Schiff's base as a stimuli-responsive linker in polymer chemistry
Yan
Xin
and
Jinying
Yuan
*
Key Lab of Organic Optoelectronics and Molecular Engineering of Ministry of Education, Department of Chemistry, Tsinghua University, Beijing, 100084, P. R. China. E-mail: yuanjy@mail.tsinghua.edu.cn; Web: www.yuanjinyinggroup.com Tel: +86-1062783668
First published on 28th June 2012
Abstract
Schiff-base reactions are widely used in the field of chemistry. With many advantages, such as mild reaction conditions and high reaction rates, they were employed for protecting various functional groups and synthesizing a series of organic ligands. In polymer chemistry, they can serve as potential pH-responsive linkers in polymer chains because of their sensitive responses to changes in the pH value. With certain particular designs, the Schiff-base structure can cooperate with other reversible covalent bonds or supra-molecular interactions to form assemblies or gels, providing various functions and applications. This article aims to give a critical review of the recent literature on the Schiff-base reactions used in polymer chemistry and how they serve as a way of linking structures together. We will also cover some of the important developments on the functions and applications of these polymers.
 Yan Xin | Yan Xin received his B.Sc. in 2011 at the Department of Chemistry, Tsinghua University, Beijing, China. He is now a graduate student under the supervision of Prof. Jinying Yuan at the Department of Chemistry, Tsinghua University. His research interests are focused on synthesizing polymers with new stimuli-response, developing synergistic actions among different stimuli and functionalization of (pseudo)polyrotaxanes. |
 Jinying Yuan | Dr. Jinying Yuan received her B.Sc. degree from the University of Science and Technology of China (USTC) in 1987, and received her Ph.D. in the Department of Polymer Science and Engineering, USTC in 2000. After her postdoctoral research in Peking University, she joined Tsinghua University in 2002, where she became a full professor in 2011. Her research interests are focused on polymer synthesis and functional polymer materials, especially on living/controlled polymerization, stimuli-responsive polymer materials, and organic–inorganic hybrid nanomaterials. |
Introduction
The Schiff-base reaction was discovered by a German chemist, Hugo Schiff, in 1864![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and is named after him. It refers to the reaction between the class of substances containing carbonyls (aldehydes and some ketones) with amino groups (primary amine, hydroxylamine, hydrazine, etc.), the product of which contains C
1 and is named after him. It refers to the reaction between the class of substances containing carbonyls (aldehydes and some ketones) with amino groups (primary amine, hydroxylamine, hydrazine, etc.), the product of which contains C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds (Fig. 1).
N double bonds (Fig. 1).
 | ||
| Fig. 1 Scheme of the Schiff-base reaction. | ||
In this reaction, if the aldehyde or ketone are aliphatic carbonyl compounds, it is necessary to continuously remove the by-product water, because the thermodynamic balance is biased in the opposite direction. On the contrary, if the reactants are aromatic carbonyl compounds, the thermodynamic balance is biased towards the forward reaction.2,3 In these cases, simply mixing and stirring the two reactants can result in an easy reaction and produce the target product in very high yields. Furthermore, an aldehyde carbonyl group has a much higher activity than a ketone carbonyl. Unless there are some special requirements, compounds with a benzaldehyde substructure are normally selected as one of the reactants.
In aqueous solution, the pH value of the solution significantly affects the reactivity of the Schiff's base. Generally speaking, higher acidity destroys the Schiff's base. The Schiff's base becomes relatively stable in alkaline solution. With such characteristics, the Schiff's base structure can be used as a good stimuli-responsive linker in polymer chemistry.
Simple primary amines, hydroxy-substituted hydroxylamines, and hydrazides are three commonly used amino-containing reactants. The imine formed between a primary amine and an aldehyde group is less stable. In aqueous solution at a pH value below 6.5, such an imine structure can be considered to be completely decomposed. This reversibility is suitable for use in biological systems. However, with the other amines, the imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds formed are more stable. These C
N double bonds formed are more stable. These C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds only break when the pH value falls below 3. In systems where high stability is required, hydroxy-substituted hydroxylamines and hydrazides are the amines of choice.
N bonds only break when the pH value falls below 3. In systems where high stability is required, hydroxy-substituted hydroxylamines and hydrazides are the amines of choice.
The sensitivity of the imine linkage to pH value is the major responsive feature of this structure. In addition, the complexation of the imine can also be used to expand the responsiveness of the linker. The response to pH value is widely used in stimuli-responsive materials.4–16
Linker chemistry has been implemented in a wide range of applications.17 Compared to other linkers such as click chemistry, the Schiff-base reaction is much more reversible, even in mild conditions. This chemistry realizes a new way to reversibly break some chemical bonds in polymer structures, enabling the polymer with stimuli-responsive properties with pH changes, without other post-functionalization. Combined with other chemical modifications, such polymers can also exhibit collaborative responses to different stimuli. Moreover, due to the dynamic nature of such a reaction, quantities of small molecules are able to compete with the end-groups of macromolecules. So the response can be applied to specific small molecules. There are many such small molecules presented in biological systems. Therefore, this linker is applicable to biomedical fields.
Schiff-base linker in the polymer main chain
Placing linkers in the polymer main chain is a common methodology in polymer chemistry, especially in the construction of polymers with more than one block. This is often a result of the terminal groups from different blocks bonding with each other.Many reactions possess the potential for being linkers. However, certain criteria should be met to ensure the reliability in binding polymers: (1) the efficiency of the reaction should be very high, normally with a yield of greater than 90%; (2) the reaction should have high selectivity (>80%) for reacting with special functional groups and without functional group protectors; (3) the reaction should have high sensitivity and work at a very low linker concentration (∼10−5 M); (4) the linker should be small enough that the structure and function of the polymer will not be affected; (5) the linkers should be stable enough, which makes them insensitive to ordinary chemical or physical stimuli, but can be removed quantitatively and selectively with specific reagents; (6) the reaction takes place rapidly and can be completed in a short time.
Some typical reactions are widely used in chemical research as linkers, such as “click chemistry”18–26 (1,3-dipolar (3 + 2) cycloaddition reaction, Diels–Alder reaction, thiol-ene reaction, etc.) and the Schiff-base reaction. The Schiff-base reaction belongs to the family of reactions called dynamic covalent reactions.27–37 Compared with traditional linkers, the Schiff base's structure provides outstanding reversibility with changing pH value, which is a good way to control a covalent bond and quite similar to the adjustment of supramolecular systems, which made it useful in many aspects.38–43
However, designing the linker in the polymer main chain has its own weaknesses. Certain physical or chemical stimuli, no matter what they are in nature, do not easily interact with the linker.
To avoid such weaknesses, at least one block of the polymer should be easy for the reagent to pass through. Fortunately, most block copolymers synthesized, utilizing the Schiff's base as a linker, are amphiphiles; the hydrophilic block in solution can be passed through easily for the reagents H+ and OH−.
He, Zhu and their co-workers synthesized a diblock copolymer used the Schiff's base as a linker.44 An aldehyde modified polystyrene (PS-CHO) and hydrazide derivatives of polyethylene glycol mono ether (PEG-NHNH2) are the two blocks. The product is an amphiphilic dynamic diblock copolymer, which is linked through an acylhydrazone bond. The reversible acylhydrazone bond shows tuneable properties.45–50 To highlight the dynamic covalent bond, they named the copolymer PS-r-PEG, instead of PS-b-PEG (Fig. 2). Similar to the conventional amphiphilic block copolymers, the dynamic PS-r-PEG can self-assemble into diverse morphologies under neutral and alkaline conditions with high stability. By adding trifluoroacetic acid (TFA, pH = 4), the acid-triggered hydrolysis of the acylhydrazone bonds results in the disassembly of the PS-r-PEG nanoparticles. This pH-sensitive assembly encapsulation system could be used as a drug carrier as demonstrated by the sustained release of methyl porphyrin.
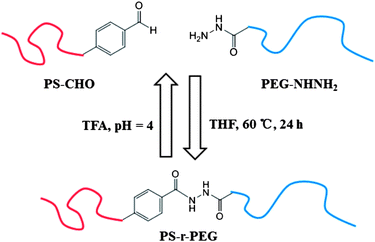 | ||
| Fig. 2 Dynamic properties of PS-r-PEG.44 | ||
Lehn and his co-workers utilized some double functionalized monomers to synthesize polymers with the help of a Schiff-base reaction.51–57 One of the four polymers (polymer 3, Fig. 3) had naphthalene and 1,4,5,8-naphthalene-tetracarboxylic diimide in its main chain.51 The donor and acceptor units can associate with each other by charge transfer interactions. In addition, the chain between the alternative groups contains oxygen and nitrogen atoms, which means that the chain will interact with alkali metal ions to some extent, just like noncyclic crown ether chains (Fig. 3). As the charge transfer interactions are not strong enough to make the donor and acceptor close in solution, the solution did not become red. However, when adding LiOTf, NaOTf, and KOTf to polymer 3 in a CD2Cl2–CD3CN mixture, the solution turned red to various degrees. Adding RbOTf and CsOTf led to similar changes. The interactions between the alkali metal ions added and the heteroatom-containing chains would bend the flexible chain; as a result, the donor and the acceptor were made to be close enough to show the charge transfer interactions between them. Macroscopically, the solutions turned red.
This provides us with a simple way to identify different alkali metal ions qualitatively through a colorimetric method. Moreover, as the ammonium ion could interact with the crown ether through dipolar interactions, some terminal functionalized polymers may be grafted to the polymer main chain through non-covalent interactions. It is possible that the grafting degree could be characterized through UV-vis spectrum directly.
Deng and his co-workers employed a triple aldehyde group modified 2-(hydroxymethyl)-2-methylpropane-1,3-diol as a cross-linking agent. When reacted with a hydrazide derivative of polyethylene (NH2NH-PEG-NHNH2), a gel was formed.58 However, simply mixing the polymer and cross-linking agent was useless; only after adding glacial acetic acid, adjusting the apparent pH to 6–7, did gelation finally occur (Fig. 4). Some basic parameters of the gel such as viscosity (η) vs. time, storage modulus (G′) and loss modulus (G′′) were characterized, which suggested the formation of covalent cross-linked networks.59 More importantly, the dynamic properties of the gel were observed in detail. After adding hydrochloric acid, the gel became a solution in 1.5 h. When triethylamine was added, the solution transformed into a gel in 20 s. Such a cycle was repeated eight times, and the sol–gel transformation was proved to be reliable. However, the phase transition process became slower and slower along with the increasing cycles, and triethylamine hydrochloride precipitated due to its low solubility in DMF, which made the gel opaque. The result showed that the gel can tolerate a relatively high ionic strength. A gel formed in 3 h without any acid catalyst in the component solvent of water and DMF, which suggested that water may promote the dynamic properties of the Schiff's base. The most conspicuous character of the gel based on the dynamic properties of the Schiff's base is its self-healing behaviour. When two plates of gel are put together, the interface will fuse with a new equilibrium between the acylhydrazone bonds and aldehyde/acylhydrazine reached. Note that the self-healing process in the gel can occur autonomously without any external intervention, making it more convenient than some normal self-repairing polymer materials60,61 for applications. The low solubility of the crosslinker may cause some problems. Modifying some polar functional groups such as hydroxy or carboxyl groups will be of great help.
Zhu and his co-workers utilized an oxime-tethered PCL (OPCL) and PEG-CHO to construct a triblock copolymer.62 The hydrophobic block OPCL was synthesized from O,O′-(propane-1,3-diyl)bis(hydroxylamine) and CHO-PCL-CHO,62,63 which indicated that the polymer possessed more than one oxime bond in its main chain (Fig. 5). The micelles formed were used as drug carriers. Their release behaviour is solely based on the dynamic properties of the oxime bond. The release properties of PEG-PCL-PEG (amide linkage) and PEG-OPCL-PEG (oxime linkage) at pH 5.0 and 7.4 are calculated. Only the PEG-OPCL-PEG (oxime linkage) had a remarkable release at pH 5.0 with some release behaviour at pH 7.4 due to the dynamic properties of the oxime bond.
 | ||
| Fig. 5 Dynamic amphiphile PEG-OPCL-PEG constructed via the Schiff-base reaction.62 | ||
Lehn and co-workers employed an exchange reaction to characterize the modulation of the optical properties in a system of two films made from hydrazone-based dynamic polymers.64 Three polymers (P1, P2 and P3) containing polyhydrazone were synthesized (Fig. 6). As the conjugated system in each polymer was unique, their diverse optical properties, such as colour and fluorescence, are well-reasoned. P1 is almost colourless, P2 is light yellow, and P3 is dark orange. To demonstrate the dynamic properties of the system, the solution of P1 and P2 were mixed in the presence of some acid as catalyst. The colour became light yellow first, and then became vivid yellow after being heated at 120 °C. The UV-visible absorption and fluorescence band showed the same change. That means that an exchange reaction happened between P1 and P2, and one of the products is exactly P3. This behaviour suggests the extensive applications of the system.
The Schiff-base reaction can also be used in conjugated systems. The product will have an expanded conjugated system, which will lead to some new optical and electrochemical properties.
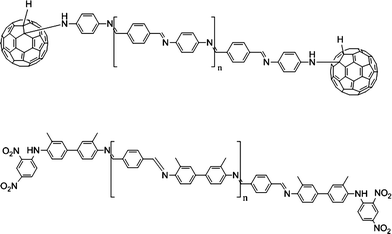
Geckeler and his co-workers,65 and Liu and his co-workers66 used similar methods to obtain conjugated polymers. As the polymers have β-cyclodextrin (β-CD) around the main chain, the system is polyrotaxane. Liu utilized STM to observe the rigid single molecular chain. Geckeler characterized the electrochemical properties of the polyrotaxane obtained by cyclovoltammograms, in which the interaction between the conjugated system and the terminal group C60 can be analysed (Fig. 7).
The dynamic properties of the Schiff-base reaction have been studied carefully. However, studies of its optical and electrical properties are few. The interactions between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds and the conjugated system change clearly before and after the formation of the imine bond. The fluorescence may change dramatically based on the reaction. In addition, the C
N double bonds and the conjugated system change clearly before and after the formation of the imine bond. The fluorescence may change dramatically based on the reaction. In addition, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds can be reversibly reduced by way of electrochemistry, which shows the possibility of its response to electricity. However, it may be difficult to construct a conducting polymer solely utilizing the Schiff-base reaction because of its relatively low stability. Traditional doping will break the bond, which will destroy the polymer.
N double bonds can be reversibly reduced by way of electrochemistry, which shows the possibility of its response to electricity. However, it may be difficult to construct a conducting polymer solely utilizing the Schiff-base reaction because of its relatively low stability. Traditional doping will break the bond, which will destroy the polymer.
Schiff-base linker outside polymer main chain
Compared with the first strategy, arranging the linker outside the polymer main chain was studied in many aspects. Many topologies of polymer can be constructed with this strategy. In addition, more than three functional groups can exist in one polymer chain, so that the crosslinking between polymers becomes a natural result. In fact, many gelating systems are constructed in this way. As mentioned before, all the gels obtained in this way have a response to changes of the pH value, and some post-modified polymer systems have other more asynchronous response modes.Zhang and his co-workers chose a double-hydrophilic block copolymer methoxy-poly(ethyleneglycol)114-block-poly(L-lysine hydrochloride)200 (PEG-b-PLKC) as one of the reactants, another component was 4-(decyloxy)benzaldehyde (DBA).67 When the pH is above 7.4, the benzaldehyde end group reacted with the primary amine groups contained in PLKC, leading to the forming of a benzoic imine bond,68–71 and the PLKC block became hydrophobic. The toothbrush-like amphipathic copolymer self-assembled into micelles. An opposite process would occur when the pH is below 6.5, leading to the disassembly of the micelles (Fig. 8). The micelles formed in a pH = 7.4 buffer and disappeared when the pH is 6.5. The zeta potential of the polymer rises when the pH value decreases, supporting the process that the imine bond breaks and the free primary amine is protonated. The cycle of zeta potential–pH value also showed good repeatability, confirming the reversibility of the Schiff-base reaction.
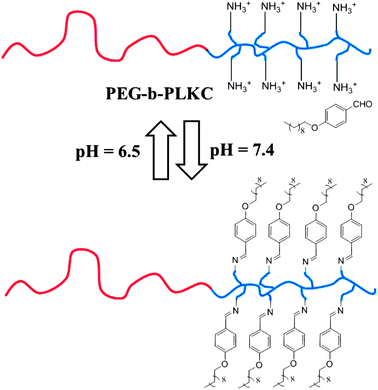 | ||
| Fig. 8 The reversible pH-responsive properties of the brush-like amphiphile via the Schiff-base reaction”.67 | ||
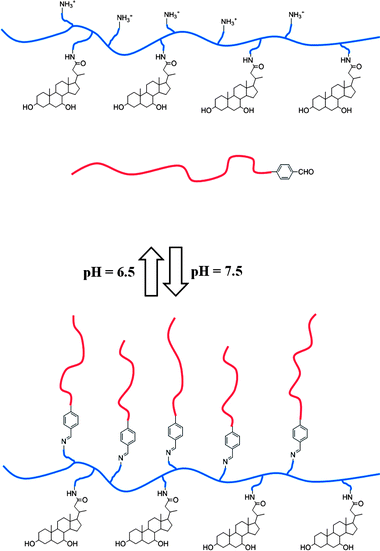
Yang et al. chose a homopolymer poly-L-lysine as the polymer main chain.72 To endow the polymer with some self-assembling abilities, it should be modified into an amphiphile structure. Its hydrophobicity was due to the grafted cholic acid. Not all the amino groups were reacted, so that the protonated amino group could provide hydrophilicity for the polymer at a low pH value. Moreover, methoxy poly(ethylene glycol)benzaldhyde (PEG-CHO) was synthesized, which would react with the amino at high pH values. At that time, it behaved as a hydrophilic block, which suggested that the PEG block acts as a halo that could be deshielded. The transforming pH value was around 7.5 and 6.5 (Fig. 9). Such a design could make full use of all the blocks. The important problem is deshielding the PEG block at the right time. Considering the environment in the cell is more or less acidic, the Schiff's base is a good choice. The key point of some similar designs is the Schiff's base.
Fulton and his co-workers synthesized two diblock copolymers with similar constructions.73 The only difference was that one is with an aldehyde group modified block, and the other is with a corresponding amino modified block. The polymers synthesized were all based on polystyrene, which makes them easy to dissolve in many different solutions such as THF (Fig. 10). Obviously, when the two kinds of polymer were mixed, a core cross-linked star (CCS) polymer was formed through imine bonding. No macroscopic gelation was observed, which means that the PS block outside prevented macroscopic crosslinking. When the polymers without a PS block were mixed, macroscopic gelation happened.
The CCS polymer is dynamic. As the molecular weight and molecular weight distribution of the styrenic CCS polymers gradually increased, the average number of polymer chains per assembly calculated rose as the percentage of total diblock copolymer weight increased. To confirm its dynamic properties, a large excess of propylamine was added to a solution of CCS polymers, then the CCS polymers disassembled into diblock copolymers, because alkyl imines are thermodynamically more stable than aniline imines.
Fulton and his co-workers synthesized two kinds of triblock copolymers via RAFT polymerization.74 A poly(N-isopropyl-acrylamide) (PNIPAM) block was introduced into the copolymers, which enables the system to respond to heat (Fig. 11). The existence of PNIPAM endowed the triblock copolymers with lower critical solution temperatures (LCSTs), but their LCSTs are different from the PNIPAM homopolymer because of the differences of relative hydrophobicity–hydrophilicity among other blocks. The CCS polymers were also sensitive to the changing of pH value. Under two pH values, 11.0 and 5.5, significant changes took place. In addition, cycles between pH = 11.0 and pH = 5.5 were shown through multi-angle laser light scattering (MALLS) experiments. The repeatability of the transformation after 5 cycles was still good. To prove the dynamic properties of the imine directly, approximately 100 equivalents of NaCNBH3 per imine bond was added to the solution at pH 11.0 and it was stirred for 2 h. After that, the CCS polymers failed to disassemble into triblock copolymers, which directly proves that the dynamic properties of the system are from the imine bond.
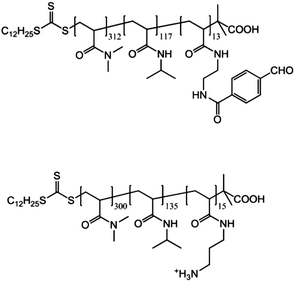
A similar triblock copolymer was synthesized to construct reversible imine shell crosslinked micelles by McCormick and his co-workers.75 The triblock copolymer, α-methoxypoly(ethylene oxide)-b-poly(N-(3-aminopropyl)methacrylamide)-b-poly(N-isopropylacrylamide) (mPEO-PAPMA-PNIPAM), was synthesized through RAFT polymerization in aqueous solution. The PNIPAM block endows the polymer with thermal responsive properties. When the temperature is above its LCST, more than one block will assemble into a core, which leads to the formation of micelles. Moreover, the PAPMA block contains some amine groups that are capable of reacting with the aldehyde groups. When adding some reactants with more than one functional group, such as terephthaldi-carboxaldehyde (TDA), the PAPMA block will be crosslinked. The result is the formation of shell crosslinked (SCL) micelles. The nanoparticles are therefore appropriate carriers for drug delivery because the shell can significantly control the release of molecules inside (Fig. 12). Due to the existence of the imine bond, the micelles manifested good dynamic properties. Their Dh decreased gradually from about 92.0 nm (swollen SCL micelles) at pH = 9.0 to about 12.0 nm (unimers) at pH = 5.0 at room temperature, just because of the breaking of the imine bonds. All the responsive patterns have a different influence on the results, which suggests that they are logically linked with each other, a bit like the logic gates in electronics.
Fulton and co-workers further utilized redox as one of the stimuli. Two copolymers, an aldehyde/disulfide-functionalized copolymer (P1) and an amine/disulfide-functionalized copolymer (P2b), were synthesized as the building blocks to form core crosslinked star (CCS) polymers.76 To further decorate the CCS polymer, hydrazide end-functionalized poly(ethylene glycol) was synthesized, which could assemble with the CCS polymer based on the formation of a hydrazone bond (Fig. 13).
The mixture of P1 and P2b crosslinked into nanoparticles in two steps: the formation of a hydrazone bond and a disulfide bond. The hydrazone bond would form automatically; meanwhile the formation of the disulfide bond needs some reagents such as dithiothreitol (DTT) in a relatively high pH value environment. What's more, the existence of some stable reaction byproducts such as 2-pyridinethione and a 6-membered ring of the oxidized form of DTT, serves as a driving force to drive the reaction to completion. The nanoparticles formed have some functional groups on or near their peripheries, which could act as suitable handles to interact with other potentially useful molecules; in other words, allowing their further functionalization by decorating their surfaces. In this way, their prospective utilities are expanded. The hydrazide end-functionalized poly(ethylene glycol) (PEG-hydrazide) was utilized to demonstrate the feasibility. In the same mechanism, a range of polymers or modified biomolecules to these CCS polymeric nanoparticles can be conjugated through the formation of hydrolytically stable C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds.
N double bonds.
Obviously, with the existence of a low pH value and a reductant such as glutathione (GSH), the nanoparticles degrade into unimers, with the release of the contained molecules, and the process can repeat in the cell. This suggests its usage in drug delivery and controlled release. The pH response and redox response present their cooperativity, acting just like an AND gate.
In addition to the crosslinking between a number of the polymer chains, only one polymer chain can also be crosslinked. In this way, nanoparticles formed from a single chain can be synthesized. Since a single polymer chain is the minimum building block in polymer self-assembly, it can be used as a typical system to discuss some basic problems in the progress.
Fulton and his co-workers employed RAFT polymerization to synthesize some polymers with aldehyde groups outside the main chain, which are poly-(vinylbenzaldehyde) (PVBA) with different molecular weights.77 A block copolymer PVBA-b-PS was synthesized on the basis of PVBA using RAFT polymerization. A small molecule containing hydrazide groups at both ends, 2,2′-((2-(tert-butyl)-1,4-phenylene)bis(oxy))di-(acetohydrazide), was used as a cross-linking agent. Other small molecules which contain only one amino group, undecenoic acid hydrazide, O-(4-tertbutylbenzyl)hydroxylamine, O-(2-hydroxyethyl)hydroxylamine, and 2-(2-hydroxyethoxy) acetohydrazide, were also synthesized (Fig. 14). Nanoparticles formed in the THF solution of the two reactants PVBA/PVBA-b-PS and 2,2′-((2-(tert-butyl)-1,4-phenylene) bis(oxy))di-(acetohydrazide) with trifluoroacetic acid as catalyst and triethylamine as terminator. The nanoparticles reacted with monohydrazide or alkoxyamine in THF under the same conditions. Different kinds of nanoparticles were obtained with a diversity of polymers and the ratio between the reactants. Moreover, by changing the order of adding the bis-hydrazide linker and monohydrazide or alkoxyamine, some white powders were also obtained. All the polymers showed much smaller molecular weights, which means crosslinking in one molecule. The polydispersity indexes of each polymer are exactly the same as reacted before, which suggests hardly any crosslinks between different polymer chains. When the nanoparticles react with monohydrazide or alkoxyamine, some crosslinking will be destroyed. The higher the concentration is, the greater damage it has.
 | ||
| Fig. 14 The reversible formation and degradation of single chain nanoparticles.77 | ||
Some other reactions are also used to crosslink a single polymer chain to gain one chain nanoparticles. Compared with other reactions, the great advantage of the Schiff-base reaction is its reversibility. However, its dynamic problems may cause some problems in this situation. The nanoparticles obtained may be crosslinked through the reaction gradually, which will have great influence on the properties of the nanoparticles. One of the solutions is adding some reducing agents. The transformation from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds to a C–N single bond leads to the disappearance of the reversibility, which will improve the stability of the nanoparticles.
N double bonds to a C–N single bond leads to the disappearance of the reversibility, which will improve the stability of the nanoparticles.
Nowadays, natural products are also used in polymer chemistry. One of them is chitosan. Each of its repeating units has one amino, which needs no modification to carry out Schiff-base reactions.
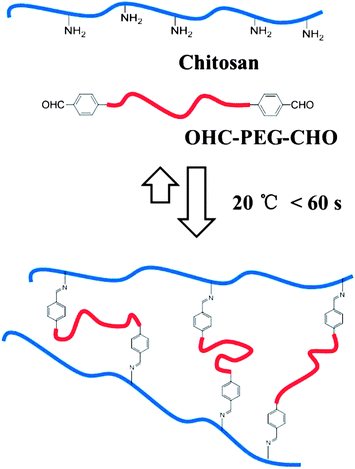
Tao, Wei and their co-workers selected chitosan as the polymer main chain, and two ends benzaldehyde modified polyethylene glycol (OHC-PEG-CHO) as a crosslinking agent to construct chitosan hydrogel (Fig. 15).78 The gel formed shows its response to changes of the pH value, and gelation still exists after six cycles. The repairing performance of the hydrogel was also outstanding. The gel can also respond to some small molecules containing amino groups in organisms, such as amino acid or vitamin B6 derivatives. Pyridoxyl hydrochloride (PL-HCl) and lysine solutions were added in the gel. The rhodamine B in the gel was used to measure the release. The release was much more obvious than that in water. The gel transformed into solution to different extents.
Apart from modifying aldehyde groups to the terminal of the polymer chain, there are other methods to introduce aldehyde groups directly into the polymer chain, such as the reaction between polyol and periodic acid/sodium periodate.79–81
Nishinari and his co-workers chose schizophyllan (SPG) as the precursor; after a weighed amount of NaIO4 was added into SPG aqueous solution for 24 h, 2 mol of NaIO4 are consumed by 1 mol of SPG side groups, the result of which is two neighbouring hydroxyl groups being transformed into a pair of aldehyde groups. Changing the amount of NaIO4, periodate-oxidized SPG (POSPG) with different oxidation degrees can be achieved (Fig. 16).82 When mixing the POSPG and gelatin solutions rapidly under vigorous stirring and quickly degassing them by centrifugation, gelation happened gradually. The gel shows response to pH value. The gelation happens much more rapidly at higher pH values. Moreover, the final gel strength increases with increasing pH value.
 | ||
| Fig. 16 The oxidation of SPG with sodium periodate.82 | ||
Cheng, Qu, Yang and their co-workers chose glycol chitosan (GC) and a triblock copolymer benzaldehyde modified poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEO-PPO-PEO) to construct a biocompatible and injectable hydrogel.83 As the PEO-PPO-PEO has a low critical solution temperature (LCST), the hydrogel will also respond to temperature (Fig. 17). A hydrogel is formed when mixing GC and OHC-PEO-PPO-PEO-CHO aqueous solutions. The FTIR spectrum shows the transformation from aldehyde to imine bond. Meanwhile, the FTIR spectrum also shows that a certain amount of free amino groups are preserved in the hydrogel. That makes it possible for the hydrogel to interact with other molecules or to be further functionalized.
It is easy to understand that the lower critical gelation pH value of the gel decreases with the increase of the concentration of the two reactants, because the density of crosslinking becomes higher at that time. The 3D net becomes harder to degrade. The gel also has an upper critical gelation pH value. This is attributed to the complete deprotonation of the amino groups in GC, which is more or less against the formation of the Schiff's base. The LCST of the PEO-PPO-PEO is 52 °C. When the mixture solution was heated up to the LCST, the block copolymer shrank, with the functional groups packed and no gelation happening. When the mixture solution was cooled down, gelation was observed. Further heating did not cause any gel–sol transformation, which proved that the lack of gelation had scarcely any relationship with the Schiff-base reaction.
Micellar-coated drug delivery has some certain intrinsic problems. Since there are no strong interactions between the drugs and the carriers, the carriers are not a completely closed system. Therefore, part of the drug will be released from the carrier in the transport process, and the efficacy of delivery will be weakened in this way. In addition, one of the products from the breaking of the imine bonds contains aldehyde groups, which may have some potential toxic effects on organisms. These issues should be addressed in future research. One of the possible solutions is to connect the drug to the carrier with a covalent bond so that the release of the drug during the course of transportation would therefore be restrained. To avoid the introduction of aldehyde groups, making use of the carbonyl groups that might present in the drug itself is a plausible way to avoid possible side effects.
Kataoka and his co-workers utilized poly(ethylene glycol)-poly(aspartate-hydrazid) (PEG-p(Asp-Hyd), a diblock copolymer, as the matrix.84 By forming the hydrazone bond between hydrazide and carbonyl in the doxorubicine (DOX), the poly(aspartate-hydrazid) block became hydrophobic. The amphiphile then assembled into micelles, which can be used as carriers (Fig. 18). Under acidic conditions, DOX dissociated from the polymer chain and the amphiphilic diblock copolymer converts into hydrophilic chains, which leads to the collapse of the micelles and accelerates drug release. The response of the micelles to the change of the pH values was characterized by reversed-phase liquid chromatography (RPLC). The release of DOX shows both time- and pH-dependence when the pH value decreases from pH 7.4 to 3.0. The lower the pH value is, the faster the release proceeds. From pH = 6.5 to higher, the percentage of released DOX can be ignored. This is in accordance with the stability of the acylhydrazone bond.
To introduce a carrier into organisms, the release mechanism should be fit for intracellular systems. Haag explained the drug release mechanism in organisms.85 Simple diffusion through the cellular membrane is impossible for most macromolecules and nanoparticles. Most of them are transported into the cells by so-called endocytosis. In endocytosis, the cell membrane folds inward and bubble-like vesicles are separated with the surrounding materials ingested simultaneously. After the carrier is “swallowed” into the cell, the endosomes fuse with primary lysosomes and transform into secondary lysosomes. The pH value in the lysosome is significantly lower (pH ≈ 4–5) than that under physiological conditions (pH = 7.4). Under acidic conditions, the acylhydrazone bond will break, which leads to the release of DOX.
Compared with the linker in the polymer main chain, modifying the linker on the branches is much more variable. The topologies of the polymer become full of variety, and as a result, the functions of the polymers become much more pluralistic. Crosslinking between different polymer chains becomes much easier, so that gels and microgels are widely seen in this strategy.
Conclusions and future perspectives
The great advantage of the Schiff-base reaction as a linker is its simplicity in chemical synthesis. Simply mixing the reactants together and stirring them at room temperature results in completed reactions. In addition, the conditions of the end-group modification are relatively simple and mild. Moreover, if the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds formed are conjugated to the aromatic system, it is possible to form a big conjugate system, which may present quite different character from the monomers. If the dynamic covalent bond is no longer needed, it is easy to transform it into strong chemical bonds. Adding some reducing agent such as sodium borohydride to the system, the C
N double bonds formed are conjugated to the aromatic system, it is possible to form a big conjugate system, which may present quite different character from the monomers. If the dynamic covalent bond is no longer needed, it is easy to transform it into strong chemical bonds. Adding some reducing agent such as sodium borohydride to the system, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds can be transformed into C–N single bonds. Finally, with some special design, such as the use of salicylic aldehydes as a reactant, the resulting Schiff's base structure may react with some transition metal ions. This can form some new polymer structures containing metal ions and can be used potentially for metal ion response.
N double bonds can be transformed into C–N single bonds. Finally, with some special design, such as the use of salicylic aldehydes as a reactant, the resulting Schiff's base structure may react with some transition metal ions. This can form some new polymer structures containing metal ions and can be used potentially for metal ion response.
However, the Schiff-base reaction also has its deficiencies. Due to the sensitive nature of such a reaction when exposed to water, its dynamic performance may be accelerated. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond may be weakened as a result. More research is required to look into the toxicity of the aldehyde group in biological systems.
N bond may be weakened as a result. More research is required to look into the toxicity of the aldehyde group in biological systems.
Acknowledgements
The authors gratefully acknowledge financial supports from the National Basic Research Program of China (2009CB930602) and the National Natural Science Foundation of China (21174076, 51073090, 20836004).Notes and references
- H. Schiff, Justus Liebigs Ann. Chem., 1864, 131, 118 CrossRef.
- A. K. Engel, T. Yoden, K. Sanui and N. Ogata, J. Am. Chem. Soc., 1985, 107, 8308 CrossRef CAS.
- J. R. Anacona, V. E. Marquez and Y. Jimenez, J. Coord. Chem., 2009, 62, 1172 CrossRef CAS.
- L. Zhou, J. Yuan, W. Yuan, X. Sui, S. Wu, Z. Li and D. Shen, J. Magn. Magn. Mater., 2009, 321, 2799 CrossRef CAS.
- W. Yuan, Z. Zhao, J. Yuan, S. Gu, F. Zhang, X. Xie and J. Rena, Polym. Int., 2011, 60, 194 CrossRef CAS.
- L. Zhou, Z. Cai, J. Yuan, Y. Kang, W. Yuan and D. Shen, Polym. Int., 2011, 60, 1303 CAS.
- J. Tan, A. Blencowe, T. Goh, C. Dela, T. M. Irving and G. G. Qiao, Macromolecules, 2009, 42, 4622 CrossRef CAS.
- Q. Zhang, F. Xia, T. Sun, W. Song, T. Zhao, M. Liu and L. Jiang, Chem. Commun., 2008, 1199 RSC.
- K. C. Etika, M. A. Cox and J. C. Grunlan, Polymer, 2010, 51, 1761 CrossRef CAS.
- Y. Oda, S. Kanaoka and S. Aoshima, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1207 CrossRef CAS.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- Q. Yan, R. Zhou, C. Fu, H. Zhang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923 CrossRef CAS.
- D. Han, X. Tong, O. Boissière and Y. Zhao, ACS Macro Lett., 2012, 1, 57 CrossRef CAS.
- K. Zhou, J. Li, Y. Lu, G. Zhang, Z. Xie and C. Wu, Macromolecules, 2009, 42, 7146 CrossRef CAS.
- Z. Guo, Y. Feng, Y. Wang, J. Wang, Y. Wu and Y. Zhang, Chem. Commun., 2011, 47, 9348 RSC.
- T. Yu, K. Wakuda, D. L. Blair and R. G. Weiss, J. Phys. Chem. C, 2009, 113, 11546 CAS.
- A. C. Comely and S. E. Gibson, Angew. Chem., Int. Ed., 2001, 40, 1012 CrossRef CAS.
- A. Dondoni and A. Marra, Chem. Soc. Rev., 2012, 41, 573 RSC.
- T. Muller and S. Braese, Angew. Chem., Int. Ed., 2011, 50, 11844 CrossRef CAS.
- C. Chu and R. Liu, Chem. Soc. Rev., 2011, 40, 2177 RSC.
- R. Fu and G. Fu, Polym. Chem., 2011, 2, 465 RSC.
- U. Mansfeld, C. Pietsch, R. Hoogenboom, C. R. Becer and U. S. Schubert, Polym. Chem., 2010, 1, 1560 RSC.
- G. Franc and A. K. Kakkar, Chem. Soc. Rev., 2010, 39, 1536 RSC.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355 RSC.
- C. O. Kappe and E. Van der Eycken, Chem. Soc. Rev., 2010, 39, 1280 RSC.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620 CrossRef CAS.
- P. A. Brady, R. P. Bonar-Law, S. J. Rowan, C. J. Suckling and J. K. M. Sanders, Chem. Commun., 1996, 319 RSC.
- P. A. Brady and J. K. M. Sanders, Chem. Soc. Rev., 1997, 26, 327 RSC.
- J.-M. Lehn, Chem.–Eur. J., 1999, 5, 2455 CrossRef CAS.
- J. K. M. Sanders, Pure Appl. Chem., 2000, 72, 2265 CrossRef CAS.
- L. M. Greig and D. Philp, Chem. Soc. Rev., 2001, 30, 287 RSC.
- R. L. E. Furlan, S. Otto and J. K. M. Sanders, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4801 CrossRef CAS.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898 CrossRef.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652 CrossRef CAS.
- J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151 RSC.
- A. Granzhan and T. Riis-Johannessen, Angew. Chem., Int. Ed., 2010, 49, 5515 CrossRef CAS.
- E. Moulin, G. Cormosw and N. Giuseppone, Chem. Soc. Rev., 2012, 41, 1031 RSC.
- Y. H. Sohma and B. H. K. Stephen, J. Am. Chem. Soc., 2009, 131, 16313 CrossRef CAS.
- C. R. Li, R. M. Broyer and H. D. Maynard, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5004 CrossRef.
- L. C. Karen, M. R. Broyer, P. Z. Tolstyka and D. H. Maynard, J. Mater. Chem., 2007, 17, 2021 RSC.
- P. L. Miranda, H. S. Yan, W. Jason, S. C. Yun, K. Ting, G. Rod, Y. Chinn, N. Fraud, O. D. Bill, Y. Jay and W. Jill, J. Am. Chem. Soc., 2007, 129, 13153 CrossRef.
- J. C. Hua, W. Z. Guang, O. Robin and K. Rose, Bioconjugate Chem., 2003, 14, 614 CrossRef.
- S. Carmona, M. R. Jorgensen, S. Kolli, C. Crowther, F. H. Salazar, P. L. Marion, M. Fujino, Y. Natori, M. Thanou, P. Arbuthnot and A. D. Miller, Mol. Pharmaceutics, 2008, 6, 706 Search PubMed.
- L. He, Y. Jiang, C. Tu, G. Li, B. Zhu, C. Jin, Q. Zhu, D. Yan and X. Zhu, Chem. Commun., 2010, 46, 7569 RSC.
- J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814 CrossRef CAS.
- R. Nguyen, L. Allouche, E. Buhler and N. Giuseppone, Angew. Chem., Int. Ed., 2009, 48, 1093 CrossRef CAS.
- R. Nguyen, E. Buhler and N. Giuseppone, Macromolecules, 2009, 42, 5913 CrossRef CAS.
- Y. Ruff, E. Buhler, S.-J. Candau, E. Kesselman, Y. Talmon and J.-M. Lehn, J. Am. Chem. Soc., 2010, 132, 2573 CrossRef CAS.
- L. J. Zhu, Y. F. Shi, C. L. Tu, R. B. Wang, Y. Pang, F. Qiu, X. Y. Zhu, D. Y. Yan, L. He, C. Y. Jin and B. S. Zhu, Langmuir, 2010, 26, 8875 CrossRef CAS.
- J. Yuan, Y. Li, X. Li, S. Cheng, L. Jiang, L. Feng and Z. Fan, Eur. Polym. J., 2003, 39, 767 CrossRef CAS.
- S. Fujii and J.-M. Lehn, Angew. Chem., Int. Ed., 2009, 48, 7635 CrossRef CAS.
- J. F. Folmer-Andersen and J.-M. Lehn, Angew. Chem., Int. Ed., 2009, 48, 7664 CrossRef CAS.
- J. F. Folmer-Andersen, E. Buhler, S.-J. Candau, S. Joulie, M. Schmutzc and J.-M. Lehn, Polym. Int., 2010, 59, 1477 CrossRef CAS.
- T. Ono, T. Nobori and J.-M. Lehn, Chem. Commun., 2005, 1522 RSC.
- T. Ono, S. Fujii, T. Nobori and J.-M. Lehn, Chem. Commun., 2007, 46 RSC.
- C.-F. Chow, S. Fujii and J.-M. Lehn, Chem. Commun., 2007, 4363 RSC.
- C.-F. Chow, S. Fujii and J.-M. Lehn, Angew. Chem., Int. Ed., 2007, 46, 5007 CrossRef CAS.
- G. Deng, C. Tang, F. Li, H. Jiang and Y. Chen, Macromolecules, 2010, 43, 1191 CrossRef CAS.
- G. Kavanagh and S. Ross-Murphy, Prog. Polym. Sci., 1998, 23, 533 CrossRef CAS.
- S. D. Bergman and F. J. Wudl, J. Mater. Chem., 2008, 18, 41 RSC.
- D. Y. Wu, S. Meure and D. Solomon, Prog. Polym. Sci., 2008, 33, 479 CrossRef CAS.
- Y. Jin, L. Song, Y. Su, L. Zhu, Y. Pang, F. Qiu, G. Tong, D. Yan, B. Zhu and X. Zhu, Biomacromolecules, 2011, 12, 3460 CrossRef CAS.
- R. Heathcote, J. A. S. Howell, N. Jennings, D. Cartlidge, L. Cobden, S. Coles and M. Hursthouse, Dalton Trans., 2007, 1309 RSC.
- T. Ono, S. Fujii, T. Noboriab and J.-M. Lehn, Chem. Commun., 2007, 4360 RSC.
- D. Nepal, S. Samal and K. Geckeler, Macromolecules, 2003, 36, 3800 CrossRef CAS.
- Y. Liu, Y. Zhao, H. Zhang, X. Li, P. Liang, X. Zhang and J. Xu, Macromolecules, 2004, 37, 6362 CrossRef CAS.
- C. Wang, G. Wang, Z. Wang and X. Zhang, Chem.–Eur. J., 2011, 17, 3322 CrossRef CAS.
- L. Tauk, A. P. Schröder, G. Decher and N. Giuseppone, Nat. Chem., 2009, 1, 649 CrossRef CAS.
- H. Otsuka, Y. Nagasaki and K. Kataoka, Biomacromolecules, 2000, 1, 39 CrossRef CAS.
- J. Gu, W. P. Cheng, J. Liu, S. Y. Lo, D. Smith, X. Qu and Z. Yang, Biomacromolecules, 2008, 9, 255 CrossRef CAS.
- H. Saito and A. Hoffman, J. Bioact. Compat. Polym., 2007, 22, 589 CrossRef CAS.
- J. Gu, W. Cheng, J. Liu, S. Lo, D. Smith, X. Qu and Z. Yang, Biomacromolecules, 2008, 9, 255–262 CrossRef CAS.
- A. Jackson, C. Stakes and D. Fulton, Polym. Chem., 2011, 2, 2500 RSC.
- A. Jackson and D. Fulton, Chem. Commun., 2011, 47, 6807 RSC.
- X. Xu, J. D. Flores and C. L. McCormick, Macromolecules, 2011, 44, 1327 CAS.
- A. W. Jackson and D. A. Fulton, Macromolecules, 2012, 45, 2699 CrossRef CAS.
- B. Murray and D. Fulton, Macromolecules, 2011, 44, 7242 CAS.
- Y. Zhang, L. Tao, S. Li and Y. Wei, Biomacromolecules, 2011, 12, 2894 CrossRef CAS.
- B. T. Hofreiter, I. A. Wolff and C. L. Mehltretter, J. Am. Chem. Soc., 1957, 79, 6457 CrossRef CAS.
- V. Crescenzi, A. Gamini and G. Paradossi, Carbohydr. Polym., 1983, 3, 273 CrossRef CAS.
- D. Schulz and P. Rapp, Carbohydr. Res., 1991, 222, 223 CrossRef CAS.
- Y. Fang, R. Takahashi and K. Nishinari, Biomacromolecules, 2005, 6, 3202 CrossRef CAS.
- C. Ding, L. Zhao, F. Liu, J. Cheng, J. Gu, S. Dan, C. Liu, X. Qu and Z. Yang, Biomacromolecules, 2010, 11, 1043 CrossRef CAS.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed., 2003, 42, 4640 CrossRef CAS.
- R. Haag, Angew. Chem., Int. Ed., 2004, 43, 278 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |