Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A ferroelectric proton conductor with colossal polarization induced by in-plane symmetry breaking in a two-dimensional coordination polymer†
Yanqing Songa,
Yuta Tsuji b,
Kunihisa Sugimotoc,
Takashi Kikuchid,
Yuxin Shia,
Yusuke Murakamie,
Kotaro Hiramatsua,
Benjamin Le Ouay
b,
Kunihisa Sugimotoc,
Takashi Kikuchid,
Yuxin Shia,
Yusuke Murakamie,
Kotaro Hiramatsua,
Benjamin Le Ouay a,
Masaaki Ohba
a,
Masaaki Ohba *a and
Ryo Ohtani
*a and
Ryo Ohtani *a
*a
aDepartment of Chemistry, Faculty of Science, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: ohtani@chem.kyushu-univ.jp
bFaculty of Engineering Sciences, Kyushu University, Kasuga, Fukuoka 816-8580, Japan
cDepartment of Chemistry, Kindai University, 3-4-1 Kowakae, Higashi-osaka, Osaka 577-8502, Japan
dRigaku Corporation, 3-9-12 Matsubara-cho, Akishima-shi, Tokyo 196-8666, Japan
ePh.D. Program in Humanics, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8577, Japan
First published on 26th June 2025
Abstract
Noncentrosymmetric two-dimensional (2D) coordination polymers/metal–organic frameworks (CPs/MOFs) are very rare and their functionalities have not been explored. Herein, we report the first 2D ferroelectric proton conductor based on cyanido-bridged undulating 2D CPs. [Mn(salen)]2[ReN(CN)4(MeCN)]·H2O (MnReMeCN·H2O) crystallized in the Pna21 space group and underwent in-plane symmetry breaking upon incorporation of water within the crystal layers. Thus, the dehydrated MnReMeCN layers were centrosymmetric and exhibited reversible switching of second harmonic generation induced by water vapor. The ferroelectricity of MnReMeCN·H2O was strongly coupled with ion conduction, yielding a colossal polarization of 21 mC cm−2, which was determined via positive-up–negative-down measurements at 0.005 Hz and 298 K for single crystals. Moreover, MnReMeCN exhibited anisotropic thermal expansion based on the undulation change, while the zigzag angle changes of the layers switched between a decrease and an increase at around 300 K. These transformations corroborate the characteristic relationship between the zigzag pitch and the interlayer interaction of the undulating layered structures.
Introduction
Two-dimensional (2D) coordination polymers/metal–organic frameworks (CPs/MOFs) are modern functional materials consisting of coordination layered structures,1–5 in which the anisotropic structural design of the layers allows tailoring functionalities such as magnetic,6,7 electronic,8–12 and adsorption properties.13,14 Among the various structural motifs of the layers, noncentrosymmetric layers constitute a fascinating structure with symmetry breaking that provides polar functionalities such as ferro-, pyro-, and piezoelectricity and second harmonic generation (SHG) activity.15–21 However, the design and synthesis of such noncentrosymmetric structures is difficult, which renders the synthesis of noncentrosymmetric 2D CPs/MOFs and the investigation of their ferroelectricity challenging tasks.Recently, multifunctionalities based on polarity have been investigated by combining magnetism, luminescence, ion conduction, and guest responsivity.22–29 Metal-complex-based solid-state materials are composed of characteristic flexible frameworks with coordination networks, yielding host–guest polar systems. Notably, the incorporation of guest species such as water within the lattice of polarity-switchable crystals has been demonstrated to induce symmetry breaking.28–31 In addition, the presence of guest water in polar crystals leads to the formation of specific hydrogen bonding, which results in unique functionalities such as super-ion conduction32 and ferroelectricity.33,34 Importantly, recent experimental and theoretical investigations of such ion-conductive polar systems suggested that long-range ion displacement in crystals causes anomalous polarization phenomena, giving rise to ferroelectric ion conductors.35,36 This long-range ion displacement constitutes a new polarization mechanism involving strong correlation between the conduction ions and the polar skeleton. Compared with conventional displacement-type and order–disorder-type transitions, a large polarization greater than 1 mC cm−2 is achieved. Thus, exploring new systems underpinned by long-range ion displacement is important for the development of advanced ferroelectric materials. However, coupling ferroelectricity with ion conduction in polar crystals is challenging and still rare.33,34
Our group investigated undulating layer–type 2D CPs/MOFs, in particular [M(salen)]2[M′(CN)4(solvent)] (M = Mn and Fe; M′ = MnN, ReN, Pt, and PtI2; solvent = MeOH and MeCN), and explored their anisotropic properties, such as thermal expansion, compression, and ion conduction.37–39 Unlike flat layers, undulating layers contain tunable alignments of dipoles via in-plane distortion (Fig. 1a). Notably, the [ReN(CN)4]2− unit, which has been extensively studied as a luminescent complex,40–44 was found to be a characteristic building block to construct undulating layers owing to its unique umbrella-shaped geometry with a dipole. For example, [MnIII(salen)]2[ReV(CN)4(H2O)2]·H2O incorporated in-plane distortion due to interlayer interaction, resulting in dipole cancellation; therefore, centrosymmetric crystals were obtained.45 This result indicated that effectively controlling the direction of in-plane distortion within undulating 2D CP structures could lead to polar structures exhibiting ion conduction. On the basis of this design idea, we further explored the polar structures and their functionalities via layer modification using other solvent molecules (Fig. 1a).
 | ||
| Fig. 1 (a) Structural design of noncentrosymmetric layers based on undulation. (b) Schematic of the polarization inversion of a 2D ferroelectric ion conductor based on long-range ion displacement. | ||
In this study, [MnIII(salen)]2[ReVN(CN)4(MeCN)]·H2O (MnReMeCN·H2O) was synthesized as the first 2D ferroelectric proton conductor. This compound exhibited water-dependent SHG-switching via in-plane symmetry breaking of layers and ferroelectricity with an extremely large polarization (21 mC cm−2, 0.005 Hz) coupled with ion transport (Fig. 1b). Moreover, dehydrated nonpolar MnReMeCN showed switching of the anisotropic thermal expansion (ATE) with undulation changes at 300 K, resulting in a characteristic relationship between the layer undulation and interlayer distances.
Results and discussion
Crystal structures and polarity switching
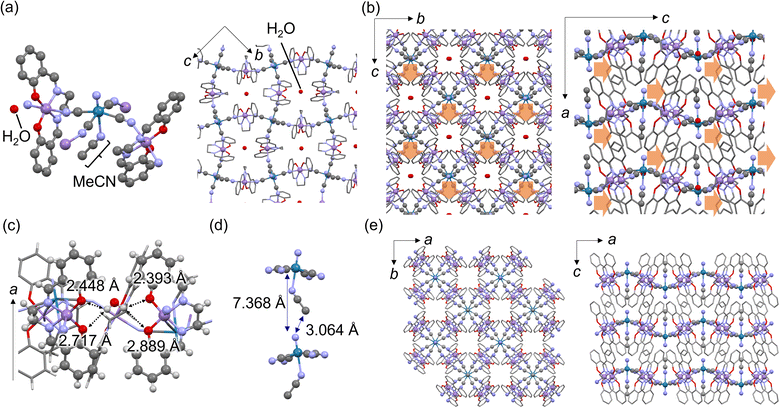
Block-type single crystals of MnReMeCN·H2O were synthesized by slowly mixing MeCN solutions of (PPh4)2[ReN(CN)4(MeOH)]·3MeOH44 and water solutions of [Mn(salen)(H2O)2]Cl (Fig. S1†). At 100 K, MnReMeCN·H2O crystallized in the orthorhombic noncentrosymmetric Pna21 space group (Table S1†). MnReMeCN·H2O consists of cyanido-bridged undulating layers expanding in the bc plane and stacking in the a axis direction (Fig. 2a and b). MeCN coordinates to the axial position of the [ReN(CN)4]2− units. Water exists at the center of the grids and forms hydrogen bonds with the oxygen atoms of the salen ligands (Fig. 2c). This water is removed at 350 K, as confirmed by TG-DTA (Fig. S2†). Uniquely, the symmetry breaking of the layered structures of MnReMeCN·H2O involves in-plane distortion caused by the interlayer interaction between nitrido groups and MeCN moieties (Fig. 2d). Due to their repulsion, the Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group is tilted at 4.3° along the c-axis direction and MeCN is tilted from the a axis. The polarization value of MnReMeCN·H2O was calculated to be 0.57 μC cm−2 on the basis of its crystal structure (Fig. S3†).
N group is tilted at 4.3° along the c-axis direction and MeCN is tilted from the a axis. The polarization value of MnReMeCN·H2O was calculated to be 0.57 μC cm−2 on the basis of its crystal structure (Fig. S3†).
The lattice water within the grid was found to be responsible for the symmetry breaking of the layers. Dehydrated MnReMeCN crystallized in the P4/ncc space group at 400 K (Fig. 2e and Table S2†). The cyanido-bridged layers remained intact, but the in-plane distortion disappeared with the removal of the lattice water. This demonstrates that MnReMeCN is a polarity-switchable 2D CP based on displacive-type polarization in response to water adsorption. MnReMeCN gradually adsorbed water molecules with a hysteresis at room temperature, indicating a slow response to water vapor likely due to its dense structure (Fig. S4†). The cyclability of the reversible polarity switching associated with structural transformations induced by water adsorption was confirmed via SHG, PXRD and water adsorption measurements (Fig. S4–S6†).
A comparison between MnReMeCN and its [Mn(salen)]2[MnN(CN)4(MeCN)] (MnMnMeCN)46 and [Fe(salen)]2[MnN(CN)4(MeCN)] (FeMnMeCN)37 analogs suggested that the water responsivity associated with the structural transformation of the undulating layers affects the relationship between the undulating layer size and the flexibility. In particular, MnReMeCN exhibited the largest layers, reflecting its large metal-ion size (MnIII > FeIII and ReV > MnV; Fig. S7†). The medium-sized grids of MnMnMeCN accommodate water molecules, resulting in the conversion of the space group from P4/ncc to Pccn without tilting MeCN,46 whereas the smallest grids of FeMnMeCN provide no space for accommodating water molecules.37 Meanwhile, [Mn(salen)]2[ReN(CN)4(H2O)]·2H2O exhibits a similar in-plane distortion but crystallizes in the nonpolar Pbcn space group.45 These results indicate that the rigidity of MeCN within the large layers accounts for the resultant in-plane symmetry breaking of the coordination layers.
Ferroelectricity coupled with ion conduction
Noncentrosymmetric MnReMeCN·H2O exhibited a notable ferroelectricity with a colossal remnant polarization of 21 mC cm−2 at room temperature and a relative humidity of 85%, as revealed by a positive-up–negative-down (PUND) measurement performed at 0.005 Hz on single crystals (Fig. 3). The voltage for the polarization inversion was 0.04 kV cm−1. Uniquely, as the frequency became faster, the polarization inversion voltage became higher, whereas the polarization value decreased considerably (Fig. 3a and b and S8†). Moreover, no hysteresis was observed at 10–1000 Hz (Fig. 3c). In addition to the characteristic frequency-dependent hysteretic behavior, the proton conduction of MnReMeCN·H2O was confirmed by conducting variable-temperature AC impedance measurements on a crystal, which yielded a conductivity of 3.53 × 10−6 S cm−1 at 298 K and an activation energy (Ea) of 0.61 eV (Fig. 4a and b). By studying the isotope effect using D2O on conductivity (Fig. S9†), we confirmed that proton conduction was caused by proton hopping via water molecules in the lattice.47,48 Note that the water–layer interaction via hydrogen bonds facilitates efficient proton dissociation for conduction. The cyanido and nitrido groups of the layers would connect the pathways through which protons flow. These results demonstrate that MnReMeCN·H2O is a ferroelectric proton conductor33 based on long-range ion displacement, whose ferroelectricity is strongly correlated with proton conduction (Fig. 1b). The proton flow within the lattice amplifies the current involved in the ferroelectric response; that is, the number of conducting and trapped protons increases at slower frequencies, resulting in an increase in the polarization value of the ferroelectricity (Fig. 3b and S10†). As a consequence, the polarization value of MnReMeCN·H2O is much higher than that calculated using the crystal structure (0.57 μC cm−2). Moreover, the decrease in the voltage of polarization inversion at slower frequency is in accord with the protons reaching the electrode interface (Fig. S8†), which strongly supports the proton conduction behavior.Ferroelectric measurements conducted on several single crystals of MnReMeCN·H2O revealed that several crystals exhibited no hysteresis but IV curves based on ion conduction (Fig. 4d). This result is in accordance with the results observed for the [110] and [1−10] directions of the selected crystals. The proton conductivity in the nonpolar direction was 1.55 × 10−6 S cm−1 at 298 K and Ea = 0.51 eV (Fig. 4b and c). The crystal morphology changed considerably after high-voltage (100 V) measurements along the [001] direction (Fig. S11†). Such macroscopic changes are most likely associated with the electric-field-induced structural transformation leading to polarization inversion, indicating the weakness of the 2D crystal formed via van der Waals interactions between the layers. This hypothesis is supported by the fact that no such morphological changes were observed when the P–E measurements were performed along the nonpolar axis (i.e., the [110] or [1–10] direction).
To gain insight into the synergistic properties of polarity inversion and proton flow, the frequency dependency on the current was investigated using the first and second pulses in PUND measurements, demonstrating that the matching dynamics of the skeletons and conduction ions responding to the electric fields are responsible for the anomalous polarization. Specifically, the ratio of the calculated polarization value using the second pulse (Psecond = Isecond pulse × t) and the remnant polarization value of the first pulse (Pfirst remnant) changed nonlinearly (Fig. 3d). Psecond/Pfirst remnant showed the lowest value of 0.11 at 0.05 Hz and values of 0.25 and 0.19 at 0.5 and 0.005 Hz, respectively. Isecond pulse is caused by the conduction of free ions within the channel; that is, when the frequency exceeds a certain threshold, the Psecond/Pfirst remnant increases as more untrapped ions return from the electrode interface where the ions are biased. Thus, the maximum amount of ions involved in the polarization of the ferroelectric ion conductor is determined by the characteristic interaction between the polar skeletons and the conduction ions.
Although a similar proton-conduction-coupled ferroelectricity was demonstrated for K2MnN(CN)4·H2O,33 the voltage for the polarization inversion of MnReMeCN·H2O (0.09 kV cm−1 at 0.1 Hz) was considerably lower than that of K2MnN(CN)4·H2O (0.5 kV cm−1 at 0.1 Hz). This decrease in voltage indicates the occurrence of different polarization mechanisms in MnReMeCN·H2O and K2MnN(CN)4·H2O. In K2MnN(CN)4·H2O, polarization proceeds through a combination of order–disorder and displacive mechanisms with nitrido migration via bond cleavage and reformation. Conversely, MnReMeCN·H2O only displays displacive-type polarization, which requires a change in the layer distortion direction for the polarization inversion, resulting in a low ferroelectric voltage. In addition, the single-pulse and PUND measurements gave relatively close polarization values of 26 and 21 mC cm−2, respectively, for MnReMeCN·H2O. In contrast, for K2MnN(CN)4·H2O, the polarization obtained via the PUND method (56 mC cm−2) was half of that obtained via the single-pulse method (120 mC cm−2). These facts suggest that compared with K2MnN(CN)4·H2O, MnReMeCN·H2O incorporates fewer untrapped protons in the polar skeleton and its leakage current is smaller. The leakage current in ferroelectric behavior with long-range ion displacement is associated with the irreversibility of proton migration. Thus, the Ea of proton conduction would be significant for such characteristic ferroelectric responses. MnReMeCN·H2O and K2MnN(CN)4·H2O exhibited noticeably different Ea values owing to their proton path structures, i.e., hydrophobic interlayer narrow spaces (Ea = 0.61 eV) and hydrophilic 1D channels surrounded with cyanido groups (Ea = 0.48 eV), respectively. This suggests that a high Ea could be beneficial for efficiently using the conduction ions for polarization, although it is not favorable for proton conduction.
Water diffusion behavior
Guest diffusion in crystals is an important phenomenon involved in various functions, such as adsorption, separation, guest-induced structural changes, and ion conduction.49–52 In this sense, it is important to clarify the effect of noncentrosymmetric structures on such diffusion behavior. Thus, to obtain insight into the water migration in the MnReMeCN·H2O framework, the energy differences associated with water flow were calculated by changing the water position between the +c- and −c-axis directions and between the a and b axes (Fig. 5 and S12†). It was found that water flows more easily in the nonpolar a- and b-axis directions than in the c-axis direction. Importantly, the calculated Ea values along the c-axis are different in the +c- and −c-axis directions, with the Ea in the −c direction being lower than that in the +c direction. This directional migration behavior occurs due to the existence of different migration paths stemming from the noncentrosymmetric layered structure with the tilted N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Re–MeCN moieties; specifically, water molecules pass through the Re
Re–MeCN moieties; specifically, water molecules pass through the Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N side in the +c axis and through the MeCN side in the −c-axis direction (Fig. 5b and S13†). Note that directional migration is related to rectification, which has been widely explored in various fields for the construction of sophisticated functional devices that actively transport ions and molecules.53–55 In this context, we recently reported that the rectified water migration behavior of K2MnN(CN)4·H2O also occurred due to the different molecular motions of water depending on the direction along the noncentrosymmetric 1D channel.56 These structure–guest migration relationships prove that polar structures cause unique molecular dynamics and intracrystalline directional diffusion behaviors.
N side in the +c axis and through the MeCN side in the −c-axis direction (Fig. 5b and S13†). Note that directional migration is related to rectification, which has been widely explored in various fields for the construction of sophisticated functional devices that actively transport ions and molecules.53–55 In this context, we recently reported that the rectified water migration behavior of K2MnN(CN)4·H2O also occurred due to the different molecular motions of water depending on the direction along the noncentrosymmetric 1D channel.56 These structure–guest migration relationships prove that polar structures cause unique molecular dynamics and intracrystalline directional diffusion behaviors.
Anisotropic thermal expansion (ATE)
The strong interlayer interaction in the MnReMeCN framework was found to affect considerably the thermally induced changes of the undulating layered structures associated with ATE. The thermal expansion of coordination frameworks has been actively investigated because of their structural flexibility based on dynamic connections and structural anisotropy.57–65 In our previous investigation of the ATE of a series of [M(salen)]2[M′(CN)4(solvent)] compounds (M = Mn and Fe; M′ = MnN, ReN, Pt and PtI2; solvent = MeOH and MeCN), we demonstrated how undulation changes and interlayer interactions influence the ATE behavior.37 In the present study, variable-temperature SC-XRD revealed an anomaly in the ATE of MnReMeCN at around 300 K (Fig. 6 and Table 1). More specifically, the space group changed between P4/ncc at higher temperatures (HT) and P4/n at lower temperatures (LT) (Fig. 6a and Table S2†). Accordingly, although the volume changes were nearly linear at 150–420 K (αV = dV/VdT = 100–103 MK−1; V = crystal volume, T = temperature, M = 10−6), this phase transition at 300 K largely affected the ATE behavior linked with the structural changes of the undulating layers. Structural analyses revealed the unique tendency of the undulation change at 300 K; that is, the undulation angle of the layers decreased in the LT region and oppositely increased in the HT region with increasing temperature (Fig. 6b). Since the structural transformations around the [Mn(salen)]+ moieties were similar and the layer area expanded at high and low temperatures, the interlayer repulsion between the nitrido and MeCN moieties due to the narrow layer stacking of MnReMeCN accounts for this unique anisotropy switch during the thermal expansion (TE) behavior (Fig. S14†). Specifically, MnReMeCNLT, showing a short stacking distance with strong interlayer repulsion, exhibited a high αc (dlc/lcdT = +100 M K−1; lc = c-axis value) and a displacement of the [ReN(CN)4(MeCN)]2− units along the c-axis direction to decrease the zigzag angles of the layers. This structural transformation largely differs from that observed for [Mn(salen)]2[MN(CN)4] analogs (M = Mn and Re), which exhibit similar decreases in the zigzag angles but a negative area TE.45 Meanwhile, in the HT region where the interlayer repulsion is weak, the large area TE (αa = dla/ladT = +48 MK−1; la = a axis value) of MnReMeCNHT caused a large decrease in the layer thickness, yielding a nearly zero αc (+4.5 MK−1) by counterbalancing the interlayer space expansion. Note that this low αc of MnReMeCNHT was also observed for an analog of MnMnMeCN (Table 1).37,46Conclusions
This work reports the first 2D ferroelectric proton conductor consisting of cyanido-bridged undulating coordination layers exhibiting polarity switching and a colossal polarization of 21 mC cm−2 based on long-range ion displacement. The undulating layers with in-plane distortion are a unique structural motif capable of inducing polarization inversion. Such a unique property is not observed in Janus-type layered structures, which are other noncentrosymmetric motifs in 2D materials.66 Thus, understanding and controlling distortion within the undulating layers allows modulating the characteristic symmetry breaking of the layers for the construction of novel functional polar materials. This work also illustrates that 2D materials offer a powerful platform for the development of future ferroelectric ion conductors by coupling anisotropic ion conduction with polarity because layered structures enable the design of anisotropic functionalities based on the in-plane and out-of-plane directions.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for MnReMeCN·H2O (100 K) and MnReMeCN (150, 170, 200, 250, 270, 300, 330, 350, 400 and 420 K) have been deposited at the CCDC under 2410485–2410495 and can be obtained from http://www.ccdc.cam.ac.uk/data_request/cif.Author contributions
R. O. conceived and designed the project. Y. Song, Y. Shi, R. O. and B. L. O. performed all synthetic and characterization experiments. K. S. performed synchrotron radiation measurements for MnReMeCN·H2O. R. O. and T. K. analysed crystal structures. Y. M. and K. H. performed SHG measurements. R. O. and M. O. supervised the project. Y. Song and R. O. wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Grant-in-Aid for Transformative Research Areas (A) “Supra-ceramics” (JSPS KAKENHI grant numbers JP22H05144, JP22H05145 and JP22H05146) and JST, PRESTO Grant Number JPMJPR24M2. This work was also supported by the JSPS KAKENHI grant numbers JP24K21784, JP24K01457, JP21K18936, JP24K01499, JP22K19052, and JP24K08447. This work was also supported by JST FOREST (JPMJFR216R), JSPS Grant-in-Aid for Scientific Research (B) (JP23K23297), and the Konica Minolta Science and Technology Foundation. This work was partially supported by the Cooperative Research Program of the ‘Network Joint Research Center for Materials and Devices’, the Asahi Glass-, the Sumitomo-, the Casio- and the Murata- Foundations. The computations in this work were performed using the computer facilities at the Research Institute for Information Technology, Kyushu University, at the Supercomputer Center, the Institute for Solid State Physics, the University of Tokyo, and at the Cyberscience Center, Tohoku University. The synchrotron radiation experiments were performed at the BL02B1 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2022B0544 and 2022B1633). We thank Prof. Osamu Sato and Dr Shu-Qi Wu for kind support on the magnetic measurements.References
- G. Chakraborty, I. H. Park, R. Medishetty and J. J. Vittal, Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications, Chem. Rev., 2021, 121, 3751–3891 CrossRef CAS PubMed.
- Y. S. Zheng, F. Z. Sun, X. Han, J. L. Xu and X. H. Bu, Recent Progress in 2D Metal-Organic Frameworks for Optical Applications, Adv. Opt. Mater., 2020, 8, 2000110 CrossRef CAS.
- J. Nicks, K. Sasitharan, R. R. R. Prasad, D. J. Ashworth and J. A. Foster, Metal-Organic Framework Nanosheets: Programmable 2D Materials for Catalysis, Sensing, Electronics, and Separation Applications, Adv. Funct. Mater., 2021, 31, 2103723 CrossRef CAS.
- A. Dhakshinamoorth, A. M. Asiri and H. Garcia, 2D Metal-Organic Frameworks as Multifunctional Materials in Heterogeneous Catalysis and Electro/Photocatalysis, Adv. Mater., 2019, 31, 1900617 CrossRef PubMed.
- Y. Peng, Y. S. Li, Y. J. Ban, H. Jin, W. M. Jiao, X. L. Liu and W. S. Yang, Metal-organic framework nanosheets as building blocks for molecular sieving membranes, Science, 2014, 346, 1356–1359 Search PubMed.
- J. Li and R. Q. Wu, Metal-organic frameworks: possible new two-dimensional magnetic and topological materials, Nanoscale, 2020, 12, 23620–23625 Search PubMed.
- P. Perlepe, I. Oyarzabal, A. Mailman, M. Yquel, M. Platunov, I. Dovgaliuk, M. Rouzieres, P. Negrier, D. Mondieig, E. A. Suturina, M. A. Dourges, S. Bonhommeau, R. A. Musgrave, K. S. Pedersen, D. Chernyshov, F. Wilhelm, A. Rogalev, C. Mathoniere and R. Clerac, Metal-organic magnets with large coercivity and ordering temperatures up to 242 degrees C, Science, 2020, 370, 587–592 CrossRef CAS PubMed.
- M. Ko, L. Mendecki and K. A. Mirica, Conductive two-dimensional metal-organic frameworks as multifunctional materials, Chem. Commun., 2018, 54, 7873–7891 RSC.
- C. Park, J. W. Baek, E. Shin and I. Kim, Two-Dimensional Electrically Conductive Metal-Organic Frameworks as Chemiresistive Sensors, ACS Nanosci. Au, 2023, 3, 353–374 CrossRef CAS PubMed.
- P. Apostol, S. M. Gali, A. Su, D. Tie, Y. Zhang, S. Pal, X. D. Lin, V. R. Bakuru, D. Rambabu, D. Beljonne, M. Dinca and A. Vlad, Controlling Charge Transport in 2D Conductive MOFs-The Role of Nitrogen-Rich Ligands and Chemical Functionality, J. Am. Chem. Soc., 2023, 145, 24669–24677 CAS.
- Y. Lu, Y. Y. Zhang, C. Y. Yang, S. Revuelta, H. Y. Qi, C. H. Huang, W. L. Jin, Z. C. Li, V. Vega-Mayoral, Y. N. Liu, X. Huang, D. Pohl, M. Polozij, S. Q. Zhou, E. Cánovas, T. Heine, S. Fabiano, X. L. Feng and R. H. Dong, Precise tuning of interlayer electronic coupling in layered conductive metal-organic frameworks, Nat. Commun., 2022, 13, 7240 CrossRef CAS PubMed.
- T. Kambe, R. Sakamoto, K. Hoshiko, K. Takada, M. Miyachi, J. H. Ryu, S. Sasaki, J. Kim, K. Nakazato, M. Takata and H. Nishihara, π-Conjugated Nickel Bis(dithiolene) Complex Nanosheet, J. Am. Chem. Soc., 2013, 135, 2462–2465 CrossRef CAS PubMed.
- S. Sakaida, K. Otsubo, O. Sakata, C. Song, A. Fujiwara, M. Takata and H. Kitagawa, Crystalline coordination framework endowed with dynamic gate-opening behaviour by being downsized to a thin film, Nat. Chem., 2016, 8, 377–383 CrossRef CAS PubMed.
- D. Tanaka, A. Henke, K. Albrecht, M. Moeller, K. Nakagawa, S. Kitagawa and J. Groll, Rapid preparation of flexible porous coordination polymer nanocrystals with accelerated guest adsorption kinetics, Nat. Chem., 2010, 2, 410–416 CrossRef CAS PubMed.
- M. Runowski, D. Marcinkowski, K. Soler-Carracedo, A. Gorczynski, E. Ewert, P. Wozny and I. R. Martín, Noncentrosymmetric Lanthanide-Based MOF Materials Exhibiting Strong SHG Activity and NIR Luminescence of Er: Application in Nonlinear Optical Thermometry, ACS Appl. Mater. Interfaces, 2023, 15, 3244–3252 CrossRef CAS PubMed.
- K. Taniguchi, M. Nishio, N. Abe, P. J. Huang, S. Kimura, T. H. Arima and H. Miyasaka, Magneto-Electric Directional Anisotropy in Polar Soft Ferromagnets of Two-Dimensional Organic-Inorganic Hybrid Perovskites, Angew. Chem., Int. Ed., 2021, 60, 14350–14354 CrossRef CAS PubMed.
- Y. Lee, J. Park, S. Cho, Y. E. Shin, H. Lee, J. Kim, J. Myoung, S. Cho, S. Kang, C. Baig and H. Ko, Flexible Ferroelectric Sensors with Ultrahigh Pressure Sensitivity and Linear Response over Exceptionally Broad Pressure Range, ASC Nano., 2018, 12, 4045–4054 CrossRef CAS PubMed.
- S. D. Nguyen, J. Yeon, S. H. Kim and P. S. Halasyamani, BiO(IO3): A New Polar Iodate that Exhibits an Aurivillius-Type (Bi2O2)(2+) Layer and a Large SHG Response, J. Am. Chem. Soc., 2011, 133, 12422–12425 CrossRef CAS PubMed.
- Y. Sekine, R. Akiyoshi and S. Hayami, Recent advances in ferroelectric metal complexes, Coord. Chem. Rev., 2022, 469, 214663 CrossRef CAS.
- P. P. Shi, Y. Y. Tang, P. F. Li, W. Q. Liao, Z. X. Wang, Q. Ye and R. G. Xiong, Symmetry breaking in molecular ferroelectrics, Chem. Soc. Rev., 2016, 45, 3811–3827 RSC.
- W. Zhang and R. G. Xiong, Ferroelectric Metal-Organic Frameworks, Chem. Rev., 2012, 112, 1163–1195 CrossRef CAS PubMed.
- V. Jornet-Mollá, Y. Duan, C. Giménez-Saiz, Y. Y. Tang, P. F. Li, F. M. Romero and R. G. Xiong, A Ferroelectric Iron(II) Spin Crossover Material, Angew. Chem., Int. Ed., 2017, 56, 14052–14056 CrossRef PubMed.
- G. Yuan, Y. Kimura, T. Kobayashi, T. Takeda, N. Hoshino and T. Akutagawa, Ion polarisation-assisted hydrogen-bonded ferroelectrics in liquid crystalline domains, Chem. Sci., 2021, 12, 13520–13529 RSC.
- S. D. Zhu, J. J. Hu, L. Dong, H. R. Wen, S. J. Liu, Y. B. Lu and C. M. Liu, Multifunctional Zn(II)-Yb(III) complex enantiomers showing second-harmonic generation, near-infrared luminescence, single-molecule magnet behaviour and proton conduction, J. Mater. Chem. C, 2020, 8, 16032–16041 RSC.
- M. Li, M. J. Pietrowski, R. A. De Souza, H. R. Zhang, I. M. Reaney, S. N. Cook, J. A. Kilner and D. C. Sinclair, A family of oxide ion conductors based on the ferroelectric perovskite Na0.5Bi0.5TiO3, Nat. Mater., 2014, 13, 31–35 CrossRef CAS PubMed.
- W. J. Xu, P. F. Li, Y. Y. Tang, W. X. Zhang, R. G. Xiong and X. M. Chen, A Molecular Perovskite with Switchable Coordination Bonds for High-Temperature Multiaxial Ferroelectrics, J. Am. Chem. Soc., 2017, 139, 6369–6375 Search PubMed.
- T. Kimura, T. Goto, H. Shintani, K. Ishizaka, T. Arima and Y. Tokura, Magnetic control of ferroelectric polarization, Nature, 2003, 426, 55–58 CrossRef CAS PubMed.
- F. Kobayashi, M. Gemba, S. Hoshino, K. Tsukiyama, M. Shiotsuka, T. Nakajima and M. Tadokoro, Polarity and Dielectric Property Control Triggered by a Coordinated Solvent Molecule Exchange in Luminescent Mononuclear Aluminium(III) Complexes, Chem.–Eur. J., 2023, 29, e202203937 CrossRef CAS PubMed.
- F. Kobayashi, R. Akiyoshi, D. Kosumi, M. Nakamura, L. F. Lindoy and S. Hayami, Solvent vapor-induced polarity and ferroelectricity switching, Chem. Commun., 2020, 56, 10509–10512 RSC.
- J. Yanagisawa, K. Tanaka, H. Kano, K. Miyata, B. Le Ouay, R. Ohtani and M. Ohba, Vapor-Induced Conversion of a Centrosymmetric Organic-Inorganic Hybrid Crystal into a Proton-Conducting Second-Harmonic-Generation-Active Material, Inorg. Chem., 2022, 61, 15638–15644 CrossRef CAS PubMed.
- H. B. Cui, Z. M. Wang, K. Takahashi, Y. Okano, H. Kobayashi and A. Kobayashi, Ferroelectric porous molecular crystal, [Mn(HCOO)](CHOH), exhibiting ferrimagnetic transition, J. Am. Chem. Soc., 2006, 128, 15074–15075 CrossRef CAS PubMed.
- S. Ohkoshi, K. Nakagawa, K. Imoto, H. Tokoro, Y. Shibata, K. Okamoto, Y. Miyamoto, M. Komine, M. Yoshikiyo and A. Namai, A photoswitchable polar crystal that exhibits superionic conduction, Nat. Chem., 2020, 12, 338–344 Search PubMed.
- J. Yanagisawa, T. Aoyama, K. Fujii, M. Yashima, Y. Inaguma, A. Kuwabara, K. Shitara, B. Le Ouay, S. Hayami, M. Ohba and R. Ohtani, Strongly Enhanced Polarization in a Ferroelectric Crystal by Conduction-Proton Flow, J. Am. Chem. Soc., 2024, 146, 1476–1483 CrossRef CAS PubMed.
- J. L. Lu, R. Luo, J. Y. Zhou, M. N. Hao, C. C. Chai, T. P. Ying, Y. R. Gao, S. F. Jin and X. L. Chen, High, Multiple, and Nonvolatile Polarizations in Organic-Inorganic Hybrid [(CH)(CHCHCl)N]InCl·H2O for Memcapacitor, J. Am. Chem. Soc., 2024, 146, 281–288 CrossRef CAS PubMed.
- X. C. Wang, Y. Y. Ren and M. H. Wu, Unconventional Ferroelectricity with Quantized Polarizations in Ionic Conductors: High-Throughput Screening, J. Phys. Chem. Lett., 2022, 13, 9552–9557 CrossRef CAS PubMed.
- Y. X. Sheng, M. H. Wu and J. M. Liu, Ferroelectricity with Long ion Displacements in Crystals of Non-Polar Point Groups, Adv. Funct. Mater., 2024, 34, 2404665 CrossRef CAS.
- R. Ohtani, J. Yanagisawa, Y. Iwai, B. Le Ouay and M. Ohba, Negative Thermal Expansion of Undulating Coordination Layers through Interlayer Interaction, Inorg. Chem., 2022, 61, 21123–21130 CrossRef CAS PubMed.
- Y. Iwai, S. Kusumoto, R. Suzuki, M. Tachibana, K. Komatsu, T. Kikuchi, S. I. Kawaguchi, H. Kadobayashi, Y. Masubuchi, Y. Yamamoto, Y. Ozawa, M. Abe, K. Hirai, B. Le Ouay, M. Ohba and R. Ohtani, Mechanical Properties of Modulative Undulating Layers in Two-Dimensional Metal-Organic Frameworks, Chem. Mater., 2024, 36, 5446–5455 CrossRef CAS.
- Y. Shi, S. Kimura, Y. Iwai, Y. Tsuji, B. L. Ouay, M. Ohba and R. Ohtani, Experimental and theoretical investigation of anisotropic ion conduction of two-dimensional metal-organic frameworks, Inorg. Chem., 2024, 63, 22194–22202 CrossRef CAS PubMed.
- R. Ohtani, J. E. Xu, J. Yanagisawa, Y. Iwai, T. Ehara, K. Miyata, K. Onda, J. Pirillo, Y. Hijikata, T. Hiraoka, S. Hayami, B. Le Ouay and M. Ohba, Structural-transformation-induced Drastic Luminescence Changes in an Organic-Inorganic Hybrid [ReN(CN)] Salt Triggered by Chemical Stimuli, Angew. Chem., Int. Ed., 2023, 62, e202306853 CrossRef CAS PubMed.
- M. Liberka, J. J. Zakrzewski, M. Heczko, M. Reczynski, S. Ohkoshi and S. Chorazy, Solvent- and Temperature-Driven Photoluminescence Modulation in Porous Hofmann-Type Sr-Re Metal-Organic Frameworks, Inorg. Chem., 2021, 60, 4093–4107 CrossRef CAS PubMed.
- M. Seike, K. Nagata, H. Ikeda, A. Ito, E. Sakuda, N. Kitamura, A. Shinohara and T. Yoshimura, Synthesis and Photoluminescence of Tetracyanidonitridorhenium(V) Complexes with Five-Membered N-Heteroaromatic Ligands and Photoluminescence-Intensity Change, ACS Omega, 2019, 4, 21251–21259 CrossRef CAS PubMed.
- H. Ikeda, A. Ito, E. Sakuda, N. Kitamura, T. Takayama, T. Sekine, A. Shinohara and T. Yoshimura, Excited-State Characteristics of Tetracyanidonitridorhenium(V) and -technetium(V) Complexes with N-Heteroaromatic Ligands, Inorg. Chem., 2013, 52, 6319–6327 CrossRef CAS PubMed.
- H. Ikeda, T. Yoshimura, A. Ito, E. Sakuda, N. Kitamura, T. Takayama, T. Sekine and A. Shinohara, Photoluminescence Switching with Changes in the Coordination Number and Coordinating Volatile Organic Compounds in Tetracyanidonitridorhenium(V) and -technetium(V) Complexes, Inorg. Chem., 2012, 51, 12065–12074 CrossRef CAS PubMed.
- R. Ohtani, H. Yoshino, J. Yanagisawa, H. Ohtsu, D. Hashizume, Y. Hijikata, J. Pirillo, M. Sadakiyo, K. Kato, Y. Shudo, S. Hayami, B. Le Ouay and M. Ohba, Flexibility Control of Two-Dimensional Coordination Polymers by Crystal Morphology: Water Adsorption and Thermal Expansion, Chem.–Eur. J., 2021, 27, 18135–18140 CrossRef CAS PubMed.
- R. Ohtani, A. Grosjean, R. Ishikawa, R. Yamamoto, M. Nakamura, J. K. Clegg and S. Hayami, Zero in-Plane Thermal Expansion in Guest-Tunable 2D Coordination Polymers, Inorg. Chem., 2017, 56, 6225–6233 CrossRef CAS PubMed.
- A. S. Nowick and A. V. Vaysleyb, Isotope effect and proton hopping in high-temperature protonic conductors, Solid State Ionics, 1997, 97, 17–26 CrossRef CAS.
- H. Arcis, J. Plumridge and P. R. Tremaine, Limiting Conductivities of Strong Acids and Bases in D2O and H2O: Deuterium Isotope Effects on Proton Hopping over a Wide Temperature Range, J. Phys. Chem. B, 2022, 126, 8791–8803 CrossRef CAS PubMed.
- Y. Su, J. J. Zheng, K. Otake, N. Hosono, S. Kitagawa and C. Gu, Controlling Guest Diffusion by Local Dynamic Motion in Soft Porous Crystals to Separate Water Isotopologues and Similar Gases, Acc. Chem. Res., 2024, 57, 3455–3464 CrossRef CAS PubMed.
- D. W. Lim, M. Sadakiyo and H. Kitagawa, Proton transfer in hydrogen-bonded degenerate systems of water and ammonia in metal-organic frameworks, Chem. Sci., 2019, 10, 16–33 RSC.
- B. Zheng, L. L. Wang, L. Du, Y. Pan, Z. Lai, K. W. Huang and H. L. Du, Diffusion as a function of guest molecule length and functionalization in flexible metal-organic frameworks, Mater. Horiz., 2016, 3, 355–361 RSC.
- K. D. Kreuer, A. Rabenau and W. Weppner, Vehicle Mechanism, a New Model for the Interpretation of the Conductivity of Fast Proton Conductors, Angew. Chem. Int. Ed., 1982, 21, 208–209 CrossRef.
- J. F. Lu, Y. Yoshida, M. Maesato and H. Kitagawa, High-Performance All-Solid-State Proton Rectifier Using a Heterogeneous Membrane Composed of Coordination Polymer and Layered Double Hydroxide, Angew. Chem., Int. Ed., 2022, 61, e202213077 CrossRef CAS PubMed.
- J. Lu, H. C. Zhang, J. Hou, X. Y. Li, X. Y. Hu, Y. X. Hu, C. D. Easton, Q. Y. Li, C. H. Sun, A. W. Thornton, M. R. Hill, X. W. Zhang, G. P. Jiang, J. Z. Liu, A. J. Hill, B. D. Freeman, L. Jiang and H. T. Wang, Efficient metal ion sieving in rectifying subnanochannels enabled by metal-organic frameworks, Nat. Mater., 2020, 19, 767–774 CrossRef CAS PubMed.
- Q. Liu, K. Xiao, L. P. Wen, H. Lu, Y. H. Liu, X. Y. Kong, G. H. Xie, Z. Zhang, Z. S. Bo and L. Jiang, Engineered Ionic Gates for Ion Conduction Based on Sodium and Potassium Activated Nanochannels, J. Am. Chem. Soc., 2015, 137, 11976–11983 CrossRef CAS PubMed.
- Y. Tsuji and R. Ohtani, Rectified water migration behavior in the noncentrosymmetric channels of a ferroelectric proton conductor, Inorg. Chem., 2025, 64, 3868–3874 CrossRef PubMed.
- Q. L. Gao, J. Q. Wang, A. Sanson, Q. Sun, E. J. Liang, X. R. Xing and J. Chen, Discovering Large Isotropic Negative Thermal Expansion in Framework Compound AgB(CN) via the Concept of Average Atomic Volume, J. Am. Chem. Soc., 2020, 142, 6935–6939 CrossRef CAS PubMed.
- A. F. Sapnik, H. S. Geddes, E. M. Reynolds, H. H. M. Yeung and A. L. Goodwin, Compositional inhomogeneity and tuneable thermal expansion in mixed-metal ZIF-8 analogues, Chem. Commun., 2018, 54, 9651–9654 RSC.
- Z. N. Liu, Q. L. Gao, J. Chen, J. X. Deng, K. Lin and X. R. Xing, Negative thermal expansion in molecular materials, Chem. Commun., 2018, 54, 5164–5176 RSC.
- S. A. Hodgson, J. Adamson, S. J. Hunt, M. J. Cliffe, A. B. Cairns, A. L. Thompson, M. G. Tucker, N. P. Funnell and A. L. Goodwin, Negative area compressibility in silver(I) tricyanomethanide, Chem. Commun., 2014, 50, 5264–5266 RSC.
- N. Lock, Y. Wu, M. Christensen, L. J. Cameron, V. K. Peterson, A. J. Bridgeman, C. J. Kepert and B. B. Iversen, Elucidating Negative Thermal Expansion in MOF-5, J. Phys. Chem. C, 2010, 114, 16181–16186 CrossRef CAS.
- Y. Wu, A. Kobayashi, G. J. Halder, V. K. Peterson, K. W. Chapman, N. Lock, P. D. Southon and C. J. Kepert, Negative Thermal Expansion in the Metal-Organic Framework Material Cu(1,3,5-benzenetricarboxylate), Angew. Chem., Int. Ed., 2008, 47, 8929–8932 CrossRef CAS PubMed.
- P. Lama, A. Hazra and L. J. Barbour, Accordion and layer-sliding motion to produce anomalous thermal expansion behaviour in 2D-coordination polymers, Chem. Commun., 2019, 55, 12048–12051 RSC.
- A. S. Sergeenko, J. S. Ovens and D. B. Leznoff, Designing anisotropic cyanometallate coordination polymers with unidirectional thermal expansion (TE): 2D zero and 1D colossal positive TE, Chem. Commun., 2018, 54, 1599–1602 RSC.
- S. J. Hibble, A. M. Chippindale, A. H. Pohl and A. C. Hannon, Surprises from a simple material–The structure and properties of nickel cyanide, Angew. Chem., Int. Ed., 2007, 46, 7116–7118 CrossRef CAS PubMed.
- Y. Iwai, Y. Imamura, M. Nakaya, M. Inada, B. Le Ouay, M. Ohba and R. Ohtani, Janus-Type Mixed-Valent Copper-Cyanido Honeycomb Layers, Inorg. Chem., 2023, 62, 18707–18713 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental sections, crystal parameters, TG-DTA curves, adsorption isotherms, SHG measurements, calculation results, PXRD patterns, and I–E curves of the PUND measurements. CCDC 2410485–2410495. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08700c |
| This journal is © The Royal Society of Chemistry 2025 |