sadhukha, T. S. Wiedmann and J. Panyam, Biomaterials, 2014, 35, 7860–7869 CrossRef CAS PubMed.
- S. Rose, A. Prevoteau, P. Elziere, D. Hourdet, A. Marcellan and L. Leibler, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- H. Tran, K. L. Killops and L. M. Campos, Soft Matter, 2013, 9, 6578–6586 RSC.
- J. Hodak, Z. J. Chen, S. Wu and R. Etchenique, Anal. Chem., 2016, 88, 1468–1475 CrossRef CAS PubMed.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS PubMed.
- J. C. Boyer and F. C. van Veggel, Nanoscale, 2010, 2, 1417–1419 RSC.
- American National Standard for safe use of lasers, Laser Institute of America, Orlando, FL, 2000 Search PubMed.
- Laser safety handbook, Northwestern University, 2011 Search PubMed.
- Y. F. Wang, G. Y. Liu, L. D. Sun, J. W. Xiao, J. C. Zhou and C. H. Yan, ACS Nano, 2013, 7, 7200–7206 CrossRef CAS PubMed.
- X. J. Xie, N. Y. Gao, R. R. Deng, Q. Sun, Q. H. Xu and X. G. Liu, J. Am. Chem. Soc., 2013, 135, 12608–12611 CrossRef CAS PubMed.
- Y. T. Zhong, G. Tian, Z. J. Gu, Y. J. Yang, L. Gu, Y. L. Zhao, Y. Ma and J. N. Yao, Adv. Mater., 2014, 26, 2831–2837 CrossRef CAS PubMed.
- J. Shen, G. Y. Chen, A. M. Vu, W. Fan, O. S. Bilsel, C. C. Chang and G. Han, Adv. Opt. Mater., 2013, 1, 644–650 CrossRef.
- H. L. Wen, H. Zhu, X. Chen, T. F. Hung, B. L. Wang, G. Y. Zhu, S. F. Yu and F. Wang, Angew. Chem., Int. Ed., 2013, 52, 13419–13423 CrossRef CAS PubMed.
- Z. Hou, K. Deng, C. Li, X. Deng, H. Lian, Z. Cheng, D. Jin and J. Lin, Biomaterials, 2016, 101, 32–46 CrossRef CAS PubMed.
- S. Y. Han, R. R. Deng, X. J. Xie and X. G. Liu, Angew. Chem., Int. Ed., 2014, 53, 11702–11715 CrossRef CAS PubMed.
- W. Feng, L. D. Sun and C. H. Yan, Chem. Commun., 2009, 4393–4395 RSC.
- N. J. Greybush, M. Saboktakin, X. C. Ye, C. Della Giovampaola, S. J. Oh, N. E. Berry, N. Engheta, C. B. Murray and C. R. Kagan, ACS Nano, 2014, 8, 9482–9491 CrossRef CAS PubMed.
- N. Mauser, D. Piatkowski, T. Mancabelli, M. Nyk, S. Mackowski and A. Hartschuh, ACS Nano, 2015, 9, 3617–3626 CrossRef CAS PubMed.
- N. Liu, W. Qin, G. Qin, T. Jiang and D. Zhao, Chem. Commun., 2011, 47, 7671–7673 RSC.
- Z. Yin, Y. Zhu, W. Xu, J. Wang, S. Xu, B. Dong, L. Xu, S. Zhang and H. Song, Chem. Commun., 2013, 49, 3781–3783 RSC.
- J. H. Lin, H. Y. Liou, C. D. Wang, C. Y. Tseng, C. T. Lee, C. C. Ting, H. C. Kan and C. C. Hsu, ACS Photonics, 2015, 2, 530–536 CrossRef CAS.
- L. Liang, Y. Liu, C. Bu, K. Guo, W. Sun, N. Huang, T. Peng, B. Sebo, M. Pan, W. Liu, S. Guo and X.-Z. Zhao, Adv. Mater., 2013, 25, 2174–2180 CrossRef CAS PubMed.
- Y. Li, G. Wang, K. Pan, B. Jiang, C. Tian, W. Zhou and H. Fu, J. Mater. Chem., 2012, 22, 20381–20386 RSC.
- C. Yuan, G. Chen, P. N. Prasad, T. Y. Ohulchanskyy, Z. Ning, H. Tian, L. Sun and H. Agren, J. Mater. Chem., 2012, 22, 16709–16713 RSC.
- M. Zhang, Y. Lin, T. J. Mullen, W.-f. Lin, L.-D. Sun, C.-H. Yan, T. E. Patten, D. Wang and G.-y. Liu, J. Phys. Chem. Lett., 2012, 3, 3188–3192 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2017 |