Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An anion exchange membrane of layered silicalite nanosheets with surface quaternary ammonium cations†
Yukta
Sharma
,
Devraj
Singh
,
Zishu
Cao‡
,
Jan
Gabski
and
Junhang
Dong
 *
*
Department of Chemical and Environmental Engineering, University of Cincinnati, Cincinnati, OH 45221, USA. E-mail: dongj@ucmail.uc.edu
First published on 12th February 2025
Abstract
Silicalite nanosheets (SNs) with surfaced quaternary ammonium cations of the template molecules are demonstrated as a new paradigm of building blocks for anion exchange membranes (AEMs). A multilayered SN membrane is fabricated on porous PVDF to achieve 3 A cm−2 at 2.35 V and coulombic efficiency of 97% in alkaline water electrolysis.
AEM water electrolyzers (AEM-WEs) have great potential for cost-effective production of green hydrogen. To date, a few commercial AEMs have been tested for AEM-WEs, but none has been adopted by industry mainly because of their inadequate chemical stability and mechanical strength, large resistance, and high costs.1 The development of AEMs has been largely focused on synthesizing new anion exchange polymers (AEPs) and innovating strategies for blending ionic and nonionic polymers to reduce the electrical resistance while enhancing the stability and mechanical resistance.2 Some notable progress has been reported on the AEMs of aromatic ionic polymers such as the benzyl trimethylammonium, guanidinium, imidazolium, phosphonium, and metal–organic complex groups, etc.3 In general, increasing the ionic group density in the AEP chains enhances the conductivity but reduces the mechanical stability and vice versa because the hydroxide ion (OH−) attacks the carbon-backbone and the quaternary ammonium groups by intramolecular nucleophilic substitutions and Hoffman elimination. These destructive reactions are thermodynamically favored and worsen under low hydration.4 Thus, improvement of AEM mechanical strength by blending AEPs with nonionic polymers is also limited because the latter reduces water uptake to decrease the stability and conductivity.5
Zeolites are crystalline aluminosilicates containing ordered pores of sizes ranging from 0.3 nm to over 1.0 nm depending on the crystallographic structures. The open-pore zeolite membranes have been demonstrated for proton-conduction while rejecting hydrated metal ions by size-exclusion in aqueous redox flow batteries (RFBs).6 The membranes of MFI-type crystal structure (Fig. S1a and b, ESI†) are particularly desirable for proton conduction because of their excellent stability in both basic and acidic solutions and pore size (∼0.56 nm) nearing the maximum for exclusion of hydrated metal ions (size > 0.6 nm).7 The recent success in the synthesis of MFI zeolite nanosheets (ZNs) has enabled ultrathin ZN-laminated membranes on polymer supports and mixed matrix membranes of preferentially aligned ZNs as proton conductors.8,9 These ZN–polymer composite membranes can achieve high proton selectivity while reducing conduction resistance by shortening the proton transport pathways to enhance the efficiency of aqueous RFBs. However, the sub-nanometer zeolitic channels are unsuitable for anion transport due to strong steric hindrance.
Herein, we demonstrate the silicalite (pure-silica MFI) nanosheets (SNs) with surface quaternary ammonium cations (QAC) of the template molecules as a new type of anionic building block for high-performance AEMs. In this case, the zeolitic channels are occupied by diquaternary bis-1,5(tripropyl ammonium)pentamethylene diiodide (dC5) molecules, which are templates directing the growth of SN.10 The QAC groups of dC5 are exposed at defects of incomplete cell structure (Fig. S1c and d, ESI†) that generate free OH− by ionization in alkaline solutions, i.e., (![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+·OH−)surface →(
N+·OH−)surface →(![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+)surface + OH−aqueous. An SN-layered membrane (SNLM) is coated on a porous PVDF substrate (SNLM–PVDF) and demonstrated for application as an efficient AEM for alkaline WE.
N+)surface + OH−aqueous. An SN-layered membrane (SNLM) is coated on a porous PVDF substrate (SNLM–PVDF) and demonstrated for application as an efficient AEM for alkaline WE.
The SNs for membrane coating were obtained by epitaxial growth of two-unit cell-thick nanosheets from silicalite nanoparticle seeds using dC5 as the template followed by extensive cleaning and mechanical removal of the seed-evolved cores (Fig. S2, ESI†).11 The silicalite seeds with dia. ∼30 nm (Fig. S3a, ESI†) were synthesized by TPAOH-templated in situ crystallization. The seeded growth of SN crystals used a precursor containing 80 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.75 dC5
3.75 dC5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 KOH
20 KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9500 H2O.10,12 The resultant crystals were rhombus-shaped (Fig. S3b, ESI†) and were mechanically disintegrated to recover the flat SN flakes by sedimentation removal of the seed-evolved cores.11
9500 H2O.10,12 The resultant crystals were rhombus-shaped (Fig. S3b, ESI†) and were mechanically disintegrated to recover the flat SN flakes by sedimentation removal of the seed-evolved cores.11
The SNLM layer was formed on a porous PVDF film, which was selected for its thermal and chemical stabilities and commercial availability, by vacuum filtration coating (VFC, Fig. S4, ESI†) using a suspension containing 0.02 wt% SN and 0.06 wt% dissolved PVDF in a mixed solvent of 33 wt% DMSO and 67 wt% EtOH.9,11 The VFC-coated SNLM–PVDF was immediately dried in a vacuum oven at 80 °C and ∼1.5 kPa for 2 h and subsequently cured at 120 °C and ∼24 kPa for 3 h. The PVDF binder intrinsically fused the SNLM layer with the PVDF substrate surface to create strong and stable adhesion.9,11
Characterization and tests. The SNs were examined by HR TEM and zeta potential (ζ) analysis in comparison with the SNs after dC5 removal by firing in air at 550 °C for 6 h. The SNLM–PVDF membrane was examined by SEM, XRD, ATR-FTIR, and strain–stress test. The area-specific resistance (ASR) of the SNLM–PVDF was measured for various KOH solution concentrations (CKOH) at different temperatures. A commercially obtained FAA3-50® (δm ∼ 65 μm) AEM was parallelly tested. The EIS measurements and WE tests were performed using a system schematically shown in Fig. S5 (ESI†).
The membrane-electrode assembly (MEA) used 1 cm × 1 cm square electrodes, which were placed in the center of an AEM installed in the 2 cm × 2 cm gasket window (Fig. S5, ESI†). The anode was loaded with 2 mg cm−2 of a commercial NiFe2O4 catalyst (Thermo Scientific Chemicals) and the cathode was loaded with 0.5 mg cm−2 of a commercial Pt/C catalyst (Pt-Vulcan 40%, Fuel Cell Store), both coated on 1 cm × 1 cm Ni felts following the literature procedures (Fig. S6, ESI†). These electrodes were employed because of their reported good performance in AEM WE.13,14 Photos of the actual electrodes are given in Fig. S5c (ESI†) and the SEM images of the electrodes are presented in Fig. S6 (ESI†). The KOH solutions were circulated at a flowrate of 20 mL min−1 on both sides. The performance of the WE cells equipped with the SNLM–PVDF and FAA3-50 AEMs were investigated by polarization curve measurements and stability tests in long-term WE using a 5 wt% KOH electrolyte.
SNs. The as-synthesized SN crystals were of typical rhombus shape (Fig. 1a) where the flat areas are known to have a uniform thickness of two unit-cells (i.e., ∼4 nm) in (010) orientation (i.e., the b-direction of crystal cell).10–12 The intensively cleaned flat SNs were highly perforated (Fig. 1b). The HR TEM images of the SNs revealed large numbers of surface defects (Fig. 1c) of incomplete zeolitic cells where the QAC groups of dC5 are exposed for ionization upon hydration to enhance the anion conductivity. These lattice defects are the result of variations in crystal growth stage during the multicomponent multiphase reactions, which inevitably involve localized nonuniformity of reactant composition, temperature, and crystallization rates. The nonuniform crystallization is also the likely cause of holes in the SNs especially when the secondary growth is terminated prematurely within 4 days.10,12 These holes are desirable for constructing the AEM because more defects and hence surface QACs exist in the edge areas. These holes also shorten the pathways for ion transport across the layered SNs by diffusion through the holes instead of along the entire SN surface.
The surface QAC-OH groups can be readily ionized in the KOH solution, i.e., (![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+·OH−)
N+·OH−) ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) (
(![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+) + OH−. A suspension of dC5-free SNs was also prepared after template removal.11 The surfaced QAC groups (
N+) + OH−. A suspension of dC5-free SNs was also prepared after template removal.11 The surfaced QAC groups (![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+·OH−) in the nonactivated SN were evident by its significantly smaller ζ-potential (ζ = −16.00 ± 1.73 mV) as compared to the activated SNs (ζ = −31.92.0 ± 1.74 mV) in pure water (pH ∼ 7.0). The smaller ζ of the nonactivated SNs in neutral pH is apparently caused by the ionized OH−, which is nonexistent in the activated SNs. The nonactivated and activated SNs had about the same ζ values at pH = 3–4, i.e., ζ = 23.12 ± 2.76 mV for the former and ζ = 26.99 ± 3.40 mV for the latter, because the silica surfaces of both samples are protonated while the exposed QAC-OH in the nonactivated SNs are neutralized in the strongly acidic solution.
N+·OH−) in the nonactivated SN were evident by its significantly smaller ζ-potential (ζ = −16.00 ± 1.73 mV) as compared to the activated SNs (ζ = −31.92.0 ± 1.74 mV) in pure water (pH ∼ 7.0). The smaller ζ of the nonactivated SNs in neutral pH is apparently caused by the ionized OH−, which is nonexistent in the activated SNs. The nonactivated and activated SNs had about the same ζ values at pH = 3–4, i.e., ζ = 23.12 ± 2.76 mV for the former and ζ = 26.99 ± 3.40 mV for the latter, because the silica surfaces of both samples are protonated while the exposed QAC-OH in the nonactivated SNs are neutralized in the strongly acidic solution.
SNLM–PVDF. The amount of SN (mSN) loaded over the coating area (AM) of 7.55 cm2 was ∼0.6 mg, which would form a dense film thickness (δm) of 462 nm based on the silicalite density ρs ∼ 1.72 g cm−3, i.e., δm = mSN/ρs·AM. However, the SNLM on the macropore PVDF surface (Fig. 2a) had a uniform thickness (δm) of 1.0 ± 0.2 μm (Fig. 2b and Fig. S7a, ESI†). Thus, the SN-to-PVDF volume ratio (VSN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VPVDF) within the SNLM layer was estimated to be about 1
VPVDF) within the SNLM layer was estimated to be about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in dry conditions. The dry SNLM layer appeared to be wrinkled but conformed to the PVDF substrate surface without cracking (Fig. 2c) because of the mechanical flexibility of the large 4-nm-thin SNs well-aligned along the a–c plane (Fig. S8, ESI†). The FTIR spectrum of the SNLM–PVDF is apparently convolution of the SN and PVDF spectra with no appreciable sign of new chemical bonds (Fig. 2d), indicating a physically retained SNLM by the PVDF binder.15 The SNLM–PVDF exhibited a tensile stress and a strain at break of 82.3 ± 6.0 MPa and 25.5 ± 2.5%, respectively, that were slightly improved from those of the PVDF substrate, which were 75.2 ± 5.5 MPa and 20.1 ± 3.0%, respectively (Fig. S7b, ESI†). This could be attributed to the minimal PVDF in the suspension coated on the substrate. The FAA3-50 membrane had a very small strain tolerance of ∼2.5% although with a large tensile stress of >1000 MPa.
1 in dry conditions. The dry SNLM layer appeared to be wrinkled but conformed to the PVDF substrate surface without cracking (Fig. 2c) because of the mechanical flexibility of the large 4-nm-thin SNs well-aligned along the a–c plane (Fig. S8, ESI†). The FTIR spectrum of the SNLM–PVDF is apparently convolution of the SN and PVDF spectra with no appreciable sign of new chemical bonds (Fig. 2d), indicating a physically retained SNLM by the PVDF binder.15 The SNLM–PVDF exhibited a tensile stress and a strain at break of 82.3 ± 6.0 MPa and 25.5 ± 2.5%, respectively, that were slightly improved from those of the PVDF substrate, which were 75.2 ± 5.5 MPa and 20.1 ± 3.0%, respectively (Fig. S7b, ESI†). This could be attributed to the minimal PVDF in the suspension coated on the substrate. The FAA3-50 membrane had a very small strain tolerance of ∼2.5% although with a large tensile stress of >1000 MPa.
AEM ASR. In the hydrated SNLM layer, the QAC-OH groups (![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+·OH−) exposed at the SN surface defects are ionized to release exchangeable OH− (Fig. 3a); and meanwhile, the entering and diffusion of cations (e.g., K+) through the SN/PVDF interfacial boundary may be hindered by steric effects of the positively charged (i.e.,
N+·OH−) exposed at the SN surface defects are ionized to release exchangeable OH− (Fig. 3a); and meanwhile, the entering and diffusion of cations (e.g., K+) through the SN/PVDF interfacial boundary may be hindered by steric effects of the positively charged (i.e., ![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) N+) SN surface in the boundary. The SNLM layer had a smaller conductivity (σm = δm/ASR = 0.14–0.21 mS cm−1) than the FAA3-50 (σFAA = 7.08–8.03 mS cm−1), which was consistent with literature values.16 The small ASR of the SNLM–PVDF is a result of the SNLM thinness (δm ∼ 1 μm) and the high conductivity of the electrolyte-filled PVDF substrate (σs = 54–66 mS cm−1). The ASR of the SNLM–PVDF and FAA3-50 decreased when increasing the temperature (Fig. 3b) because of the activated ion transport diffusivity (DOH−). However, the temperature-dependency of ASR was more pronounced for the SNLM–PVDF than for the FAA3-50, suggesting a smaller width of the SN/PVDF boundary and a greater activation energy barrier than the water channels in the AEP. The SNLM–PVDF ASR was slightly larger at <40 °C but became smaller than the FAA3-50 at >40 °C (Fig. 3b).
N+) SN surface in the boundary. The SNLM layer had a smaller conductivity (σm = δm/ASR = 0.14–0.21 mS cm−1) than the FAA3-50 (σFAA = 7.08–8.03 mS cm−1), which was consistent with literature values.16 The small ASR of the SNLM–PVDF is a result of the SNLM thinness (δm ∼ 1 μm) and the high conductivity of the electrolyte-filled PVDF substrate (σs = 54–66 mS cm−1). The ASR of the SNLM–PVDF and FAA3-50 decreased when increasing the temperature (Fig. 3b) because of the activated ion transport diffusivity (DOH−). However, the temperature-dependency of ASR was more pronounced for the SNLM–PVDF than for the FAA3-50, suggesting a smaller width of the SN/PVDF boundary and a greater activation energy barrier than the water channels in the AEP. The SNLM–PVDF ASR was slightly larger at <40 °C but became smaller than the FAA3-50 at >40 °C (Fig. 3b).
The electrolyte-filled PVDF substrate had a low ASR decreasing with increasing CKOH. The ASR of SNLM–PVDF was drastically lowered by over 40% when CKOH increased from 5 wt% to 10 wt% (Fig. 3c), indicating that the OH− concentration (COH−) in SNLM increased accordingly to enhance the σm,6
 | (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH + OH− →
Si–OH + OH− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O− + H2O) in the extremely high COH− that creates negatively charged surfaces to hinder the OH− diffusion.
Si–O− + H2O) in the extremely high COH− that creates negatively charged surfaces to hinder the OH− diffusion.
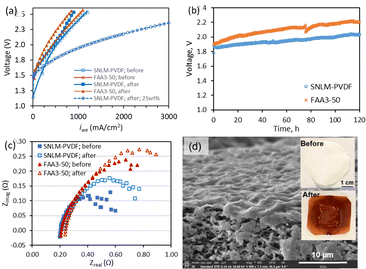
Water electrolysis. The SNLM–PVDF-equipped WE was first tested for alkaline WE at 60 °C using a 5 wt% KOH solution where its ARS was lower than the FAA3-50. The polarization curves (Fig. 4a) show lower operation voltages for WE with the SNLM–PVDF than that with the FAA3-50. In the 120-h WE at a current density (iWE) of 400 mA cm−2, the former operated at a voltage from 1.88 to 2.00 V, which was lower than the latter which operated from 1.91 to 2.20 V (Fig. 4b). The lower operation voltage of the SNLM–PVDF WE may be attributed to its smaller ASR at 60 °C (Fig. 3b) that reduced the Ohmic overpotential (ΔVohm = iWE·ASRcell) in WE. The SNLM–PVDF WE reached a coulombic efficiency of ∼97% (CE = AmiWE/2FrH2; rH2 flowrate of H2) that evidenced negligible internal current and gas crossover through the membrane. The SNLM–PVDF performance was also comparable to AEM WE with similar electrodes in the literature (Table S1, ESI†). The ASR of SNLM–PVDF WE increased slightly from 0.81 to 0.94 Ω cm2 while that of the FAA3-50 WE increased from 0.85 to 0.97 Ω cm2 over 120 h (Fig. 4c). These small increases in cell ASR were largely reversible after resting for 4 h. A polarization curve was then measured for a 25 wt% KOH solution. The WE with SNLM–PVDF achieved remarkable iWE of 1000, 2000, and 3000 mA cm−2 at 1.94, 2.18, and 2.35 V, respectively (Fig. 4a). The SNLM–PVDF was physically undamaged with brown coloration (Fig. 4d) after WE operations due to limited loss of –HF groups from the stable PVDF.17 The SNLM–PVDF showed good reproducibility in WE performance (Fig. S6, ESI†).
In summary, SNs with surface QAC groups were synthesized to fabricate the SNLM–PVDF, which exhibited low ASR for anion conduction and outperformed the commercial FAA3-50 AEM in alkaline WE with lower operation voltages. The results demonstrate that the QAC-templated SNs can be a new class of building blocks for high-performing AEMs for alkaline WE. Our ongoing efforts are focused on developing unsupported SN–polymer mixed membranes with the SN surface aligned along the thickness or in house-of-cards structures to further enhance the ion conduction through the SN/polymer interfaces.
Y. S.: investigation, formal analysis, and data curation. D. S.: investigation, and data curation. Z. C.: investigation. J. G.: resources. J. D.: conceptualization, supervision, formal analysis, writing.
Research supported by the U.S. NSF Grant #CBET-1935205 and the George Rieveschl Jr. Endowment Funds.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- (a) D. Henkensmeier, M. Najibah, C. Harms, J. Žitka, J. Hnát, K. Bouzek and J. Electrochem, Energy Convers. Storage, 2021, 18, 024001 CAS; (b) Y. C. Lei, J. Zhou, W. Zhou, Y. Wang, M. Zhang, A. Zhang and L. Wang, Chem. Commun., 2024, 60, 11000 RSC.
- (a) C. Santoro, A. Lavacchi, P. Mustarelli, V. Di Noto, L. Elbaz, D. R. Dekel and F. Jaouen, ChemSusChem, 2022, 15, 202200027 CrossRef PubMed; (b) Y. S. Kim, ACS Appl. Polym. Mater., 2021, 3, 1250 CrossRef CAS; (c) Z. Zakaria and S. K. Kamarudin, Int. J. Energy Res., 2021, 45, 18337 CrossRef CAS.
- (a) M. G. Marino and K. D. Kreuer, ChemSusChem, 2015, 8, 513 CrossRef CAS PubMed; (b) D. S. Kim, C. H. Fujimoto, M. R. Hibbs, A. Labouriau, Y. K. Choe and Y. S. Kim, Macromolecules, 2013, 46, 7826 CrossRef CAS; (c) Z. Liu, S. D. Sajjad, Y. Gao, H. Yang, J. J. Kaczur and R. I. Masel, Int. J. Hydrogen Energy, 2017, 42, 29661 CrossRef CAS; (d) K. M. Hugar, H. A. Kostalik and G. W. Coates, J. Am. Chem. Soc., 2015, 137, 8730 CrossRef CAS PubMed; (e) Y. Zha, M. L. Disabb-miller, Z. D. Johnson, M. A. Hickner and G. N. Tew, J. Am. Chem. Soc., 2012, 134, 4493 CrossRef CAS PubMed.
- (a) J. Müller, A. Zhegur, U. Krewer, J. R. Varcoe and D. R. Dekel, ACS Mater. Lett., 2020, 2, 168 CrossRef PubMed; (b) P. Shirvanian, A. Loh, S. Sluijter and X. Li, Electrochem. Commun., 2012, 132, 107140 CrossRef.
- Y. Zheng, U. Ash, R. P. Pandey, A. G. Ozioko, J. Ponce-González, M. Handl, T. Weissbach, J. R. Varcoe, S. Holdcroft, M. W. Liberatore, R. Hiesgen and D. R. Dekel, Macromolecules, 2018, 51, 3264 CrossRef CAS.
- (a) Z. Xu, I. Michos, X. Wang, R. Yang, X. Gu and J. Dong, Chem. Commun., 2014, 50, 2416 RSC; (b) Z. Xu, I. Michos, Z. Cao, W. Jing, X. Gu, K. R. Kinkel, S. Murad and J. Dong, J. Phys. Chem. C, 2016, 120, 26386 CrossRef CAS.
- (a) K. R. Hinkle, C. J. Jameson and S. Murad, J. Phys. Chem. C, 2014, 118, 23803 Search PubMed; (b) S. H. Jamali, T. J. H. Vlugt and L. C. Lin, J. Phys. Chem. C, 2017, 121, 11273 CrossRef CAS.
- Y. Xia, H. Cao, F. Xu, Y. Chen, Y. Xia, D. Zhang, L. Dai, K. Qu, C. Lian, K. Huang, W. Xing, W. Jin and Z. Xu, Nat. Sustainable, 2022, 5, 1080 CrossRef.
- L. Iskhakova, Z. Cao, X. Sun, J. Gabski and J. Dong, J. Membr. Sci., 2023, 669, 121328 CrossRef CAS.
- M. Y. Jeon, D. Kim, P. Kumar, P. S. Lee, N. Rangnekar, P. Bai, M. Shete, B. Elyassi, H. S. Lee, K. Narasimharao, S. N. Basahel, S. Al-Thabaiti, W. Xu, H. J. Cho, E. O. Fetisov, R. Thyagarajan, R. F. Dejaco, W. Fan, K. A. Mkhoyan, J. I. Siepmann and M. Tsapatsis, Nature, 2017, 543, 690 CrossRef CAS PubMed.
- Z. Cao, L. Iskhakova, X. Sun, Z. Tang and J. Dong, ACS Appl. Nano Mater., 2012, 4, 2895 Search PubMed.
- Z. Cao, S. Zeng, Z. Xu, A. Arvanitis, S. Yang, X. Gu and J. Dong, Sci. Adv., 2018, 4, eaau8634 CrossRef CAS PubMed.
- S. Kamali, M. Zhiani and H. Tavakol, Renewable Energy, 2020, 154, 1122 Search PubMed.
- (a) G. F. Li, D. Yang and P. Y. Abel Chuang, ACS Catal., 2018, 8(12), 11688 CrossRef CAS; (b) A. S. Pushkarev, I. V. Pushkareva, S. P. du Preez and D. G. Bessarabov, Catalysts, 2023, 13(3), 554 Search PubMed; (c) A. Carbone, S. C. Zignani, I. Gatto, S. Trocino and A. S. Aricò, Int. J. Hydrogen Energy, 2020, 45(16), 9285 CrossRef CAS; (d) Y. S. Park, J. Jeong, Y. Noh, M. J. Jang, J. Lee, K. H. Lee, D. C. Lim, M. H. Seo, W. B. Kim, J. Yang and S. Choi, Appl. Catal., B, 2021, 292, 120170 CrossRef CAS.
- (a) V. Vijayakumar, T. Y. Son, K. S. Im, J. E. Chae, H. J. Kim, T. H. Kim and S. Y. Nam, ACS Omega, 2021, 6, 10168 CrossRef CAS PubMed; (b) D. Ion-Ebras, R. D. Andrei, S. Enache, S. C. C. C. Negrilă, C. Jianu, A. Enache, I. Boeras, E. Carcadea, M. Varlam, B. S. Vasile and J. Ren, Materials, 2021, 14, 4952 CrossRef PubMed.
- C. Lo Vecchio, A. Carbone, I. Gatto and V. Baglio, Polymers, 2023, 15, 1555 CrossRef CAS PubMed.
- Y. Jin, H. Chen, G. Wen, J. Zhou, Z. Wang, F. Zhang and Z. Cui, Eur. Polym. J., 2024, 207, 112816 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: SN synthesis procedure, AEM WE system and cell components, microscopic pictures of electrodes, membrane coating apparatus, strain–stress test results, and table of WE performance summary. See DOI: https://doi.org/10.1039/d4cc06709f |
| ‡ Current address: Department of Chemical Engineering, University of Texas at Tyler, Tyler, TX 75799, USA. |
| This journal is © The Royal Society of Chemistry 2025 |