Re-healable polyimine thermosets: polymer composition and moisture sensitivity†
Philip
Taynton‡
,
Chengpu
Zhu‡
,
Samuel
Loob
,
Richard
Shoemaker
,
James
Pritchard
,
Yinghua
Jin
and
Wei
Zhang
*
Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309, USA. E-mail: wei.zhang@colorado.edu
First published on 7th November 2016
Abstract
Moisture sensitivity of polyimines is systematically studied by incorporating various diamine moieties into the polymer networks. Significant variations in moisture responsiveness and property changes were observed. Hydrophilic polyimines can be re-healed at ambient conditions, whereas more hydrophobic polyimines require heat to be healed.
Covalently cross-linked polymer networks are traditionally described as thermosets,1,2 obtaining this name from the fact that they cannot flow upon heating and thus cannot be reshaped or recycled.1 Very recently, this picture has been changed by incorporating dynamic covalent bonds3–7 into polymer networks.8–21 Such polymers, referred as “covalently adaptable networks”9,12,22,23 are still covalently cross-linked, but the presence of dynamic covalent bonds24–26 allows the rearrangement of the polymer network through bond breaking and reformation, which results in macroscopic stress relaxation and ultimately material flow.12–15,27–30 Covalently adaptable networks represent an exciting breakthrough in novel polymer development as they open the door to new material processing paradigms for thermosets including thermoforming,13 welding,28 self-healing,8,10 and recycling.30
The imine-linked polymers,31,32 which form through Imine condensation,33,34 are attractive for the development of dynamic covalent networks. Since imine condensation simply requires an amine and an aldehyde or ketone, there is a wide variety of suitable monomers commercially available. In addition, the reaction can take place at reasonable rates at elevated temperatures even in the absence of a catalyst.35 We have demonstrated that polyimines fundamentally behave like a classic thermoset at ambient conditions yet can be reshaped and molded by the application of either water or heat in the absence of any catalyst.29 We showed that heat or water greatly facilitates imine exchange reactions, leading to the malleability and rehealability of polyimines.29 Although polyimines hold a great potential for developing simple, easily accessible, truly green, and inexpensive malleable polymer networks, there have been only few reports on this interesting type of materials.36,37 One interesting feature of polyimine materials is their moisture sensitivity. Although they can be stable against hydrolysis and retain their structural integrity even when soaked in water, the mechanical and malleable properties of the networks can be strongly influenced by water and atmospheric moisture, which needs to be evaluated for practical applications. Herein, we demonstrate the moisture sensitivity of polyimines can be modulated through judicious selection of monomers. Highly hydrophilic to hydrophobic polyimines with a broad range of mechanical/thermal properties and moisture sensitivity have been obtained by simply varying amine-containing monomers. We also sought to understand to what extent highly hydrophilic polymers are more susceptible to hydrolysis. We also found rehealability of polyimines is directly influenced by their hydrophilicity.
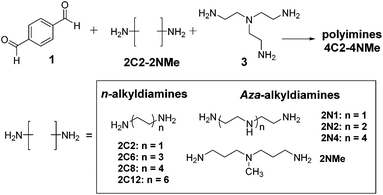
Polyimine networks were prepared from diamine and dialdehyde monomers combined with triamine crosslinkers (Scheme 1). Because of the relative abundance of inexpensive commercially available diamines, we chose to investigate the impact of changing the composition of diamine monomers. A series of diamines (2C2-2NMe) were explored, which include alkyl diamines (2C2–2C12) consisting of alkyl chains of varied length and the aza-analogues (2N1–2N4) consisting of oligo-ethyleneimine or oligo-propyleneimine of varying length. In order to examine the effect of possible hydrogen bonding, we also chose to use 2NMe, in which the secondary amine group in the backbone of diamine monomer is methylated. The polyimines (4C2-4NMe) were obtained from a diamine (2C2-2NMe), terephthaldehyde (1), and tris(2-aminoethyl)amine (3), as shown in Scheme 1. In a typical procedure, diamine 2, dialdehyde 1 and crosslinker 3 in a stoichiometric ratio of 0.450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.000
1.000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.367 were mixed in solvent (EtOH for 4N1-4NMe, EtOH/CH2Cl2/H2O for 4C2–4C12) in a tray made from silicone-coated release paper. The volatiles were then slowly evaporated to yield the polyimine (4C2-4NMe) as a transparent film. Prior to the property measurement, the resulting uncured film was heat pressed for 3 h at 78 °C, 1 h at 95 °C, and finally 1 h at 105 °C using a top platen-heated hand-operated heat press under nominal pressure. The crosslinking amine moieties constitute approximately 55 mol% of primary amines in the network, thus the resulting polymers can be described as 55% crosslinked. Polyimine 4N1 was prepared as 70% crosslinked using a 0.300
0.367 were mixed in solvent (EtOH for 4N1-4NMe, EtOH/CH2Cl2/H2O for 4C2–4C12) in a tray made from silicone-coated release paper. The volatiles were then slowly evaporated to yield the polyimine (4C2-4NMe) as a transparent film. Prior to the property measurement, the resulting uncured film was heat pressed for 3 h at 78 °C, 1 h at 95 °C, and finally 1 h at 105 °C using a top platen-heated hand-operated heat press under nominal pressure. The crosslinking amine moieties constitute approximately 55 mol% of primary amines in the network, thus the resulting polymers can be described as 55% crosslinked. Polyimine 4N1 was prepared as 70% crosslinked using a 0.300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.467 (2N1
0.467 (2N1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) ratio for the sake of easy comparison with the properties of the previously reported polyimine.29
3) ratio for the sake of easy comparison with the properties of the previously reported polyimine.29
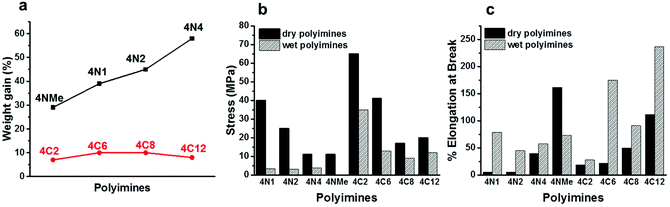
Mechanical properties of these polyimines were then investigated. Tensile testing revealed a great variation in elongation at break and tensile strength among these polyimines (Table 1, Fig. S4†). With the increase of chain length of the diamines, we observed greater elongation at break and lower tensile strength of the resulting polyimines. Tensile strength decreases in the order of 4N1 > 4N2 > 4N4, 4NMe and 4C2 > 4C6 > 4C8, 4C12, whereas elongation at break increases in the order of 4N1 < 4N2 < 4N4 < 4NMe and 4C2, 4C6 < 4C8 < 4C12. Polyimine 4C2 consisting of short ethylene chain exhibits the highest tensile strength at 65 ± 2 MPa, and a modulus of approximately 1 GPa, which are similar to previously reported 4N1.29 The polyimine 4C12 consisting of dodecyl diamine exhibits the highest percent elongation (112 ± 34% elongation at break) among polyimines consisting of alkyl diamine moieties. We found analogous polyimine 4N4 composed of aza-alkyl diamine components with the same chain length as those in 4C12 is far less elastomeric, showing ∼40% elongation at break. This result indicates the replacement of CH2 with NH group in the polyimine chain and the presence of extra hydrogen bonding have a negative effect on the elastic properties of polyimines. When the hydrogen bonding sites are removed by using N,N-bis(3-aminopropyl)methylamine (2NMe), the resultant polyimine 4NMe exhibits dramatically increased elastomeric behavior (162 ± 22% elongation at break).
| Polyimines | Tensile strength (MPa) | Elastic modulus (GPa) | Elongation at break (%) |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (°C) b (°C) |
Nitrogen contentc (mol%) | Weight gain (%) |
|---|---|---|---|---|---|---|
| a 70% crosslinked. b T g was obtained from Tan delta peak in the first scan DMA curve. c Nitrogen content (mol%) on the backbone of the polymers. | ||||||
| 4N1 | 40 | 1.0 | 4 | 110 | 19 | 63 |
| 4N2 | 25 | 0.93 | 5 | 80 | 20 | 71 |
| 4N4 | 11 | 0.21 | 40 | 95 | 22 | 90 |
| 4NMe | 11 | 0.013 | 162 | 57 | 17 | 52 |
| 4C2 | 65 | 1.05 | 19 | 120 | 18 | 7 |
| 4C6 | 41 | 1.01 | 22 | 76 | 16 | 11 |
| 4C8 | 17 | 0.014 | 50 | 61 | 15 | 6 |
| 4C12 | 20 | 0.027 | 112 | 47 | 13 | 6 |
The thermal properties of the polyimines were characterized by dynamic mechanical analysis (DMA) and thermal gravimetric analysis (TGA). The glass transition temperatures of polyimines were determined by the maximum Tan delta in DMA curves and first heating run data were used (Fig. S1 and S2†). Polyimines with short diamines exhibit higher Tg, e.g. 120 °C for 4C2 and 110 °C for 4N1. The Tg trend of polyimines containing alkyl diamines (4C2 > 4C6 > 4C8 > 4C12) is in line with the decreasing rigidity of diamine monomers, which agrees well with the notion that Tg is associated with the molecular packing density and rigidity of the polymer backbones. The Tg of polyimines containing aza-alkyl diamines with multiple amine groups does not follow an obvious trend, likely due to the combined effect of rigidity of polymer chain and inter-chain hydrogen bonding. We observed the lowest Tg (57 °C) for 4NMe, which might be attributed to the decreased hydrogen bonding density. This result indicates that inter-chain hydrogen bonding has a significant impact on the glass transition of polymers. Correspondingly, polyimine 4N2 (Tg = 80 °C) and 4N4 (Tg = 95 °C) with multiple NH groups exhibit higher glass transition temperatures compared to the analogous 4C8 (Tg = 61 °C) and 4C12 (Tg = 47 °C) in which NH groups were replaced by CH2. The thermal stabilities of the polyimines were evaluated by TGA in nitrogen atmosphere at a heating rate of 10 °C min−1. TGA analysis shows that all polyimines lose less than 5% weight at 200 °C, and the temperatures for 10% weight loss in nitrogen atmosphere were in the range of 294–326 °C for polyimines (4C2–4C12) and 238–314 °C for aza-analogues (4N1–4N4). At 600 °C, the residue weight % of 4C2 was about 66%, which was the highest among all the polyimines. The char yields of other polyimines at 600 °C were in the range of 34–47%.
In order to characterize the hydrophilicity of polyimines, we immersed each sample in deionized water for 24 h and measured the weight increase of the fully swelled sample. The swelling data provides the most relevant insight into the effects of moisture on each polyimine, as the weight increase provides a reasonably accurate estimation of the water content within the polymer matrix. We found weight gains of the polyimines are mostly proportional to the density of amine/imine bonds in the polymer matrix in most cases, e.g. the order of weight gains in polyimines (4N4 > 4N2 > 4N1 > 4NMe) agrees well with the order of nitrogen mol% in polyimines (4N4 > 4N2 > 4N1 > 4NMe) (Table 1). More hydrophilic polyimines (4N1, 4N2, and 4N4), which have imine/amine bonds for every two carbons, absorb considerable amount of water, showing greater than 60% weight increase. Polyimines (4C2–4C12) with alkyl diamines show less than 11% weight gain after immersion in water. Polyimine 4C12 consisting of hydrophobic docecyldiamine moieties absorbs only 6 wt% of water upon saturation. Our results clearly show the water sensitivity of polyimines can be tuned by judicious selection of monomers, and both hydrophilic and hydrophobic polymers can be accessible.
Next, the mechanical properties of the wet polyimine films were examined after 24 h immersion in water. After absorbing water, most of the polyimines become more elastomeric with the exception of polyimine 4NMe (>2 fold decrease), which was originally the most elastic. The polyimines made critical gains in elongation ranging from 150% (4C2, or 4N4) to approximately 20 fold in the case of polyimine 4N1. The polyimine 4C2 consisting of ethylenediamine moieties shows the least change of elongation at break (50%). We found that polyimines (4C2–4C12) retain considerable toughness (∼50% original tensile strength) in the wet state, whereas polyimines (4N1–4N4) lose >90% original tensile strength (Fig. 1b). Our study suggests that for most polyimines water mainly acts as plasticizer, reducing the stiffness and increasing the elongation at break by interposing itself between polymer chains and reducing their intermolecular bond strength, which is consistent with plasticizing effect of water on many other polymers.38 It is interesting to note that wet sample of polyimine 4NMe consisting of N-methyldipropylenetriamine essentially loses all mechanical strength, showing only 45% of its original elongation, and 2% of its initial tensile strength.
In order to gauge the impact of hydrophilicity on the hydrolytic stability of the polymers, the solid-state 13C-NMR of the polyimines 4N4 and 4C12 were measured in the wet and dry state. Hydrolysis of imine bonds in the polymer matrix was determined by measuring the change in the ratio of imine to aldehyde carbon peaks (159 ppm and 192 ppm, respectively). As shown in the 13C-NMR spectra (Fig. S5–S9†), there is no observable decrease in the intensity of the imine carbon peak (159 ppm) when either of the polymers is completely saturated with water. This means that even very hydrophilic polyimine, which readily absorbs nearly 90% of its weight in water, does not exhibit any measureable hydrolysis in the swollen state. In order to further test whether any decomposition of polyimines occurs during the soaking process, we measured the weight change of samples before and after soaking. Polyimine 4N1 was soaked in aqueous solutions of three different pH values (pH = 4, 7, and 10) for 24 h. Nearly no weight loss was observed for all the samples after drying, which indicates polyimine 4N1 is stable and does not undergo any decomposition under a broad range of pH conditions (pH = 4–10).
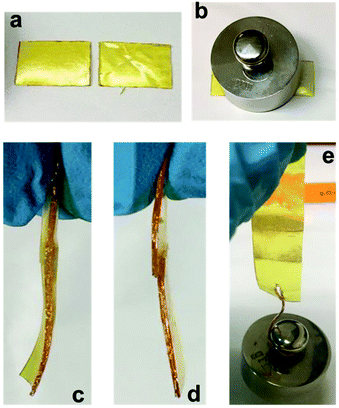
The reversibility of dynamic imine bonds allows these polyimines to be rehealed at the molecular level when pressed together at room temperature. We found hydrophobic and hydrophilic polyimines exhibit different rehealability. As shown in Fig. 2, when two broken pieces of hydrophilic polyimine 4N4 was pressed together with 200 g loading for 1 h, they were physically inseparable (Fig. 2d). When the two broken pieces were covered with a piece of moist paper towel and pressed together with 200 g loading, we obtained better re-healed materials with no discernible interface, which could hold 50 g of weight (Fig. 2c and e). This result indicates water catalyzes the bond exchange reaction in the polyimine, and atmosphere moisture might be enough to heal polyimines under extended time and higher pressure. On the contrary, more hydrophobic polyimine 4C12 cannot be re-healed at room temperature under the same conditions even in the presence of water. However, when the two broken pieces of 4C12 were pressed together under heat (77 °C) for 1 h, we obtained a completely healed piece that can hold 50 g of weight. Our study indicates polymer composition and hydrophobicity directly influence the rehealability of these polyimines.
In conclusion, we have demonstrated that the moisture-sensitivity of polyimines can be modulated by the incorporation of hydrophilic and hydrogen bonding amine moieties into their backbones. Simple variation of monomers among commercially available diamines can produce malleable polyimine networks with a wide range of thermal, mechanical, and moisture-induced properties, such as semicrystalline polymers with high strength and thermal stability, and tough elastomeric materials with low temperature malleability. Some of these polyimines are re-healable at ambient temperature using only water or even atmosphere moisture, which represents a highly green approach for materials processing and repair. With easy means of tuning the mechanical, thermal, and moisture-induced properties, polyimine vitrimers serve as a novel class of catalyst-free malleable thermosets with great potential to be adapted to a large number of potential applications.
The authors thank Prof. Yifu Ding and Prof. Christopher Bowman for providing instrumental support to the project. This work is supported by the National Science Foundation (CMMI-1404627) and Colorado AIA grant program.
Notes and references
- M. Doi, Introduction to polymer physics, Oxford University Press, Oxford, 1996 Search PubMed.
- J. J. Alkonis and W. J. MacKnight, Introduction to polymer viscoelasticity, Wiley, New York, 2nd edn, 1983 Search PubMed.
- E. K. Bang, M. Lista, G. Sforazzini, N. Sakai and S. Matile, Chem. Sci., 2012, 3, 1752–1763 RSC.
- A. Wilson, G. Gasparini and S. Matile, Chem. Soc. Rev., 2014, 43, 1948–1962 RSC.
- S. Liu, K. T. Dicker and X. Jia, Chem. Commun., 2015, 51, 5218–5237 RSC.
- J. R. Davidson, G. A. Appuhamillage, C. M. Thompson, W. Voit and R. A. Smaldone, ACS Appl. Mater. Interfaces, 2016, 8, 16961–16966 CAS.
- J. Lee, B. B. Rajeeva, T. Y. Yuan, Z. H. Guo, Y. H. Lin, M. Al-Hashimi, Y. B. Zheng and L. Fang, Chem. Sci., 2016, 7, 881–889 RSC.
- X. X. Chen, M. A. Dam, K. Ono, H. B. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed.
- T. F. Scott, A. D. Schneider, W. D. Cook and C. N. Bowman, Science, 2005, 308, 1615–1617 CrossRef CAS PubMed.
- Y. Zhang, A. A. Broekhuis and F. Picchioni, Macromolecules, 2009, 42, 1906–1912 CrossRef CAS.
- A. J. Inglis, L. Nebhani, O. Altintas, F. G. Schmidt and C. Barner-Kowollik, Macromolecules, 2010, 43, 5515–5520 CrossRef CAS.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS PubMed.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed.
- Y. X. Lu, F. Tournilhac, L. Leibler and Z. B. Guan, J. Am. Chem. Soc., 2012, 134, 8424–8427 CrossRef CAS PubMed.
- P. W. Zheng and T. J. McCarthy, J. Am. Chem. Soc., 2012, 134, 2024–2027 CrossRef CAS PubMed.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC.
- H. H. Jin, K. R. Hart, A. M. Coppola, R. C. Gergely, J. S. Moore, N. R. Sottos and S. R. White, Self-Healing Polymers: From Principles to Applications, 2013, pp. 361–380 Search PubMed.
- K. A. Williams, D. R. Dreyer and C. W. Bielawski, MRS Bull., 2008, 33, 759–765 CrossRef CAS.
- D. Y. Wu, S. Meure and D. Solomon, Prog. Polym. Sci., 2008, 33, 479–522 CrossRef CAS.
- A. Rekondo, R. Martin, A. R. d. Luzuriaga, G. Cabañero, H. J. Grandea and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- J. J. Cash, T. Kubo, A. P. Bapat and B. S. Sumerlin, Macromolecules, 2015, 48, 2098–2106 CrossRef CAS.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- C. N. Bowman and C. J. Kloxin, Angew. Chem., Int. Ed., 2012, 51, 4272–4274 CrossRef CAS PubMed.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC.
- S. J. Rowan, S. J. Cantrill, G. R. Cousins, J. K. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J. L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed.
- M. Capelot, M. Unterlass, F. Tournihac and L. Leibler, ACS Macro Lett., 2012, 1, 789–792 CrossRef CAS.
- M. Capelot, D. Montarnal, F. Tournilhac and L. Leibler, J. Am. Chem. Soc., 2012, 134, 7664–7667 CrossRef CAS PubMed.
- P. Taynton, K. Yu, R. Shoemaker, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS PubMed.
- K. Yu, P. Taynton, W. Zhang, M. Dunn and H. J. Qi, RSC Adv., 2014, 4, 10108–10117 RSC.
- D. Janeliunas, P. van Rijn, J. Boekhoven, C. B. Minkenberg, J. H. van Esch and R. Eelkema, Angew. Chem., Int. Ed., 2013, 52, 1998–2001 CrossRef CAS PubMed.
- J. Leclaire, G. Husson, N. Devaux, V. Delorme, L. Charles, F. Ziarelli, P. Desbois, A. Chaumonnot, M. Jacquin, F. Fotiadu and G. Buono, J. Am. Chem. Soc., 2010, 132, 3582–3593 CrossRef CAS PubMed.
- Y. H. Jin, Y. L. Zhu and W. Zhang, CrystEngComm, 2013, 15, 1484–1499 RSC.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- T. Ono, T. Nobori and J. M. Lehn, Chem. Commun., 2005, 12, 1522–1524 RSC.
- P. Taynton, H. G. Ni, C. P. Zhu, K. Yu, S. Loob, Y. H. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2016, 28, 2904–2909 CrossRef CAS PubMed.
- J. M. Whiteley, P. Taynton, W. Zhang and S. H. Lee, Adv. Mater., 2015, 27, 6922–6927 CrossRef CAS PubMed.
- N. A. J. Platzer, Plasticization and Plasticizer Processes, American Chemical Society, 1965 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, mechanical and thermal property tests, and NMR spectra. See DOI: 10.1039/c6py01395c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |